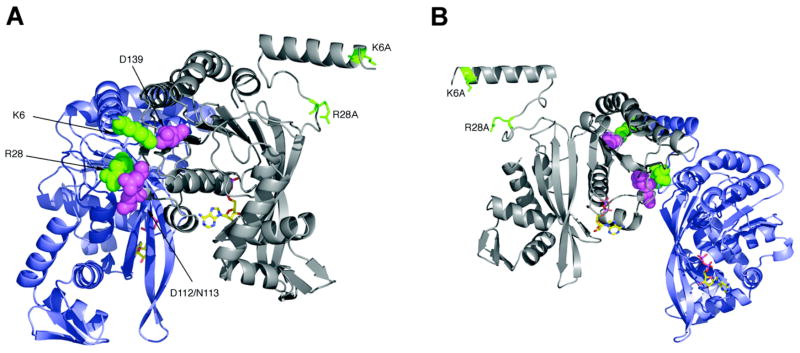
Figure 1.
RecA dimer structure. These views show (A) the outside surface and (B) inside surface of a RecA dimer as it exists within the helical RecA protein filament. The α-carbon backbones of the two subunits are colored gray and blue, with side chains of residues Lys6 and Arg28 in green, Asn112, Asp113 and Asp139 in violet, and ADP in atom colors. The Lys6Ala and Arg28Ala in the N-terminal subunit of the fused dimer are indicated. The images were created using PyMOL (DeLano Scientific LLC) and the pdb file 1REA.