Summary
Paxillin is a multi-domain scaffold protein that localizes to the intracellular surface of sites of cell adhesion to the extracellular matrix. Through the interactions of its multiple protein-protein binding modules, many of which are regulated by phosphorylation, paxillin serves as a platform for the recruitment of numerous regulatory and structural proteins that together control the dynamic changes in cell adhesion, cytoskeletal reorganization and gene expression that are necessary for cell migration and survival. In particular, paxillin plays a central role in coordinating the spatial and temporal action of the Rho family of small GTPases, which regulate the actin cytoskeleton by recruiting an array of GTPase activator, suppressor and effector proteins to cell adhesions. When paxillin was first described eighteen years ago, the amazing complexity of cell adhesion organization, dynamics and signaling was yet to be realized. Herein we highlight our current understanding of how paxillin’s multiple protein interactions contribute to the coordination of cell adhesion function.
Introduction
The interaction of a cell with its microenvironment provides important chemical and spatial cues that contribute to the regulation of processes such as embryonic development, wound healing, immune surveillance and tissue homeostasis, through the modulation of a diverse array of cellular functions such as migration, differentiation and proliferation. The principal cell-surface proteins that are responsible for regulating the binding of a cell to components of the external environment are the integrins. Integrins are transmembrane proteins that comprise an α and a β subunit, which together produce 24 distinct heterodimers (Hynes, 2002) that serve as bridges between the extracellular matrix (ECM) and the intracellular signalling machinery and actin cytoskeleton. Upon their interaction with the extracellular matrix, integrins cluster and recruit a wide variety of intracellular proteins (Figure 1). These macromolecular foci were originally observed in cultured fibroblasts as electron-dense regions of the plasma membrane (Abercrombie et al., 1971) that were termed attachment plaques (Heggeness et al., 1978; Hynes and Destree, 1978) and have subsequently been defined as focal complexes, focal adhesions and fibrillar adhesions (Geiger et al., 2001) depending on their size, cellular localisation or dependency on different members of the Rho GTPase family (Chrzanowska-Wodnicka and Burridge, 1992; Nobes and Hall, 1995; Small et al., 1999; Zamir et al., 2000).
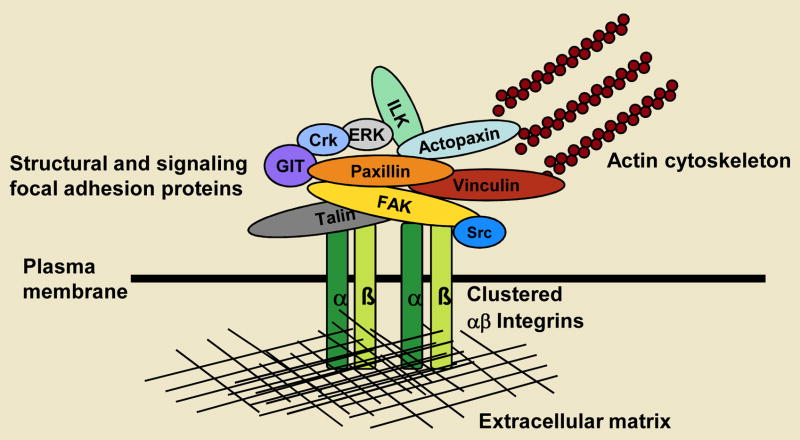
Fig. 1. Focal adhesions provide both a structural and signaling link between the extracellular matrix and the actin cytoskeleton.
Cell adhesion to the extracellular matrix, via transmembrane integrin αβ heterodimers, leads to integrin activation and the recruitment of numerous intracellular proteins to the plasma membrane. Focal adhesions now comprise over 125 proteins (only selected examples are depicted), including both structural and regulatory molecules that mediate a physical link to the actin cytoskeleton and also play a major role in regulating actin dynamics necessary for productive cell migration. Molecules such as paxillin serve as scaffold proteins to facilitate the functional integration of these different categories of focal adhesion proteins.
Integrin cytoplasmic domains have no intrinsic enzymatic activity and so must recruit a variety of proteins to adhesion plaques or contacts to enable these foci to serve as conduits for the transmission of force necessary for cell migration and for bidirectional signalling between the cell interior and its local microenvironment. Originally, the list of focal-adhesion-localised proteins was limited to structural proteins, such as talin and vinculin, that were believed to mediate the anchorage of the integrin cytoplasmic domain to the actin cytoskeleton (Burridge et al., 1988). Now, approximately 37 years after the visualisation of the first cell-adhesion contact, the apparent molecular complexity of focal adhesions continues to increase. Focal adhesions are now reported to comprise upwards of 125 individual proteins, many of which exhibit multiple protein-protein interactions (Turner, 2000; Zaidel-Bar et al., 2007a). Thus, understanding the interactions that govern focal-adhesion function, the regulation of these multiple interactions and their role in coordinating bidirectional signalling remains a considerable challenge.
In 1990, paxillin joined the integrins (Hynes, 1992; Zaidel-Bar et al., 2007a), talin (Burridge and Connell, 1983) and vinculin (Geiger et al., 1980) as one of the earliest members of the “focal adhesion proteome” (Turner et al., 1990). Having first been identified as a 68-kDa protein that exhibited increased tyrosine phosphorylation following the transformation of chick embryonic fibroblasts by the v-Src-expressing Rous sarcoma virus (Glenney and Zokas, 1989), paxillin was subsequently purified from smooth muscle tissue and characterised as a direct binding partner for the vinculin tail domain (Turner et al., 1990). In keeping with the prevailing dogma of the time, in which focal adhesions were believed to function solely as passive, structural links between the ECM and the actin cytoskeleton, the name ‘paxillin’ was coined: it derives from the Latin term paxillus (a peg or stake) and suggests a function that is somewhat analogous to a tent peg in tethering actin stress-fiber cables to the adhesion site (Brown and Turner, 2004; Turner et al., 1990). The discovery that paxillin, along with the recently described focal adhesion kinase (FAK) (Parsons, 2003; Schaller et al., 1992) was also tyrosine phosphorylated in non-transformed cells upon their adhesion to the ECM (Burridge et al., 1992) and in the developing embryo (Turner, 1991; Turner et al., 1993) provided the first indications that focal-adhesion proteins were actively signalling to the cell interior. The early embryonic lethality of mice deficient in paxillin, FAK and fibronectin further reinforced the essential role that focal adhesion proteins play in vivo (Hagel et al., 2002; Ilic et al., 1995). The subsequent analysis of fibroblasts derived from paxillin-deficient embryos has indicated that defects in cell migration might be the primary cause of the severe developmental phenotype.
In this commentary we will first detail the basic molecular architecture of the paxilin molecule and explain its classification as a molecular scaffold. Secondly, we will discuss how paxillin, through tightly regulated interaction with multiple structural and signalling molecules, serves as a nexus for the control and function of the Rho family of GTPases in their capacity as essential regulators of the actin cytoarchitecture and adhesion dynamics. The importance of emerging microscopy technologies and model systems in further unravelling paxillin function and directing future avenues of investigation will also be discussed.
Paxillin structure and the paxillin ‘interactome’
The molecular cloning of paxillin and subsequent peptide-sequence analysis revealed that it comprises numerous discrete structural domains (Brown et al., 1996; Turner and Miller, 1994) that have since been identified as protein-protein binding modules (Brown and Turner, 2004; Turner, 2000). In turn, this has led to the current classification of paxillin as a molecular adaptor or scaffold protein, the primary function of which is to serve as a nexus to coordinate, integrate and facilitate efficient cell signalling, through direct and indirect interactions with multiple signalling and structural proteins that constitute the paxillin “interactome”. The C-terminal half of paxillin contains four Lin11, Isl-1, Mec-3 (LIM) domains, which are double-zinc finger motifs that mediate protein-protein interactions (Perez-Alvarado et al., 1994; Schmeichel and Beckerle, 1994) and are found in all eukaryotes but are absent from prokaryotes (Kadrmas and Beckerle, 2004). The LIM2 and LIM3 domains of paxillin are essential for targeting the protein to focal adhesions (Brown et al., 1996). It has been established that phosphorylation of these domains contributes to the regulation of paxillin focal-adhesion targeting (Brown et al., 1998b), but the identity of the docking protein for paxillin has so far remained elusive, despite the fact that the localisation of paxillin at focal adhesions is an absolute requirement for most paxillin-mediated processes. The LIM domains of paxillin also serve as binding sites for several structural and regulatory proteins, including tubulin and the tyrosine phosphatase PTP-PEST (also known as PTPN12) (Cote et al., 1999; Herreros et al., 2000), and these interactions have important roles in controlling focal-adhesion dynamics (Efimov et al., 2008; Webb et al., 2004).
The N-terminus of paxillin controls most of its signalling activity. It contains five leucine- and aspartate-rich LD motifs (LD1-LD5) which are protein-binding modules that have the consensus sequence LDXLLXXL (Tumbarello et al., 2002). LD motifs were originally identified when the binding sites on paxillin for vinculin and FAK were mapped (Brown et al., 1996; Turner and Miller, 1994) and have since been identified in several proteins of diverse function (Brown et al., 1998a). Molecular modelling predicted that the short LD peptides would probably form amphipathic α-helices, in which the leucine side chains were arranged on a single, hydrophobic face of the helix (Brown et al., 1998a; Sattler et al., 2000; Tumbarello et al., 2002). This secondary structure has since been confirmed by structural NMR studies (Hoellerer et al., 2003). Interestingly, despite their small size and high level of sequence conservation, the individual paxillin LD motifs are able to mediate multiple protein interactions that are both overlapping and specific (Brown and Turner, 2004; Turner, 2000; Turner et al., 1999). The paxillin N-terminus also contains a proline-rich region that was originally identified as a docking site for the SH3 domain of the non-receptor tyrosine kinase Src (Weng et al., 1993). The proline-rich region has subsequently been shown to form a polyproline-II helix upon interaction with the vinculin binding protein ponsin, and this interaction might be important for the formation of costameres, sites of actin –membrane interactions in cardiac muscle (Gehmlich et al., 2007; Zhang et al., 2006).
Multiple tyrosine, serine and threonine phosphorylation sites exist throughout the paxillin molecule (Brown and Turner, 2004; Turner and Miller, 1994; Webb et al., 2005), and are targeted by a diverse array of kinases that are activated in response to various adhesion stimuli and by growth factors. These include, but are not limited to, p21-activated kinase (PAK) (Nayal et al., 2006), FAK-Src (Thomas et al., 1999), receptor for activated C kinase 1 (RACK1; also known as GNBL2L1) (Doan and Huttenlocher, 2007), c-Jun N-terminal kinase (JNK) (Huang et al., 2004b; Huang et al., 2003), extracellular-signal-regulated kinase (ERK; also known as MAPK1) (Ishibe et al., 2003; Ku and Meier, 2000), p38 mitogen-activated protein kinase (p38 MAPK) (Huang et al., 2004a; Huang et al., 2004b), cyclin-dependent kinase 5 (CDK5) (Miyamoto et al., 2007) and c-Abl (Lewis and Schwartz, 1998). Phosphorylation contributes to the complexity of the paxillin interactome by regulating the interactions of various proteins with its protein-binding modules or, as in the case of tyrosine phosphorylation, by providing additional docking sites for other structural and signalling components (Brown and Turner, 2004).
Paxillin interactions in the regulation of Rho GTPases and cell migration
The ability of cells to coordinate the complicated and tightly regulated process of cell migration is fundamental for wound healing, the immune response, angiogenesis and embryogenesis (Li et al., 2005). The spatiotemporal regulation of the Rho family of small GTPases, which includes Cdc42, Rac1 and RhoA, and in turn the localised activation of a wide variety of effector proteins by these GTPases, is essential for controlling the dynamics of the actin cytoskeleton and of actin-associated adhesions during polarised cell migration (Raftopoulou and Hall, 2004; Ridley, 2001a; Ridley, 2001b). Specifically, Cdc42 is required for cell polarization and the formation of filopodia, narrow finger like actin-rich projections at the front of the migrating cell, while Rac promotes the extension of broad membrane sheets or lamellipodia, also at the cell’s leading edge. Rho stimulates actin stress fiber formation necessary for cell contractility and translocation. Cdc42 and Rac also stimulate the assembly of nascent adhesion complexes, while Rho promotes both adhesion maturation at the leading edge and adhesion disassembly at the rear of the cell (Ridley, 2001b)(Raftopoulou and Hall, 2004). In the 18 years since paxillin was first identified as a component of focal adhesions it has emerged as a key coordinator of the Rho GTPase family and their signalling processes in the context of cell spreading and migration (Brown and Turner, 2004; Price et al., 1998).
Paxillin phosphorylation in Rho GTPase signalling
Rho-family GTPases function as molecular switches by cycling between an inactive (GDP-bound) and an active (GTP-bound) state. Cycling is controlled by a large group of guanine-nucleotide-exchange factors (GEFs) that catalyse the exchange of GDP for GTP, and by GTPase-activating proteins (GAPs) that promote the hydrolysis of GTP to GDP (Hoffman and Cerione, 2002). Paxillin contributes to the regulation of the Rho GTPase family, and therefore to the coordination of their downstream signalling to the cytoskeleton, by indirectly recruiting various GEFs, GAPs and effector proteins to cell-ECM contact sites. For example, upon the ligation of an integrin to either a fibronectin or collagen substrate, paxillin becomes tyrosine phosphorylated, primarily on tyrosine residues 31 and 118 (Bellis et al., 1997; Burridge et al., 1992; Petit et al., 2000), in a FAK- and Src-dependent manner ((Schaller and Parsons, 1995). The CrkII-DOCK180-ELMO complex regulates Rac signaling in organisms as diverse as C. elegans, D. melanogaster to higher eukaryotes (Grimsley et al., 2004). This complex can interact with the phosphorylated Y31 and Y118 residues of paxillin via the SH2 domain of Crk2 (Birge et al., 1993; Petit et al., 2000; Valles et al., 2004) and, by means of the unconventional Rac1- and Cdc42-GEF activity of DOCK180 (Brugnera et al., 2002), activates Rac1 to promote cell migration. Interestingly, the phosphorylation Y31 and Y118 has also been shown to regulate RhoA activity. Phosphorylated paxillin binds directly to p120RasGAP, which diminishes the interaction of p120RasGAP with p190RhoGAP at the plasma membrane; this culminates in the suppression of localized RhoA activity by p190RhoGAP (Tsubouchi et al., 2002). Therefore, paxillin that is phosphorylated at Y31 and Y118 can indirectly activate Rac1 and inhibit RhoA, and both of these activities are necessary for efficient leading edge protrusion during cell migration. As phosphorylated Y31 and Y118 cannot bind simultaneously to both CrkII and p120RasGAP, it will be important to determine what additional signals, perhaps in the form of posttranslational modifications or recruitment of additional binding partners, are necessary to control the specificity of these interactions with paxillin. In this regard, it is interesting to note that tyrosine phosphorylation of Y31 and Y118 indirectly enhances the binding of FAK to the adjacent LD motifs of paxillin and has been implicated in focal-adhesion maturation (Zaidel-Bar et al., 2007b).
The paxillin LD4 motif
The LD4 motif of paxillin is a particularly important region of the protein for the regulation of Rho GTPase signalling and focal adhesion turnover. The motif recruits a molecular complex that comprises the proteins G-protein-coupled receptor kinase interacting protein2 (GIT2; also known as paxillin kinase linker (PKL)), PAK-interacting exchange factor (PIX), p21-activated serine/threonine kinase (PAK) and NCK to adhesion sites at the leading edge of migrating cells (Turner et al., 1999; West et al., 2001; Zhao et al., 2000). GIT2 and the closely related GIT1 bind directly to the paxillin LD4 motif (Turner et al., 1999; West et al., 2001; Zhao et al., 2000) and are members of the ArfGAP family. Arf GAPs are negative regulators of Arfs, another family of small GTPases that control membrane trafficking and can in some cases also indirectly stimulate Rac activity and modulate actin dynamics (Premont et al., 2000; Zhao et al., 2000). Alpha and beta-PIX (Manser et al., 1998) display Rac1- and Cdc42-GEF activity and in turn bind to the Cdc42-Rac1 effector PAK (Bokoch, 2003; Brown et al., 2002; Feng et al., 2002; Premont et al., 2004; Turner et al., 1999; West et al., 2001; Zhao et al., 2000). Importantly, fibroblasts that express a paxillin mutant that lacks the LD4 motif, blocking recruitment of the GIT1/2-PIX-PAK-NCK complex to focal adhesions, exhibit abnormal membrane-protrusion dynamics that are caused by sustained global Rac1 activity (West et al., 2001) (Figure 2). These cells are also defective in polarized cell migration (Brown and Turner, 2004; West et al., 2001) and focal-adhesion turnover (Webb et al., 2004).
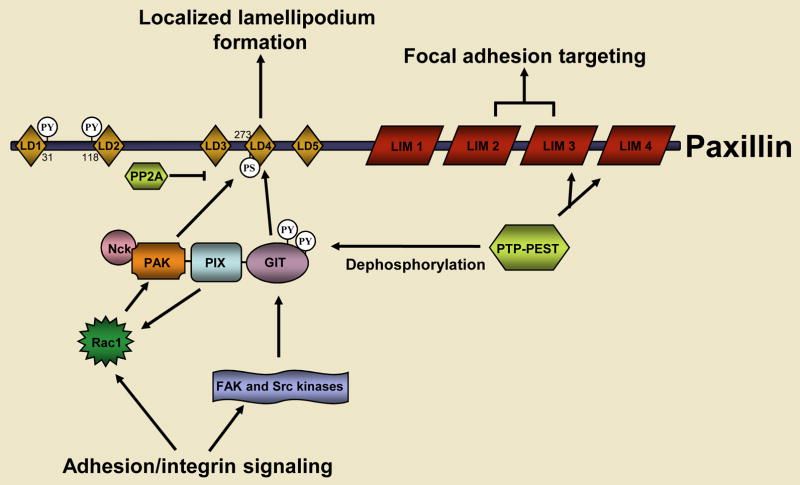
Fig. 2. The LD4 motif of paxillin regulates cell protrusion and retraction.
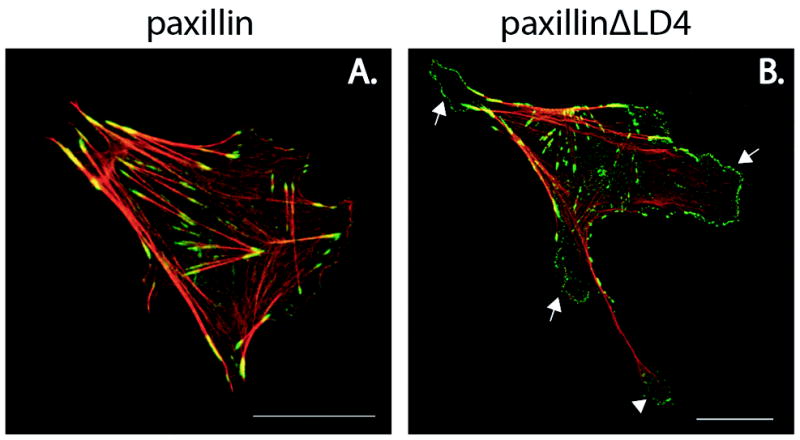
CHO.K1 cells stably transfected with (A) wild-type paxillin or (B.) a mutant of paxillin that lacks the LD4 motif (ΔLD4) were spread on 10μg/ml fibronectin for 2 hours in the presence of serum. Cells were fixed and stained for F-actin (red) and paxillin (green). Deletion of the LD4 domain of paxillin results in extensive peripheral protrusive activity (arrows) and defective tail retraction (arrowhead). It is also of note that the protrusions observed in cells that express paxillin ΔLD4 contain numerous dot-like paxillin-rich focal complexes at their periphery, which are less prevalent in cells that express wild-type paxillin. Scale bar = 20 μm.
The recruitment of the GIT-PIX-PAK-NCK complex to paxillin, and the activity of the complex, are tightly regulated (Figure 3), which is consistent with its crucial role in coordinating Rac1 signalling. The adhesion-dependent activation of Cdc42 and Rac1, which is mediated, in part, by the GEF activity of PIX, combined with FAK-Src-dependent tyrosine phosphorylation of GIT2 promotes a functional unmasking of its paxillin binding site and thus the stable recruitment of the entire GIT-PIX-PAK-NCK complex to nascent focal complexes at the leading edge of the cell (Brown et al., 2005; Brown et al., 2002; Loo et al., 2004; Turner et al., 1999). The kinase activity of PAK is also stimulated upon its binding to GTP-bound Rac1 or Cdc42 (Bokoch, 2003) and, once it is targeted to the cell periphery as a member of the GIT2 complex PAK contributes to focal-adhesion and actin-cytoskeleton remodelling in the developing lamellipodium by phosphorylating LIM kinase and myosin-light-chain kinase (Bokoch, 2003). Recently, the kinase activity of PAK has also been shown to enhance the interaction between the related GIT1-PIX-PAK complex and paxillin; PAK directly phosphorylates serine 273 within the paxillin LD4 motif, which further stimulates local Rac1 signalling (Nayal et al., 2006). Conversely, other studies suggest that the interaction of the GIT1/2-PIX-PAK complex with paxillin might also serve as a termination signal for Rac activity, by means of a mechanism that requires the ArfGAP activity of GIT proteins (Nishiya et al., 2005; West et al., 2001). It is important to note that, although they are closely related, the GIT1 and GIT2 proteins are not functionally redundant (Brown et al., 2005; Frank et al., 2006), and this distinction provides further potential for differential signalling via the binding of the two GIT proteins to paxillin. For instance, it remains to be determined if phosphorylation of S273 by PAK influences GIT2, as well as GIT1 binding to paxillin.
Fig. 3. The coordination of Rac signaling by the paxillin LD4 motif.
Paxillin is one of the earliest proteins that is recruited to nascent focal adhesions and is necessary for the turnover of focal adhesions during cell migration. The engagement of integrins with the extracellular matrix results in the localized activation of Rac and the Src and FAK tyrosine kinases. Together, Rac, Src and FAK promote the recruitment of the GIT-PIX-PAK-NCK complex to focal adhesions by means of an interaction between the paxillin binding subdomain 2 (PBS2) of GIT2 and the paxillin LD4 motif. Rac mediates this process by binding to and activating its effector PAK, which stimulates a multi-step conformational remodeling of the GIT-PIX-PAK-NCK complex and the PAK-dependent phosphorylation of the paxillin LD4 motif at S273. Src-FAK-dependent phosphorylation of GIT is also necessary for its binding to paxillin. The Rac GEF activity of PIX might function in a feed-forward loop to further promote localized Rac signaling to PAK at the cell’s leading edge. The recruitment of the GIT-PIX-PAK-NCK complex to paxillin also serves as a termination signal for Rac activity. This is also regulated at multiple levels, including the PTP-PEST-dependent tyrosine dephosphorylation of GIT, paxillin and FAK, as well as the GIT-dependent inhibition of Arf6 signaling to Rac (not shown). The serine/threonine phosphatase PP2A might also be recruited to paxillin to dephosphorylate S273.
Paxillin and FAK
Interestingly, the phosphorylation of the paxillin LD4 motif at S273 not only promotes GIT1 binding, but also reduces the affinity of FAK for the motif (Nayal et al., 2006). This provides a mechanism by which paxillin might promote GIT1-mediated signalling while simultaneously attenuating the activity and/or localisation of FAK, which has a key role in regulating RhoA activity (Hanks et al., 2003). Structural NMR studies of the LD4 motif provide a potential mechanism for this regulation. In solution, and when phosphorylated at S273, the LD4 peptide exists primarily as a random coil, whereas the non-phosphorylated peptide folds into an ordered α-helix upon its interaction with the C-terminal focal-adhesion-targeting (FAT) domain of FAK (Bertolucci et al., 2005; Bertolucci et al., 2008; Hildebrand et al., 1995; Hoellerer et al., 2003). However, unlike GIT proteins, FAK interacts with the LD2 motif of paxillin in addition to the LD4 motif (Brown et al., 1996; Turner et al., 1999). The FAT domain of FAK is composed of a four-helical bundle, which is stabilised by hydrophobic interactions (Arold et al., 2002; Hayashi et al., 2002). The LD2 and LD4 motifs of paxillin bind preferentially to opposite faces of the FAT helical bundle and interact with hydrophobic patches at the interface of helices 1 and 4, and helices 2 and 3, respectively (Bertolucci et al., 2005) which suggests that paxillin and FAK interact with a stoichiometry of 1:1. Recently, it has been shown that FAK requires only one functional LD motif (LD2 or LD4) for its appropriate localisation to focal adhesions, but must bind to both for maximal activation and downstream signalling (Scheswohl et al., 2008). Therefore, it seems likely that paxillin can fine-tune the balance between the local activity of FAK and Rac1 activity through the phosphorylation of its LD4 motif, which regulates its interaction with the GIT1-PIX-PAK-NCK complex (and possibly GIT2); at the same time, paxillin can maintain its interaction with suboptimally active FAK in adhesion contacts.
Paxillin and PTP-PEST
Not unexpectedly, the action of phosphatases is also essential for adhesion turnover and cell migration (Angers-Loustau et al., 1999; Garton and Tonks, 1999; Stoker, 2005; Webb et al., 2004). A further link between paxillin and Rho GTPase regulation involves the tyrosine phosphatase PTP-PEST, which is recruited to focal adhesions by binding to the paxillin LIM3 and LIM4 domains (Cote et al., 1999). PTP-PEST regulates cell spreading, cell migration and protrusion by decreasing Rac1 activity (Sastry et al., 2002). It does this by interacting directly with paxillin, and inhibiting signalling cascades that are mediated by the LD4 motif and the phosphorylation of Y31 and Y118 (Jamieson et al., 2005). While the mechanism of action of PTP-PEST in this context is incompletely understood, GIT2 has been shown to be a substrate for PTP-PEST (Jamieson et al., 2005); through the dephosphorylation of GIT2, PTP-PEST might act to destabilize the GIT2-PIX-PAK complex and its interaction with paxillin to suppress localized Rac1 activation. PTP-PEST can also dephosphorylate and thereby inactivate both Vav2 (a Rac1 GEF) and p190RhoGAP (a RhoA GAP) and so might modulate the balance between Rac1activity (which drives cell protrusion) and RhoA activity (which stabilises adhesions at the leading edge and promotes cell-rear detachment) (Sastry et al., 2006). Interestingly, preliminary evidence suggests that paxillin also binds indirectly to Vav2 (Jamieson and Turner, unpublished observations). In addition to PTP-PEST, other phosphatases, including PP2A (Ito et al., 2000; Jackson and Young, 2003; Young et al., 2003) and SHP-2 (also known as PTPN11) (Manes et al., 1999), have been reported to bind and dephosphorylate paxillin and undoubtedly have important roles in controlling paxillin interactions, for instance during cell metastasis (Young et al., 2003).
Paxillin, ILK and actopaxin
The paxillin LD1 motif regulates cell adhesion signalling by interacting directly with the integrin-linked kinase (ILK) (Nikolopoulos and Turner, 2001) and with actopaxin (Nikolopoulos and Turner, 2000) (a member of the parvin family of focal-adhesion proteins) (Gimona et al., 2002; Olski et al., 2001) to help recruit both proteins to focal adhesions (Nikolopoulos and Turner, 2002). When complexed with the adapter protein PINCH, ILK and actopaxin play a necessary role in the stabilization of interactions between the plasma membrane and the actin cytoskeleton in motile and non-motile cells (Legate, 2006; Wu, 2004; Zervas et al., 2001), as well as in the regulation of cell survival, proliferation and tissue morphogenesis (Legate, 2006). Both actopaxin and ILK are thought to regulate cell migration by modulating Rho GTPase signalling (Clarke et al., 2004; Filipenko et al., 2005; Khyrul et al., 2004). Parvin family members bind both to PIX (Mishima et al., 2004) and to CdGAP (LaLonde et al., 2006), which activate (PIX) and inhibit (CdGAP) Cdc42 and Rac. Perturbation of these interactions affects the subcellular localization and activity of both PIX and CdGAP, which results in defects in cell spreading and migration (Filipenko et al., 2005; LaLonde et al., 2006; Mishima et al., 2004).
The evolution of the paxillin-Rho GTPase interaction
The role of paxillin in the regulation of Rho family GTPase signaling and cell migration is evolutionarily well conserved. In the yeast Saccharomyces cerevisiae, a paxillin-like protein, Pxl1p, has been described that appears to regulate cell polarity and bud formation by coordinating the activity of RhoA and Cdc42 (Gao et al., 2004; Mackin et al., 2004). Interestingly, the Schizosaccharomyces pombe homologue Pxl1 has also been shown to modulate RhoA activity during cytokinesis (Ge and Balasubramanian, 2008; Pinar et al., 2008). The cellular slime mold Dictyostelium discoideum expresses two isoforms of paxillin, PaxA and PaxB, of which PaxB exhibits closer homology to mammalian paxillin (Bukharova et al., 2005). The deletion of PaxB reveals that it has a key role in regulating cell adhesion and migration during the multicellular phases of D. discoideum development (Bukharova et al., 2005). Paxillin also plays an important role in cell migration and Rho GTPase signaling during development in Drosophila melanogaster (Chen et al., 2005; Llense and Martin-Blanco, 2008) and Xenopus laevis (Iioka et al., 2007). It is also expressed throughout Zebrafish (Danio rerio) development (Crawford et al., 2003), where it exhibits robust changes in tyrosine phosphorylation (Lemeer et al., 2007), similar to that observed during development in higher eukaryotes (Turner, 1991). The precise role of paxillin in Rho GTPase signaling in these model organisms deserves further study.
Paxillin and adhesion dynamics
Adhesion contacts are dynamic structures (Ballestrem et al., 2001; Laukaitis et al., 2001; Webb et al., 2002; Wiseman et al., 2004) that must assemble, disassemble or mature at the extending leading edge, and disassemble at the retracting cell rear, for efficient cell migration to occur (Webb et al., 2002). Paxillin is one of the earliest proteins that is detected in nascent adhesions at the cell’s leading edge, where it is rapidly organised (Digman et al., 2007), which suggests that paxillin plays an important role in promoting the assembly of adhesions and in defining their molecular composition. Integrin clustering cannot be visualised in these paxillin-rich nascent adhesions by using standard approaches (Laukaitis et al., 2001), but the use of image correlation microscopy (ICM) and fluorescence recovery after photobleaching (FRAP) has revealed that integrins do indeed cluster at these sites and are less mobile there than in the surrounding area; this is consistent with integrin engagement with the ECM and/or the cytoskeleton (Wiseman et al., 2004). Ratio image analysis of newly formed adhesions during fibroblast spreading has revealed that paxillin is particularly enriched at the periphery of these small dot-like adhesions (Zimerman et al., 2004). By contrast, the adhesion core is enriched in actin and has high levels of tyrosine phosphorylated protein throughout, which suggests the existence of nascent microarchitecture (Zimerman et al., 2004).
Upon the application of tensile force and the subsequent activation of RhoA, some focal complexes can mature into focal adhesions, thereby providing a physical link to the contractile actomyosin machinery that is required for cell translocation (Balaban et al., 2001; Rottner et al., 1999; Zaidel-Bar et al., 2003). The powerful combination of total internal reflection fluorescence microscopy (TIRFM) and fluorescent speckle microscopy (FSM) indicates that, despite these robust links to the cytoskeleton, individual focal-adhesion components exhibit variable dynamic retrograde flow that appears to relate to whether they interact directly with actin. The retrograde flow of paxillin has a low correlation with actin retrograde flow, whereas the flow of the actin-binding proteins vinculin and α-actinin is more tightly coupled to the motion of actin (Hu et al., 2007). This suggests that, despite biochemical evidence for a direct interaction between paxillin and vinculin (Turner et al., 1990), the dynamic behaviour of the two proteins in focal adhesions can be uncoupled. FRAP analysis confirms this observation, as the dynamics of paxillin in focal adhesions appear to be independent of its association with vinculin (Humphries et al., 2007). One plausible explanation for this apparent contradiction is that the interaction of paxillin with vinculin could serve to structurally stabilise nascent adhesions by providing an early link to the cortical actin network at the cell’s leading edge, while in mature focal adhesions their association is primarily transient and so has little or no influence on the dynamic behaviour of the proteins; this does not however preclude a signaling role for such short-lived encounters.
In addition to its role in the assembly of focal adhesions, paxillin also regulates cell migration by contributing to efficient focal-adhesion disassembly, as indicated by the stabilization of adhesions in cells that are devoid of paxillin (Webb et al., 2004). Paxillin-mediated disassembly might be accomplished in several ways. Paxillin that is phosphorylated at Y31 and Y118 localises to dynamic adhesions (Ballestrem et al., 2006; Laukaitis et al., 2001) and the mutation of Y31 and Y118 to non-phosphorylatable amino acids impairs the disassembly of adhesions at the leading edge of migrating cells (Webb et al., 2004). Expressing a paxillin-LD4-motif deletion mutant has a similar effect (Webb et al., 2004). The precise mechanism by which these regions of paxillin influence adhesion disassembly is currently unclear, but potentially involves the interactions of paxillin with ERK (Ishibe et al., 2003) and FAK-Src to regulate myosin-light-chain-kinase-dependent contractility and perhaps calpain protease activity (Carragher et al., 2003; Webb et al., 2004; Zaidel-Bar et al., 2007b). Indeed, the proteolysis of paxillin by the calcium-dependent cysteine protease calpain 2 (Yamaguchi et al., 1994) has been reported to induce the disassembly of adhesion contacts in smooth muscle cells (Carragher et al., 1999) and to stimulate protrusive activity in fibroblasts (Franco et al., 2004). Conversely, the serine phosphorylation of paxillin at residues 188 and 190 (Bellis et al., 1997) has been shown to stimulate cell migration, and presumably adhesion turnover, by preventing the polyubiquitination of paxillin and its subsequent degradation (Abou Zeid et al., 2006). Furthermore, in X. laevis, the regulation of paxillin stability by polyubiqitination is essential for mesodermal cell migration during the process of convergent extension (Iioka et al., 2007). Finally, it has also been suggested that the targeting of microtubules to adhesions triggers paxillin-mediated disassembly or ‘catastrophes’ of these macromolecular foci, either directly through the binding of tubulin to the paxillin LIM2 and LIM3 domains (Brown and Turner, 2002; Herreros et al., 2000) or through an as-yet-unidentified paxillin-associated factor (Efimov et al., 2008).
Although it is clear that paxillin can employ several mechanisms to regulate adhesion contacts and concomitant cell migration, it has yet to be ascertained whether each of the paxillin-mediated migration control mechanisms are employed at distinct times and subcellular locations in a single migrating cell or whether they are cell-type- or environmental-context-specific. Further complexity has been suggested by the use of emerging, highly sensitive, nano-scale microscopy techniques, which are providing a more detailed dissection of focal-adhesion dynamics and submolecular organisation. Evidence is growing that adhesions are not homogenous clusters of proteins but instead contain subdomains that are enriched in specific focal-adhesion components or display ‘hot spots’ of enzymatic activity. For example, in αvβ3 integrin-mediated adhesions, local variations in the degree of co-localization between the integrin, paxillin and vinculin have been observed (Zimerman et al., 2004). FAK is another protein that is known to interact directly with paxillin and to exhibit heterogeneity in distribution within adhesions. The visualisation of FAK activity using a FRET based biosensor also reveals ‘hot spots’ of FAK activation, which are independent of the fluorescence intensity of FAK or the focal-adhesion size and might therefore indicate potential sub-domains of kinase activity (Cai et al., 2008). Vinculin and paxillin, when conjugated to the spectrally distinct photoactivatable fluorescent proteins Dronpa (Ando et al., 2004) and EosFP (Wiedenmann et al., 2004), can be localised to spatially distinct aggregates within adhesion contacts using high resolution two-color photoactivated localisation microscopy (PALM) (Shroff et al., 2007). It is also of note that the adhesion disassembly that is induced by microtubule targeting, as visualised by time-lapse microscopy of GFP-paxillin expressed in fibroblasts, did not occur homogenously within adhesion contacts, but rather in a punctate manner (Ezratty et al., 2005). The dynamic motion of adhesion proteins, as analysed by fluorescence speckle microscopy, also shows heterogeneity within individual focal adhesions (Hu et al., 2007). There is therefore the potential for a high level of complexity in the spatio-temporal regulation of paxillin and its interactions within a single adhesion contact.
The evolving picture of focal adhesions, from mere homogenous structural plaques to complex, discretely heterogeneous, signalling foci, emphasises the need for continued study to enable greater understanding of the dynamics of paxillin and the architecture of adhesion contacts. This will provide invaluable information on how adhesion contacts are regulated during the cell migration-dependent processes of wound healing, the immune response and embryogenesis, as well as pathological processes such as invasion, metastasis and angiogenesis.
Paxillin and cell survival
Regulated programmed cell death, or apoptosis, and cell proliferation are essential for normal embryonic development. Apoptosis also provides protection against cancer, which can be induced by genetic instability (Hanahan and Weinberg, 2000). The Bcl-2 family of proteins have been shown to provide anti- and pro-apoptotic signals (Youle and Strasser, 2008) and are important in embryogenesis, the immune response and tissue morphogenesis. The interaction of Bcl-2 with the LD4 domain of paxillin promotes cell survival and is required for nephrogenesis during kidney development, although the mechanism of action is as yet unclear (Sheibani et al., 2008; Sorenson, 2004). The structure of Bcl-2 has been solved and, interestingly, reveals an amphipathic helical-bundle-like topology (Petros et al., 2001) that is closely analogous to the characterised LD-binding proteins vinculin (Bakolitsa et al., 1999; Izard et al., 2004), GIT1 (Schmalzigaug et al., 2007) and FAK (Hayashi et al., 2002; Liu et al., 2002).
The interaction of vinculin with the LD1 and LD2 motifs of paxillin (Turner et al., 1999) has also been shown to regulate cell survival. It has been proposed that the tail domain of vinculin competes with FAK for paxillin binding and thereby promotes ERK signalling through FAK or paxillin to prevent apoptosis (Subauste et al., 2004 et al.). Paxillin has also been linked to apoptosis through its identification as a substrate for caspase-3. The cleavage of paxillin at six different aspartic acid residues by caspase-3 (Chay et al., 2002) was proposed to inhibit integrin-mediated cell-survival signals (Frisch and Francis, 1994) and promote apoptosis or anoikis caused by the detachment of cells from the ECM. In cardiomyocytes, apoptosis that is induced by the overexpression of the FAK homologue PYK2 can be inhibited by the coexpression of paxillin, which further implies that pro-survival signals are mediated by paxillin (Melendez et al., 2004). Thus, the involvement of paxillin in regulating cell survival and apoptosis suggests that it plays an essential role during normal developmental processes and could also provide a novel cellular target for cancer therapeutics.
Conclusions and perspectives: what next for paxillin?
In this brief review of paxillin’s formative years, from its humble origins as a structural “peg”, we have focused primarily on highlighting advances in our understanding of the role of paxillin in coordinating the regulation of the cell’s motile machinery, as it is through this function that paxillin exerts the most significant impact on development and tissue morphogenesis (Brown and Turner, 2004; Hagel et al., 2002; Ishibe et al., 2003), as well as on pathologies such as tumor cell invasion (Azuma et al., 2005), fibrosis and atherosclerosis (Mehta and Griendling, 2007). However, the role of paxillin in regulating gene expression (Brown and Turner, 2004) through its interactions with ERK (Ishibe et al., 2003), Poly-A-binding protein (Woods et al., 2002), Abl (Lewis et al., 1996) and steroid receptors, as well as through its own ability to undergo nucleocytoplasmic shuttling (Brown and Turner, 2004) should not be overlooked.
As paxillin comes of age, it is clear that it is important as a nexus for the coordination of numerous signalling pathways through its function as a molecular scaffold. However, there are several instances in which a number of proteins must compete for a single binding domain or motif on the paxillin molecule (Turner, 2000). Thus an important focus of future studies must be the identification of the mechanism(s) by which the cell orchestrates these interactions in a spatio-temporal and context-specific manner. To that end, invaluable information will be obtained from the complete structural determination of paxillin in complex with its interacting partners: this remains a significant technical challenge due to the inherent flexibility of the paxillin amino-terminus. In combination with biochemical and microscopy-based direct binding analyses, this will enable the development of bioinformatics-based modelling of paxillin and its interactome. The recent identification of adhesion sub-domains underscores the complexity of the interactions of paxillin with other proteins, and high-resolution microscope-based technologies will be required to fully understand paxillin’s spatio-temporal modulation of protein-protein interactions, and therefore its regulation of downstream signalling pathways, in two- and three-dimensional environments.
The increasing utilization of more in-vivo-relevant, 3D ECM model systems has identified aspects of cell morphology, focal-adhesion organization and signalling processes that are distinct from those observed using 2D substrates (Figure 4) (Berrier and Yamada, 2007; Cukierman et al., 2001; Damianova et al., 2007; Pankov et al., 2005; Wozniak et al., 2003). It will be important to employ these 3D systems in conjunction with whole-animal models to ascertain the precise role of paxillin and its interactome in cell migration and survival in vivo.
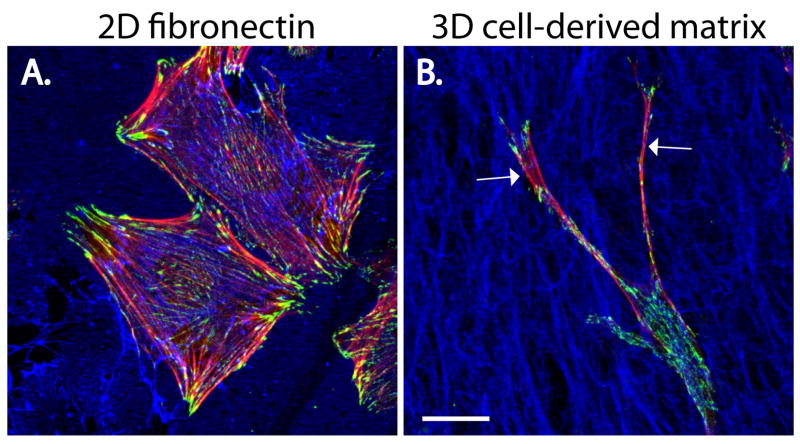
Fig. 4. Paxillin is enriched in adhesions of fibroblasts migrating on either 2D and 3D fibronectin matrices.
Mouse embryonic fibroblasts (MEFs) were allowed to migrate for 16 hours in the presence of serum on (A) a 2D fibronectin-coated surface or (B) a 3D cell-derived fibronectin-rich matrix (CDM) that is more representative of the in vivo environment. Cells were fixed and stained for F-actin (red), paxillin (green) and fibronectin (blue). Although paxillin-rich adhesions are observed in both conditions, MEFs migrating on the 2D fibronectin are well-spread and exhibit robust actin stress fibers, whereas cells migrating within the 3D CDM display long, thin protrusions (arrows) and limited actin organization. Scale bar = 20μm.
Acknowledgments
The authors wish to apologize to colleagues whose work could not be cited because of space limitations. Work in the authors’ laboratory was supported by grants GM47607 and HL070244 from the NIH.
References
- Abercrombie M, Heaysman JE, Pegrum SM. The locomotion of fibroblasts in culture. IV. Electron microscopy of the leading lamella. Exp Cell Res. 1971;67:359–67. doi: 10.1016/0014-4827(71)90420-4. [DOI] [PubMed] [Google Scholar]
- Abou Zeid N, Valles AM, Boyer B. Serine phosphorylation regulates paxillin turnover during cell migration. Cell Commun Signal. 2006;4:8. doi: 10.1186/1478-811X-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando R, Mizuno H, Miyawaki A. Regulated fast nucleocytoplasmic shuttling observed by reversible protein highlighting. Science. 2004;306:1370–3. doi: 10.1126/science.1102506. [DOI] [PubMed] [Google Scholar]
- Angers-Loustau A, Cote JF, Charest A, Dowbenko D, Spencer S, Lasky LA, Tremblay ML. Protein tyrosine phosphatase-PEST regulates focal adhesion disassembly, migration, and cytokinesis in fibroblasts. J Cell Biol. 1999;144:1019–31. doi: 10.1083/jcb.144.5.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arold ST, Hoellerer MK, Noble ME. The structural basis of localization and signaling by the focal adhesion targeting domain. Structure (Camb) 2002;10:319–27. doi: 10.1016/s0969-2126(02)00717-7. [DOI] [PubMed] [Google Scholar]
- Azuma K, Tanaka M, Uekita T, Inoue S, Yokota J, Ouchi Y, Sakai R. Tyrosine phosphorylation of paxillin affects the metastatic potential of human osteosarcoma. Oncogene. 2005;24:4754–64. doi: 10.1038/sj.onc.1208654. [DOI] [PubMed] [Google Scholar]
- Bakolitsa C, de Pereda JM, Bagshaw CR, Critchley DR, Liddington RC. Crystal structure of the vinculin tail suggests a pathway for activation. Cell. 1999;99:603–13. doi: 10.1016/s0092-8674(00)81549-4. [DOI] [PubMed] [Google Scholar]
- Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, Mahalu D, Safran S, Bershadsky A, Addadi L, et al. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat Cell Biol. 2001;3:466–72. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- Ballestrem C, Erez N, Kirchner J, Kam Z, Bershadsky A, Geiger B. Molecular mapping of tyrosine-phosphorylated proteins in focal adhesions using fluorescence resonance energy transfer. J Cell Sci. 2006;119:866–75. doi: 10.1242/jcs.02794. [DOI] [PubMed] [Google Scholar]
- Ballestrem C, Hinz B, Imhof BA, Wehrle-Haller B. Marching at the front and dragging behind: differential alphaVbeta3-integrin turnover regulates focal adhesion behavior. J Cell Biol. 2001;155:1319–32. doi: 10.1083/jcb.200107107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellis SL, Perrotta JA, Curtis MS, Turner CE. Adhesion of fibroblasts to fibronectin stimulates both serine and tyrosine phosphorylation of paxillin. Biochem J. 1997;325(Pt 2):375–81. doi: 10.1042/bj3250375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrier AL, Yamada KM. Cell-matrix adhesion. J Cell Physiol. 2007;213:565–73. doi: 10.1002/jcp.21237. [DOI] [PubMed] [Google Scholar]
- Bertolucci CM, Guibao CD, Zheng J. Structural features of the focal adhesion kinase-paxillin complex give insight into the dynamics of focal adhesion assembly. Protein Sci. 2005;14:644–52. doi: 10.1110/ps.041107205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolucci CM, Guibao CD, Zheng JJ. Phosphorylation of paxillin LD4 destabilizes helix formation and inhibits binding to focal adhesion kinase. Biochemistry. 2008;47:548–54. doi: 10.1021/bi702103n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birge RB, Fajardo JE, Reichman C, Shoelson SE, Songyang Z, Cantley LC, Hanafusa H. Identification and characterization of a high-affinity interaction between v-Crk and tyrosine-phosphorylated paxillin in CT10-transformed fibroblasts. Mol Cell Biol. 1993;13:4648–56. doi: 10.1128/mcb.13.8.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokoch GM. Biology of the p21-activated kinases. Annu Rev Biochem. 2003;72:743–81. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- Brown MC, Cary LA, Jamieson JS, Cooper JA, Turner CE. Src and FAK Kinases Cooperate to Phosphorylate Paxillin Kinase Linker, Stimulate Its Focal Adhesion Localization, and Regulate Cell Spreading and Protrusiveness. Mol Biol Cell. 2005;16:4316–28. doi: 10.1091/mbc.E05-02-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MC, Curtis MS, Turner CE. Paxillin LD motifs may define a new family of protein recognition domains. Nat Struct Biol. 1998a;5:677–8. doi: 10.1038/1370. [DOI] [PubMed] [Google Scholar]
- Brown MC, Perrotta JA, Turner CE. Identification of LIM3 as the principal determinant of paxillin focal adhesion localization and characterization of a novel motif on paxillin directing vinculin and focal adhesion kinase binding. J Cell Biol. 1996;135:1109–23. doi: 10.1083/jcb.135.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MC, Perrotta JA, Turner CE. Serine and threonine phosphorylation of the paxillin LIM domains regulates paxillin focal adhesion localization and cell adhesion to fibronectin. Mol Biol Cell. 1998b;9:1803–16. doi: 10.1091/mbc.9.7.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MC, Turner CE. Roles for the tubulin- and PTP-PEST-binding paxillin LIM domains in cell adhesion and motility. Int J Biochem Cell Biol. 2002;34:855–63. doi: 10.1016/s1357-2725(01)00154-6. [DOI] [PubMed] [Google Scholar]
- Brown MC, Turner CE. Paxillin: adapting to change. Physiol Rev. 2004;84:1315–39. doi: 10.1152/physrev.00002.2004. [DOI] [PubMed] [Google Scholar]
- Brown MC, West KA, Turner CE. Paxillin-dependent paxillin kinase linker and p21-activated kinase localization to focal adhesions involves a multistep activation pathway. Mol Biol Cell. 2002;13:1550–65. doi: 10.1091/mbc.02-02-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugnera E, Haney L, Grimsley C, Lu M, Walk SF, Tosello-Trampont AC, Macara IG, Madhani H, Fink GR, Ravichandran KS. Unconventional Rac-GEF activity is mediated through the Dock180-ELMO complex. Nat Cell Biol. 2002;4:574–82. doi: 10.1038/ncb824. [DOI] [PubMed] [Google Scholar]
- Bukharova T, Weijer G, Bosgraaf L, Dormann D, van Haastert PJ, Weijer CJ. Paxillin is required for cell-substrate adhesion, cell sorting and slug migration during Dictyostelium development. J Cell Sci. 2005;118:4295–310. doi: 10.1242/jcs.02557. [DOI] [PubMed] [Google Scholar]
- Burridge K, Connell L. A new protein of adhesion plaques and ruffling membranes. J Cell Biol. 1983;97:359–67. doi: 10.1083/jcb.97.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K, Fath K, Kelly T, Nuckolls G, Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Burridge K, Turner CE, Romer LH. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J Cell Biol. 1992;119:893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Lietha D, Ceccarelli DF, Karginov AV, Rajfur Z, Jacobson K, Hahn KM, Eck MJ, Schaller MD. Spatial and temporal regulation of focal adhesion kinase activity in living cells. Mol Cell Biol. 2008;28:201–14. doi: 10.1128/MCB.01324-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carragher NO, Levkau B, Ross R, Raines EW. Degraded collagen fragments promote rapid disassembly of smooth muscle focal adhesions that correlates with cleavage of pp125(FAK), paxillin, and talin. J Cell Biol. 1999;147:619–30. doi: 10.1083/jcb.147.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carragher NO, Westhoff MA, Fincham VJ, Schaller MD, Frame MC. A novel role for FAK as a protease-targeting adaptor protein: regulation by p42 ERK and Src. Curr Biol. 2003;13:1442–50. doi: 10.1016/s0960-9822(03)00544-x. [DOI] [PubMed] [Google Scholar]
- Chay KO, Park SS, Mushinski JF. Linkage of caspase-mediated degradation of paxillin to apoptosis in Ba/F3 murine pro-B lymphocytes. J Biol Chem. 2002;277:14521–9. doi: 10.1074/jbc.M111639200. [DOI] [PubMed] [Google Scholar]
- Chen GC, Turano B, Ruest PJ, Hagel M, Settleman J, Thomas SM. Regulation of Rho and Rac signaling to the actin cytoskeleton by paxillin during Drosophila development. Mol Cell Biol. 2005;25:979–87. doi: 10.1128/MCB.25.3.979-987.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M, Burridge K. Rho, rac and the actin cytoskeleton. Bioessays. 1992;14:777–8. doi: 10.1002/bies.950141110. [DOI] [PubMed] [Google Scholar]
- Clarke DM, Brown MC, LaLonde DP, Turner CE. Phosphorylation of actopaxin regulates cell spreading and migration. J Cell Biol. 2004;166:901–12. doi: 10.1083/jcb.200404024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote JF, Turner CE, Tremblay ML. Intact LIM 3 and LIM 4 domains of paxillin are required for the association to a novel polyproline region (Pro 2) of protein-tyrosine phosphatase-PEST. J Biol Chem. 1999;274:20550–60. doi: 10.1074/jbc.274.29.20550. [DOI] [PubMed] [Google Scholar]
- Crawford BD, Henry CA, Clason TA, Becker AL, Hille MB. Activity and distribution of paxillin, focal adhesion kinase, and cadherin indicate cooperative roles during zebrafish morphogenesis. Mol Biol Cell. 2003;14:3065–81. doi: 10.1091/mbc.E02-08-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–12. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- Damianova R, Stefanova N, Cukierman E, Momchilova A, Pankov R. Three-dimensional matrix induces sustained activation of ERK1/2 via Src/Ras/Raf signaling pathway. Cell Biol Int. 2007 doi: 10.1016/j.cellbi.2007.08.029. [DOI] [PubMed] [Google Scholar]
- Digman MA, Brown CM, Horwitz AF, Mantulin WW, Gratton E. Paxillin dynamics measured during adhesion assembly and disassembly by correlation spectroscopy. Biophys J. 2007 doi: 10.1529/biophysj.107.104984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doan AT, Huttenlocher A. RACK1 regulates Src activity and modulates paxillin dynamics during cell migration. Exp Cell Res. 2007;313:2667–79. doi: 10.1016/j.yexcr.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efimov A, Schiefermeier N, Grigoriev I, Brown MC, Turner CE, Small JV, Kaverina I. Paxillin-dependent stimulation of microtubule catastrophes at focal adhesion sites. J Cell Sci. 2008;121:196–204. doi: 10.1242/jcs.012666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezratty EJ, Partridge MA, Gundersen GG. Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nat Cell Biol. 2005 doi: 10.1038/ncb1262. [DOI] [PubMed] [Google Scholar]
- Feng Q, Albeck JG, Cerione RA, Yang W. Regulation of the Cool/Pix proteins: key binding partners of the Cdc42/Rac targets, the p21-activated kinases. J Biol Chem. 2002;277:5644–50. doi: 10.1074/jbc.M107704200. [DOI] [PubMed] [Google Scholar]
- Filipenko NR, Attwell S, Roskelley C, Dedhar S. Integrin-linked kinase activity regulates Rac- and Cdc42-mediated actin cytoskeleton reorganization via alpha-PIX. Oncogene. 2005 doi: 10.1038/sj.onc.1208737. [DOI] [PubMed] [Google Scholar]
- Franco SJ, Rodgers MA, Perrin BJ, Han J, Bennin DA, Critchley DR, Huttenlocher A. Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nat Cell Biol. 2004;6:977–83. doi: 10.1038/ncb1175. [DOI] [PubMed] [Google Scholar]
- Frank SR, Adelstein MR, Hansen SH. GIT2 represses Crk- and Rac1-regulated cell spreading and Cdc42-mediated focal adhesion turnover. Embo J. 2006;25:1848–59. doi: 10.1038/sj.emboj.7601092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–26. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao XD, Caviston JP, Tcheperegine SE, Bi E. Pxl1p, a paxillin-like protein in Saccharomyces cerevisiae, may coordinate Cdc42p and Rho1p functions during polarized growth. Mol Biol Cell. 2004;15:3977–85. doi: 10.1091/mbc.E04-01-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garton AJ, Tonks NK. Regulation of fibroblast motility by the protein tyrosine phosphatase PTP-PEST. J Biol Chem. 1999;274:3811–8. doi: 10.1074/jbc.274.6.3811. [DOI] [PubMed] [Google Scholar]
- Ge W, Balasubramanian MK. Pxl1p, a Paxillin-related Protein, Stabilizes the Actomyosin Ring during Cytokinesis in Fission Yeast. Mol Biol Cell. 2008 doi: 10.1091/mbc.E07-07-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehmlich K, Pinotsis N, Hayess K, van der Ven PF, Milting H, El Banayosy A, Korfer R, Wilmanns M, Ehler E, Furst DO. Paxillin and ponsin interact in nascent costameres of muscle cells. J Mol Biol. 2007;369:665–82. doi: 10.1016/j.jmb.2007.03.050. [DOI] [PubMed] [Google Scholar]
- Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix--cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- Geiger B, Tokuyasu KT, Dutton AH, Singer SJ. Vinculin, an intracellular protein localized at specialized sites where microfilament bundles terminate at cell membranes. Proc Natl Acad Sci U S A. 1980;77:4127–31. doi: 10.1073/pnas.77.7.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimona M, Djinovic-Carugo K, Kranewitter WJ, Winder SJ. Functional plasticity of CH domains. FEBS Lett. 2002;513:98–106. doi: 10.1016/s0014-5793(01)03240-9. [DOI] [PubMed] [Google Scholar]
- Glenney JR, Jr, Zokas L. Novel tyrosine kinase substrates from Rous sarcoma virus-transformed cells are present in the membrane skeleton. J Cell Biol. 1989;108:2401–8. doi: 10.1083/jcb.108.6.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsley CM, Kinchen JM, Tosello-Trampont AC, Brugnera E, Haney LB, Lu M, Chen Q, Klingele D, Hengartner MO, Ravichandran KS. Dock180 and ELMO1 proteins cooperate to promote evolutionarily conserved Rac-dependent cell migration. J Biol Chem. 2004;279:6087–97. doi: 10.1074/jbc.M307087200. [DOI] [PubMed] [Google Scholar]
- Hagel M, George EL, Kim A, Tamimi R, Opitz SL, Turner CE, Imamoto A, Thomas SM. The adaptor protein paxillin is essential for normal development in the mouse and is a critical transducer of fibronectin signaling. Mol Cell Biol. 2002;22:901–15. doi: 10.1128/MCB.22.3.901-915.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hanks SK, Ryzhova L, Shin NY, Brabek J. Focal adhesion kinase signaling activities and their implications in the control of cell survival and motility. Front Biosci. 2003;8:d982–96. doi: 10.2741/1114. [DOI] [PubMed] [Google Scholar]
- Hayashi I, Vuori K, Liddington RC. The focal adhesion targeting (FAT) region of focal adhesion kinase is a four-helix bundle that binds paxillin. Nat Struct Biol. 2002;9:101–6. doi: 10.1038/nsb755. [DOI] [PubMed] [Google Scholar]
- Heggeness MH, Ash JF, Singer SJ. Transmembrane linkage of fibronectin to intracellular actin-containing filaments in cultured human fibroblasts. Ann N Y Acad Sci. 1978;312:414–7. doi: 10.1111/j.1749-6632.1978.tb16822.x. [DOI] [PubMed] [Google Scholar]
- Herreros L, Rodriguez-Fernandez JL, Brown MC, Alonso-Lebrero JL, Cabanas C, Sanchez-Madrid F, Longo N, Turner CE, Sanchez-Mateos P. Paxillin localizes to the lymphocyte microtubule organizing center and associates with the microtubule cytoskeleton. J Biol Chem. 2000;275:26436–40. doi: 10.1074/jbc.M003970200. [DOI] [PubMed] [Google Scholar]
- Hildebrand JD, Schaller MD, Parsons JT. Paxillin, a tyrosine phosphorylated focal adhesion-associated protein binds to the carboxyl terminal domain of focal adhesion kinase. Mol Biol Cell. 1995;6:637–47. doi: 10.1091/mbc.6.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoellerer MK, Noble ME, Labesse G, Campbell ID, Werner JM, Arold ST. Molecular recognition of paxillin LD motifs by the focal adhesion targeting domain. Structure (Camb) 2003;11:1207–17. doi: 10.1016/j.str.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Hoffman GR, Cerione RA. Signaling to the Rho GTPases: networking with the DH domain. FEBS Lett. 2002;513:85–91. doi: 10.1016/s0014-5793(01)03310-5. [DOI] [PubMed] [Google Scholar]
- Hu K, Ji L, Applegate KT, Danuser G, Waterman-Storer CM. Differential transmission of actin motion within focal adhesions. Science. 2007;315:111–5. doi: 10.1126/science.1135085. [DOI] [PubMed] [Google Scholar]
- Huang C, Borchers CH, Schaller MD, Jacobson K. Phosphorylation of paxillin by p38MAPK is involved in the neurite extension of PC-12 cells. J Cell Biol. 2004a;164:593–602. doi: 10.1083/jcb.200307081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Jacobson K, Schaller MD. A role for JNK-paxillin signaling in cell migration. Cell Cycle. 2004b;3:4–6. [PubMed] [Google Scholar]
- Huang C, Rajfur Z, Borchers C, Schaller MD, Jacobson K. JNK phosphorylates paxillin and regulates cell migration. Nature. 2003;424:219–23. doi: 10.1038/nature01745. [DOI] [PubMed] [Google Scholar]
- Humphries JD, Wang P, Streuli C, Geiger B, Humphries MJ, Ballestrem C. Vinculin controls focal adhesion formation by direct interactions with talin and actin. J Cell Biol. 2007;179:1043–57. doi: 10.1083/jcb.200703036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Hynes RO, Destree AT. Relationships between fibronectin (LETS protein) and actin. Cell. 1978;15:875–86. doi: 10.1016/0092-8674(78)90272-6. [DOI] [PubMed] [Google Scholar]
- Iioka H, Iemura S, Natsume T, Kinoshita N. Wnt signalling regulates paxillin ubiquitination essential for mesodermal cell motility. Nat Cell Biol. 2007;9:813–21. doi: 10.1038/ncb1607. [DOI] [PubMed] [Google Scholar]
- Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–44. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- Ishibe S, Joly D, Zhu X, Cantley LG. Phosphorylation-dependent paxillin-ERK association mediates hepatocyte growth factor-stimulated epithelial morphogenesis. Mol Cell. 2003;12:1275–85. doi: 10.1016/s1097-2765(03)00406-4. [DOI] [PubMed] [Google Scholar]
- Ito A, Kataoka TR, Watanabe M, Nishiyama K, Mazaki Y, Sabe H, Kitamura Y, Nojima H. A truncated isoform of the PP2A B56 subunit promotes cell motility through paxillin phosphorylation. Embo J. 2000;19:562–71. doi: 10.1093/emboj/19.4.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izard T, Evans G, Borgon RA, Rush CL, Bricogne G, Bois PR. Vinculin activation by talin through helical bundle conversion. Nature. 2004;427:171–5. doi: 10.1038/nature02281. [DOI] [PubMed] [Google Scholar]
- Jackson JL, Young MR. Protein phosphatase-2A regulates protein tyrosine phosphatase activity in Lewis lung carcinoma tumor variants. Clin Exp Metastasis. 2003;20:357–64. doi: 10.1023/a:1024012000009. [DOI] [PubMed] [Google Scholar]
- Jamieson JS, Tumbarello DA, Halle M, Brown MC, Tremblay ML, Turner CE. Paxillin is essential for PTP-PEST-dependent regulation of cell spreading and motility: a role for paxillin kinase linker. J Cell Sci. 2005;118:5835–47. doi: 10.1242/jcs.02693. [DOI] [PubMed] [Google Scholar]
- Kadrmas JL, Beckerle MC. The LIM domain: from the cytoskeleton to the nucleus. Nat Rev Mol Cell Biol. 2004;5:920–31. doi: 10.1038/nrm1499. [DOI] [PubMed] [Google Scholar]
- Khyrul WA, LaLonde DP, Brown MC, Levinson H, Turner CE. The integrin-linked kinase regulates cell morphology and motility in a rho-associated kinase-dependent manner. J Biol Chem. 2004;279:54131–9. doi: 10.1074/jbc.M410051200. [DOI] [PubMed] [Google Scholar]
- Ku H, Meier KE. Phosphorylation of paxillin via the ERK mitogen-activated protein kinase cascade in EL4 thymoma cells. J Biol Chem. 2000;275:11333–40. doi: 10.1074/jbc.275.15.11333. [DOI] [PubMed] [Google Scholar]
- LaLonde DP, Grubinger M, Lamarche-Vane N, Turner CE. CdGAP associates with actopaxin to regulate integrin-dependent changes in cell morphology and motility. Curr Biol. 2006;16:1375–85. doi: 10.1016/j.cub.2006.05.057. [DOI] [PubMed] [Google Scholar]
- Laukaitis CM, Webb DJ, Donais K, Horwitz AF. Differential dynamics of alpha 5 integrin, paxillin, and alpha-actinin during formation and disassembly of adhesions in migrating cells. J Cell Biol. 2001;153:1427–40. doi: 10.1083/jcb.153.7.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legate KR, Montanez E, Kudlacek O, Fassler R. ILK, PINCH and parvin: the tIPP of integrin signaling. Nature Rev Med Cell Biol. 2006;7:20–31. doi: 10.1038/nrm1789. [DOI] [PubMed] [Google Scholar]
- Lemeer S, Ruijtenbeek R, Pinkse MW, Jopling C, Heck AJ, den Hertog J, Slijper M. Endogenous phosphotyrosine signaling in zebrafish embryos. Mol Cell Proteomics. 2007;6:2088–99. doi: 10.1074/mcp.M600482-MCP200. [DOI] [PubMed] [Google Scholar]
- Lewis JM, Baskaran R, Taagepera S, Schwartz MA, Wang JY. Integrin regulation of c-Abl tyrosine kinase activity and cytoplasmic-nuclear transport. Proc Natl Acad Sci U S A. 1996;93:15174–9. doi: 10.1073/pnas.93.26.15174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JM, Schwartz MA. Integrins regulate the association and phosphorylation of paxillin by c-Abl. J Biol Chem. 1998;273:14225–30. doi: 10.1074/jbc.273.23.14225. [DOI] [PubMed] [Google Scholar]
- Li S, Guan JL, Chien S. Biochemistry and biomechanics of cell motility. Annu Rev Biomed Eng. 2005;7:105–50. doi: 10.1146/annurev.bioeng.7.060804.100340. [DOI] [PubMed] [Google Scholar]
- Liu G, Guibao CD, Zheng J. Structural insight into the mechanisms of targeting and signaling of focal adhesion kinase. Mol Cell Biol. 2002;22:2751–60. doi: 10.1128/MCB.22.8.2751-2760.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llense F, Martin-Blanco E. JNK signaling controls border cell cluster integrity and collective cell migration. Curr Biol. 2008;18:538–44. doi: 10.1016/j.cub.2008.03.029. [DOI] [PubMed] [Google Scholar]
- Loo TH, Ng YW, Lim L, Manser E. GIT1 activates p21-activated kinase through a mechanism independent of p21 binding. Mol Cell Biol. 2004;24:3849–59. doi: 10.1128/MCB.24.9.3849-3859.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackin NA, Sousou TJ, Erdman SE. The PXL1 gene of Saccharomyces cerevisiae encodes a paxillin-like protein functioning in polarized cell growth. Mol Biol Cell. 2004;15:1904–17. doi: 10.1091/mbc.E04-01-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manes S, Mira E, Gomez-Mouton C, Zhao ZJ, Lacalle RA, Martinez AC. Concerted activity of tyrosine phosphatase SHP-2 and focal adhesion kinase in regulation of cell motility. Mol Cell Biol. 1999;19:3125–35. doi: 10.1128/mcb.19.4.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manser E, Loo TH, Koh CG, Zhao ZS, Chen XQ, Tan L, Tan I, Leung T, Lim L. PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol Cell. 1998;1:183–92. doi: 10.1016/s1097-2765(00)80019-2. [DOI] [PubMed] [Google Scholar]
- Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- Melendez J, Turner C, Avraham H, Steinberg SF, Schaefer E, Sussman MA. Cardiomyocyte apoptosis triggered by RAFTK/pyk2 via Src kinase is antagonized by paxillin. J Biol Chem. 2004;279:53516–23. doi: 10.1074/jbc.M408475200. [DOI] [PubMed] [Google Scholar]
- Mishima W, Suzuki A, Yamaji S, Yoshimi R, Ueda A, Kaneko T, Tanaka J, Miwa Y, Ohno S, Ishigatsubo Y. The first CH domain of affixin activates Cdc42 and Rac1 through alphaPIX, a Cdc42/Rac1-specific guanine nucleotide exchanging factor. Genes Cells. 2004;9:193–204. doi: 10.1111/j.1356-9597.2004.00717.x. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Yamauchi J, Chan JR, Okada A, Tomooka Y, Hisanaga S, Tanoue A. Cdk5 regulates differentiation of oligodendrocyte precursor cells through the direct phosphorylation of paxillin. J Cell Sci. 2007;120:4355–66. doi: 10.1242/jcs.018218. [DOI] [PubMed] [Google Scholar]
- Nayal A, Webb DJ, Brown CM, Schaefer EM, Vicente-Manzanares M, Horwitz AR. Paxillin phosphorylation at Ser273 localizes a GIT1-PIX-PAK complex and regulates adhesion and protrusion dynamics. J Cell Biol. 2006;173:587–9. doi: 10.1083/jcb.200509075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolopoulos SN, Turner CE. Actopaxin, a new focal adhesion protein that binds paxillin LD motifs and actin and regulates cell adhesion. J Cell Biol. 2000;151:1435–48. doi: 10.1083/jcb.151.7.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolopoulos SN, Turner CE. Integrin-linked kinase (ILK) binding to paxillin LD1 motif regulates ILK localization to focal adhesions. J Biol Chem. 2001;276:23499–505. doi: 10.1074/jbc.M102163200. [DOI] [PubMed] [Google Scholar]
- Nikolopoulos SN, Turner CE. Molecular dissection of actopaxin-integrin-linked kinase-Paxillin interactions and their role in subcellular localization. J Biol Chem. 2002;277:1568–75. doi: 10.1074/jbc.M108612200. [DOI] [PubMed] [Google Scholar]
- Nishiya N, Kiosses WB, Han J, Ginsberg MH. An alpha4 integrin-paxillin-Arf-GAP complex restricts Rac activation to the leading edge of migrating cells. Nat Cell Biol. 2005;7:343–52. doi: 10.1038/ncb1234. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Olski TM, Noegel AA, Korenbaum E. Parvin, a 42 kDa focal adhesion protein, related to the alpha-actinin superfamily. J Cell Sci. 2001;114:525–38. doi: 10.1242/jcs.114.3.525. [DOI] [PubMed] [Google Scholar]
- Pankov R, Endo Y, Even-Ram S, Araki M, Clark K, Cukierman E, Matsumoto K, Yamada KM. A Rac switch regulates random versus directionally persistent cell migration. J Cell Biol. 2005;170:793–802. doi: 10.1083/jcb.200503152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116:1409–16. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- Perez-Alvarado GC, Miles C, Michelsen JW, Louis HA, Winge DR, Beckerle MC, Summers MF. Structure of the carboxy-terminal LIM domain from the cysteine rich protein CRP. Nat Struct Biol. 1994;1:388–98. doi: 10.1038/nsb0694-388. [DOI] [PubMed] [Google Scholar]
- Petit V, Boyer B, Lentz D, Turner CE, Thiery JP, Valles AM. Phosphorylation of tyrosine residues 31 and 118 on paxillin regulates cell migration through an association with CRK in NBT-II cells. J Cell Biol. 2000;148:957–70. doi: 10.1083/jcb.148.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petros AM, Medek A, Nettesheim DG, Kim DH, Yoon HS, Swift K, Matayoshi ED, Oltersdorf T, Fesik SW. Solution structure of the antiapoptotic protein bcl-2. Proc Natl Acad Sci U S A. 2001;98:3012–7. doi: 10.1073/pnas.041619798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinar M, Coll PM, Rincon SA, Perez P. Schizosaccharomyces pombe Pxl1 is a Paxillin Homologue that Modulates Rho1 Activity and Participates in Cytokinesis. Mol Biol Cell. 2008 doi: 10.1091/mbc.E07-07-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premont RT, Claing A, Vitale N, Perry SJ, Lefkowitz RJ. The GIT family of ADP-ribosylation factor GTPase-activating proteins. Functional diversity of GIT2 through alternative splicing. J Biol Chem. 2000;275:22373–80. doi: 10.1074/jbc.275.29.22373. [DOI] [PubMed] [Google Scholar]
- Premont RT, Perry SJ, Schmalzigaug R, Roseman JT, Xing Y, Claing A. The GIT/PIX complex: an oligomeric assembly of GIT family ARF GTPase-activating proteins and PIX family Rac1/Cdc42 guanine nucleotide exchange factors. Cell Signal. 2004;16:1001–11. doi: 10.1016/j.cellsig.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Price LS, Leng J, Schwartz MA, Bokoch GM. Activation of Rac and Cdc42 by integrins mediates cell spreading. Mol Biol Cell. 1998;9:1863–71. doi: 10.1091/mbc.9.7.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raftopoulou M, Hall A. Cell migration: Rho GTPases lead the way. Dev Biol. 2004;265:23–32. doi: 10.1016/j.ydbio.2003.06.003. [DOI] [PubMed] [Google Scholar]
- Ridley AJ. Rho family proteins: coordinating cell responses. Trends Cell Biol. 2001a;11:471–7. doi: 10.1016/s0962-8924(01)02153-5. [DOI] [PubMed] [Google Scholar]
- Ridley AJ. Rho GTPases and cell migration. J Cell Sci. 2001b;114:2713–22. doi: 10.1242/jcs.114.15.2713. [DOI] [PubMed] [Google Scholar]
- Rottner K, Hall A, Small JV. Interplay between Rac and Rho in the control of substrate contact dynamics. Curr Biol. 1999;9:640–8. doi: 10.1016/s0960-9822(99)80286-3. [DOI] [PubMed] [Google Scholar]
- Sastry SK, Lyons PD, Schaller MD, Burridge K. PTP-PEST controls motility through regulation of Rac1. J Cell Sci. 2002;115:4305–16. doi: 10.1242/jcs.00105. [DOI] [PubMed] [Google Scholar]
- Sastry SK, Rajfur Z, Liu BP, Cote JF, Tremblay ML, Burridge K. PTP-PEST couples membrane protrusion and tail retraction via VAV2 and p190RhoGAP. J Biol Chem. 2006;281:11627–36. doi: 10.1074/jbc.M600897200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler M, Pisick E, Morrison PT, Salgia R. Role of the cytoskeletal protein paxillin in oncogenesis. Crit Rev Oncog. 2000;11:63–76. [PubMed] [Google Scholar]
- Schaller MD, Borgman CA, Cobb BS, Vines RR, Reynolds AB, Parsons JT. pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci U S A. 1992;89:5192–6. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller MD, Parsons JT. pp125FAK-dependent tyrosine phosphorylation of paxillin creates a high-affinity binding site for Crk. Mol Cell Biol. 1995;15:2635–45. doi: 10.1128/mcb.15.5.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheswohl DM, Harrell JR, Rajfur Z, Gao G, Campbell SL, Schaller MD. Multiple paxillin binding sites regulate FAK function. J Mol Signal. 2008;3:1. doi: 10.1186/1750-2187-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalzigaug R, Garron ML, Roseman JT, Xing Y, Davidson CE, Arold ST, Premont RT. GIT1 utilizes a focal adhesion targeting-homology domain to bind paxillin. Cell Signal. 2007;19:1733–44. doi: 10.1016/j.cellsig.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeichel KL, Beckerle MC. The LIM domain is a modular protein-binding interface. Cell. 1994;79:211–9. doi: 10.1016/0092-8674(94)90191-0. [DOI] [PubMed] [Google Scholar]
- Sheibani N, Tang Y, Sorenson CM. Paxillin’s LD4 motif interacts with bcl-2. J Cell Physiol. 2008;214:655–61. doi: 10.1002/jcp.21256. [DOI] [PubMed] [Google Scholar]
- Shroff H, Galbraith CG, Galbraith JA, White H, Gillette J, Olenych S, Davidson MW, Betzig E. Dual-color superresolution imaging of genetically expressed probes within individual adhesion complexes. Proc Natl Acad Sci U S A. 2007;104:20308–13. doi: 10.1073/pnas.0710517105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small JV, Kaverina I, Krylyshkina O, Rottner K. Cytoskeleton cross-talk during cell motility. FEBS Lett. 1999;452:96–9. doi: 10.1016/s0014-5793(99)00530-x. [DOI] [PubMed] [Google Scholar]
- Sorenson CM. Interaction of bcl-2 with Paxillin through its BH4 domain is important during ureteric bud branching. J Biol Chem. 2004;279:11368–74. doi: 10.1074/jbc.M310079200. [DOI] [PubMed] [Google Scholar]
- Stoker AW. Protein tyrosine phosphatases and signalling. J Endocrinol. 2005;185:19–33. doi: 10.1677/joe.1.06069. [DOI] [PubMed] [Google Scholar]
- Subauste MC, Pertz O, Adamson ED, Turner CE, Junger S, Hahn KM. Vinculin modulation of paxillin-FAK interactions regulates ERK to control survival and motility. J Cell Biol. 2004;165:371–81. doi: 10.1083/jcb.200308011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JW, Cooley MA, Broome JM, Salgia R, Griffin JD, Lombardo CR, Schaller MD. The role of focal adhesion kinase binding in the regulation of tyrosine phosphorylation of paxillin. J Biol Chem. 1999;274:36684–92. doi: 10.1074/jbc.274.51.36684. [DOI] [PubMed] [Google Scholar]
- Tsubouchi A, Sakakura J, Yagi R, Mazaki Y, Schaefer E, Yano H, Sabe H. Localized suppression of RhoA activity by Tyr31/118-phosphorylated paxillin in cell adhesion and migration. J Cell Biol. 2002;159:673–83. doi: 10.1083/jcb.200202117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumbarello DA, Brown MC, Turner CE. The paxillin LD motifs. FEBS Lett. 2002;513:114–8. doi: 10.1016/s0014-5793(01)03244-6. [DOI] [PubMed] [Google Scholar]
- Turner CE. Paxillin is a major phosphotyrosine-containing protein during embryonic development. J Cell Biol. 1991;115:201–7. doi: 10.1083/jcb.115.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CE. Paxillin interactions. J Cell Sci. 2000;113(Pt 23):4139–40. doi: 10.1242/jcs.113.23.4139. [DOI] [PubMed] [Google Scholar]
- Turner CE, Brown MC, Perrotta JA, Riedy MC, Nikolopoulos SN, McDonald AR, Bagrodia S, Thomas S, Leventhal PS. Paxillin LD4 motif binds PAK and PIX through a novel 95-kD ankyrin repeat, ARF-GAP protein: A role in cytoskeletal remodeling. J Cell Biol. 1999;145:851–63. doi: 10.1083/jcb.145.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CE, Glenney JR, Jr, Burridge K. Paxillin: a new vinculin-binding protein present in focal adhesions. J Cell Biol. 1990;111:1059–68. doi: 10.1083/jcb.111.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CE, Miller JT. Primary sequence of paxillin contains putative SH2 and SH3 domain binding motifs and multiple LIM domains: identification of a vinculin and pp125Fak-binding region. J Cell Sci. 1994;107(Pt 6):1583–91. doi: 10.1242/jcs.107.6.1583. [DOI] [PubMed] [Google Scholar]
- Turner CE, Schaller MD, Parsons JT. Tyrosine phosphorylation of the focal adhesion kinase pp125FAK during development: relation to paxillin. J Cell Sci. 1993;105(Pt 3):637–45. doi: 10.1242/jcs.105.3.637. [DOI] [PubMed] [Google Scholar]
- Valles AM, Beuvin M, Boyer B. Activation of Rac1 by paxillin-Crk-DOCK180 signaling complex is antagonized by Rap1 in migrating NBT-II cells. J Biol Chem. 2004;279:44490–6. doi: 10.1074/jbc.M405144200. [DOI] [PubMed] [Google Scholar]
- Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, Horwitz AF. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol. 2004;6:154–61. doi: 10.1038/ncb1094. [DOI] [PubMed] [Google Scholar]
- Webb DJ, Parsons JT, Horwitz AF. Adhesion assembly, disassembly and turnover in migrating cells -- over and over and over again. Nat Cell Biol. 2002;4:E97–100. doi: 10.1038/ncb0402-e97. [DOI] [PubMed] [Google Scholar]
- Webb DJ, Schroeder MJ, Brame CJ, Whitmore L, Shabanowitz J, Hunt DF, Horwitz AR. Paxillin phosphorylation sites mapped by mass spectrometry. J Cell Sci. 2005;118:4925–9. doi: 10.1242/jcs.02563. [DOI] [PubMed] [Google Scholar]
- Weng Z, Taylor JA, Turner CE, Brugge JS, Seidel-Dugan C. Detection of Src homology 3-binding proteins, including paxillin, in normal and v-Src-transformed Balb/c 3T3 cells. J Biol Chem. 1993;268:14956–63. [PubMed] [Google Scholar]
- West KA, Zhang H, Brown MC, Nikolopoulos SN, Riedy MC, Horwitz AF, Turner CE. The LD4 motif of paxillin regulates cell spreading and motility through an interaction with paxillin kinase linker (PKL) J Cell Biol. 2001;154:161–76. doi: 10.1083/jcb.200101039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedenmann J, Ivanchenko S, Oswald F, Schmitt F, Rocker C, Salih A, Spindler KD, Nienhaus GU. EosFP, a fluorescent marker protein with UV-inducible green-tored fluorescence conversion. Proc Natl Acad Sci U S A. 2004;101:15905–10. doi: 10.1073/pnas.0403668101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman PW, Brown CM, Webb DJ, Hebert B, Johnson NL, Squier JA, Ellisman MH, Horwitz AF. Spatial mapping of integrin interactions and dynamics during cell migration by image correlation microscopy. J Cell Sci. 2004;117:5521–34. doi: 10.1242/jcs.01416. [DOI] [PubMed] [Google Scholar]
- Woods AJ, Roberts MS, Choudhary J, Barry ST, Mazaki Y, Sabe H, Morley SJ, Critchley DR, Norman JC. Paxillin associates with poly(A)-binding protein 1 at the dense endoplasmic reticulum and the leading edge of migrating cells. J Biol Chem. 2002;277:6428–37. doi: 10.1074/jbc.M109446200. [DOI] [PubMed] [Google Scholar]
- Wozniak MA, Desai R, Solski PA, Der CJ, Keely PJ. ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. J Cell Biol. 2003;163:583–95. doi: 10.1083/jcb.200305010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. The PINCH-ILK-parvin complexes: assembly, functions and regulation. Biochim Biophys Acta. 2004;1692:55–62. doi: 10.1016/j.bbamcr.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Yamaguchi R, Maki M, Hatanaka M, Sabe H. Unphosphorylated and tyrosine-phosphorylated forms of a focal adhesion protein, paxillin, are substrates for calpain II in vitro: implications for the possible involvement of calpain II in mitosis-specific degradation of paxillin. FEBS Lett. 1994;356:114–6. doi: 10.1016/0014-5793(94)01246-6. [DOI] [PubMed] [Google Scholar]
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- Young MR, Liu SW, Meisinger J. Protein phosphatase-2A restricts migration of Lewis lung carcinoma cells by modulating the phosphorylation of focal adhesion proteins. Int J Cancer. 2003;103:38–44. doi: 10.1002/ijc.10772. [DOI] [PubMed] [Google Scholar]
- Zaidel-Bar R, Ballestrem C, Kam Z, Geiger B. Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J Cell Sci. 2003;116:4605–13. doi: 10.1242/jcs.00792. [DOI] [PubMed] [Google Scholar]
- Zaidel-Bar R, Itzkovitz S, Ma’ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nat Cell Biol. 2007a;9:858–67. doi: 10.1038/ncb0807-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel-Bar R, Milo R, Kam Z, Geiger B. A paxillin tyrosine phosphorylation switch regulates the assembly and form of cell-matrix adhesions. J Cell Sci. 2007b;120:137–48. doi: 10.1242/jcs.03314. [DOI] [PubMed] [Google Scholar]
- Zamir E, Katz M, Posen Y, Erez N, Yamada KM, Katz BZ, Lin S, Lin DC, Bershadsky A, Kam Z, et al. Dynamics and segregation of cell-matrix adhesions in cultured fibroblasts. Nat Cell Biol. 2000;2:191–6. doi: 10.1038/35008607. [DOI] [PubMed] [Google Scholar]
- Zervas CG, Gregory SL, Brown NH. Drosophila integrin-linked kinase is required at sites of integrin adhesion to link the cytoskeleton to the plasma membrane. J Cell Biol. 2001;152:1007–18. doi: 10.1083/jcb.152.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Liu J, Cheng A, Deyoung SM, Chen X, Dold LH, Saltiel AR. CAP interacts with cytoskeletal proteins and regulates adhesion-mediated ERK activation and motility. Embo J. 2006;25:5284–93. doi: 10.1038/sj.emboj.7601406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZS, Manser E, Loo TH, Lim L. Coupling of PAK-interacting exchange factor PIX to GIT1 promotes focal complex disassembly. Mol Cell Biol. 2000;20:6354–63. doi: 10.1128/mcb.20.17.6354-6363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimerman B, Arnold M, Ulmer J, Blummel J, Besser A, Spatz JP, Geiger B. Formation of focal adhesion-stress fibre complexes coordinated by adhesive and non-adhesive surface domains. IEE Proc Nanobiotechnol. 2004;151:62–6. doi: 10.1049/ip-nbt:20040474. [DOI] [PubMed] [Google Scholar]