Abstract
Kinetochores bind microtubules laterally in a transient fashion and stably, by insertion of plus ends. These pathways may exist to carry out distinct tasks during different stages of mitosis and likely depend on distinct molecular mechanisms. On isolated chromosomes, we found microtubule nucleation/binding depended additively on both dynein/dynactin and on the Ndc80/Hec1 complex. Studying chromosome movement in living Xenopus cells within the simplified geometry of monopolar spindles, we quantified the relative contributions of dynein/dynactin and the Ndc80/Hec1 complex. Inhibition of dynein/dynactin alone had minor effects but did suppress transient, rapid, poleward movements. In contrast, inhibition of the Ndc80 complex blocked normal end-on attachments of microtubules to kinetochores resulting in persistent rapid poleward movements that required dynein/dynactin. In normal cells with bipolar spindles, dynein/dynactin activity on its own allowed attachment and rapid movement of chromosomes on prometaphase spindles but failed to support metaphase alignment and chromatid movement in anaphase. Thus, in prometaphase, dynein/dynactin likely mediates early transient, lateral interactions of kinetochores and microtubules. However, mature attachment via the Ndc80 complex is essential for metaphase alignment and anaphase A.
Electronic supplementary material
The online version of this article (doi:10.1007/s00412-007-0135-3) contains supplementary material, which is available to authorized users.
Introduction
During cell division, chromosomes biorient to opposite spindle poles, and chromatids segregate equally between the two progeny cells, preventing the gain or loss of chromosomes (aneuploidy) with its potentially deleterious consequences. Biorientation and segregation of chromosomes depend on interactions between kinetochores and microtubules. During prometaphase, kinetochores attach to microtubules, and chromosomes congress to the metaphase plate. Rapid poleward chromosome movements, up to 24 μm/min, can occur in the early phases of chromosome capture by spindle microtubules (Rieder and Alexander 1990; Merdes and DeMey 1990).
However, these rapid interactions appear to give way to the mature form of the kinetochore where microtubule plus ends are embedded into the kinetochore and form the primary means of interaction as chromosomes move to align at the metaphase plate in prometaphase and as the separated chromatids move to the poles in anaphase (Rieder and Alexander 1990; Merdes and DeMey 1990; Dong et al. 2007; McCleland et al. 2004). Recently, it was shown that the stable interaction of microtubules with kinetochores is mediated by a protein assembly composed of the KNL-1 protein, the Mis12 complex, and the Ndc80 complex (Cheeseman et al. 2006; Wei et al. 2007). It is hypothesized that the Ndc80 complex directly mediates attachment of kinetochores to spindle microtubules (Cheeseman et al. 2006; Wei et al. 2007; Deluca et al. 2006). Previously, we showed that direct inhibition of the Ndc80 complex by antibody microinjection led to loss of most chromatid movement at anaphase (McCleland et al. 2003). However, earlier at prometaphase in the same cells, chromosomes continued to bind to the mitotic spindle microtubules and showed oscillatory movements, although normal metaphase alignment was impaired. Thus, these initial studies suggested that while Ndc80 complex-dependent mechanisms appear to be required for normal anaphase chromatid movement, other mechanisms, independent of the Ndc80 complex, can foster chromosome attachment and movement in prometaphase.
In prometaphase cells, transient attachments of kinetochores to microtubules result in rapid poleward movements. These attachments may be mediated by dynein/dynactin (Rieder and Alexander 1990; King et al. 2000). However, most evidence for dynein/dynactin in this event is indirect. Dynein accumulates on kinetochores that are not attached to microtubules and becomes depleted as initial lateral interactions of kinetochores mature into end-on attachments (King et al. 2000). Both the rapidity and direction of movement of chromosomes just after nuclear envelope breakdown are consistent with a role for dynein/dynactin. In the widely used model organism S. cerevisiae, there are six kinesins and dynein but capture and retrieval of distant chromosomes depends on one minus-end directed kinesin-14, Kar3p (Tanaka et al. 2005). In S. pombe, Klp2p, also a member of the kinesin 14 subfamily, assists in chromosomes motion toward the spindle pole body reportedly by promoting depolymerization of kinetochore fiber microtubules (Grishchuk and McIntosh 2006). It was recently observed in budding yeast as in vertebrates that chromosomes can move along lateral surfaces of microtubules (Tanaka et al. 2007).
A major hurdle in analysis of the role of dynein/dynactin function in mitosis is the exceptionally transient nature of initial kinetochore attachment in the early events of chromosome capture and movement in prometaphase. Under normal circumstances, these initial interactions are rapidly obscured by the more robust Ndc80 complex-dependent attachments of kinetochores to microtubules. To determine the potential role for dynein/dynactin in chromosome movement in mitosis of vertebrate cells, we first blocked Ndc80 complex function and then studied the role of dynein/dynactin in a system where the directionality and velocity of chromosome movements could be unambiguously quantified. Our findings suggest that rapid movements of chromosomes on microtubules in early prometaphase are mediated by dynein/dynactin but that the attachments mediated by the Ndc80 complex are required for metaphase alignment and chromatid movement in anaphase. If dynein/dynactin plays any role in anaphase, it is entirely dependent on microtubule attachment mediated through the Ndc80 complex.
Materials and methods
In vitro kinetochore–microtubule binding assays
Monolayers of Xenopus laevis tissue culture cells (XTC) were grown in 66% Leibovitz’s L-15 media containing 10% fetal calf serum, 1 mM sodium pyruvate, and antibiotics at ~21°C. Mitotic chromosomes were purified from XTC cells after a 16-h block in 10 μg/ml vinblastine (Sigma-Aldrich). Chromosomes were purified as previously described with minor modifications (Wordeman et al. 1991). Experiments were preformed essentially as described (Mitchison and Kirschner 1985). Purified mitotic chromosomes were diluted in reactions containing 10 μM tubulin in BRB80 buffer plus 1 mM guanosine triphosphate. Reactions were incubated at 37°C for 15 min in a water bath and were fixed with 10 mM ethyleneglycol-bis-succinimidyl-succinate dissolved in BRB80 for 10 min at 37°C. Reactions were layered onto a 30% glycerol cushion prepared in BRB80, and chromosome–microtubule complexes were pelleted through the cushion onto poly-l-lysine-coated coverslips. Coverslips were postfixed with ice-cold methanol for 5 min at 4°C, and processed for immunofluorescence with the DM1α anti-tubulin antibody (covalently conjugated to FITC). Antibody inhibition reactions were preformed by adding affinity-purified antibodies to reactions to block the function of the kinetochore proteins xCep57 and xNdc80. P50/dynamitin was purified as described (Wittmann and Hyman 1999) and added to the reaction to inhibit the activity of dynein/dynactin at the kinetochore.
Cell culture
X. laevis S3 cells stably transfected with α-tubulin–green fluorescent protein (GFP) were used for microinjections. Cells used for immunofluorescence were the parental X. laevis S3 cells. Cells were kept at 23°C in 70% Leibovitz’s L-15 medium containing 15% fetal bovine serum, l-glutamine, and antibiotics. For microscopy and immunostaining, cells were grown on glass coverslips. To obtain monopolar spindles, cells were incubated with 35 μM monastrol. To block mitotic exit after inhibition of the Ndc80 complex, cells also were incubated with 25 μM MG132 for 30 min before microinjections. To induce a simulated “anaphase,” cells were treated with 10 μM flavopiridol.
Microinjection and microscopy
Cells were injected with the following antibodies diluted in phosphate-buffered saline (PBS): rabbit polyclonal anti-xNuf2 at a needle concentration of 4 mg/ml, rabbit anti-xNDC80 at a needle concentration of 10 mg/ml, and rabbit polyclonal anti-Zwilch at a needle concentration of 15 mg/ml. Both anti-xNuf2 and anti-xNDC80 antibodies were characterized in the publication McCleland et al. (2003). Results from these injections were indistinguishable, and we refer to both as anti-Ndc80 complex antibodies. Control cells were injected with control rabbit IgG at a concentration of 4 mg/ml. Dynamitin was expressed in the E. coli strain BL21 (DE3 pLysS; Novagen) as a 6His-tagged protein and was purified on Ni2+-nitrilotriacetic acid agarose (Qiagen, Valencia, CA) as instructed by the manufacturer. Dynamitin was used at a needle concentration of 6 to13.5 mg/ml. The cells were analyzed using a Zeiss Axiovert 200M microscope equipped with Planapochromat 63 (N.A. 1.4) and 100× (N.A. 1.4) objectives and a Hamamatsu Orca ER CCD camera (Hamamatsu Photonics). Images were captured using Metamorph software (Molecular Devices). After single or double injections, cells were imaged every 2 or 3 s for at least 15 min.
Immunofluorescence
Cells injected with anti-xNUf2 and dynamitin or with anti-xNuf2 alone were treated with nocodazole at 50 ng/ml for 10 min to enhance the association of dynein/dynactin at kinetochores. Cells were rinsed in PBS and cofixed/extracted in PHEM 2% paraformaldehyde and 0.5% Triton X-100 in PHEM buffer (60 mM Pipes, 25 mM hydroxyethyl piperazineethanesulfonic acid, 10 mM ethylene glycol tetraacetic acid, 4 mM MgSO4, pH 6.9) for 15 min. Cells were blocked in 20% boiled normal goat serum in MBST (10 mM N-morpholinopropanesulfonic acid, 150 mM NaCl, 0.05% Tween, pH 7.4) and labeled with p150Glued mouse monoclonal antibody (BD Biosciences) at 1:500 dilution. Secondary antibodies were goat anti-mouse IgG-Cy3 (Amersham) and goat anti-rabbit IgG-FITC (Jackson ImmunoResearch Laboratories).
Chromosome movement analysis
All images were analyzed using Metamorph and Excel software. In microinjected cells with unseparated spindle poles, changes in distance from the poles to kinetochore were measured using the “Track Point” feature of Metamorph. Velocities were calculated from changes in distance from the poles to kinetochores vs time. We used the following rules in assigning directionality and processivity of chromosome movements. Changes in distance less then 0.65 μm were considered to reflect stationary or paused chromosomes because this movement is on the same order as the width of a kinetochore. The times that a chromosome spent changing direction were considered pauses. Excursions in the same direction but interrupted by pausing were considered as a separate movements, and the average velocities for those excursions were calculated separately. All p values were calculated using Student’s equal variance t test, with a two-tailed distribution.
Results
Dynein/dynactin and the Ndc80 complex additively mediate microtubule attachment to kinetochores in vitro
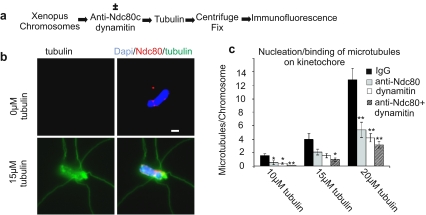
To investigate the nature of the mechanisms mediating the interaction of kinetochores with microtubules, we studied their dependence on dynein/dynactin and the Ndc80 complex in vitro. When incubated at tubulin concentrations where spontaneous microtubule nucleation is slow, kinetochores of isolated chromosomes can serve to nucleate microtubule polymerization (Mitchison and Kirschner 1985). We mixed bovine brain tubulin at three different concentrations (10, 15, and 20 μM) with chromosomes isolated from X. laevis XTC cells. Before detection of the components by immunofluorescence, we fixed and spun the chromosome–microtubule complexes onto coverslips. In control samples incubated in the presence of nonimmune IgG, the average number of microtubules assembled per chromosome was 1.5 for 10-μM tubulin, 4 for 15-μM tubulin, and 12.9 for 20-μM tubulin. Dynein/dynactin is found concentrated at the kinetochores of isolated Xenopus chromosomes as shown previously (McCleland et al. 2003). To inhibit the function of bound dynein/dynactin, we added the protein dynamitin during the microtubule nucleation/binding assay. Dynamitin is a subunit of the dynactin complex required for cytoplasmic dynein/dynactin function. Overexpression or injection of dynamitin in living cells causes inhibition of dynein/dynactin function by disrupting the dynein/dynactin complex (Echeverri et al. 1996). To inhibit the Ndc80 complex, we used function-blocking antibodies to the Ndc80 or Nuf2 proteins. Addition of either dynamitin protein or anti-Ndc80 complex antibodies decreased the number of microtubules bound to chromosomes (Fig. 1). The addition of both anti-Ndc80 antibodies and dynamitin together produced an additive effect in further reducing microtubule association with chromosomes. While the fixation, centrifugation, and labeling procedure tended to obscure our ability to precisely localize bound microtubules at the primary constriction (centromere) of the chromosomes, the fact that binding was dependent on the Ndc80 complex and on dynein/dynactin and that these proteins are concentrated at kinetochores provided confidence that the majority of attached microtubules were in fact bound at kinetochores. In a complementary assay, preassembled, taxol-stabilized microtubules also showed an additive dependence on both dynein/dynactin and the Ndc80 complex for binding to isolated chromosomes (Emanuele and Stukenberg 2007). Thus, isolated chromosomes contain two independent, additive activities for binding microtubules, one dependent on dynein/dynactin and the other on the Ndc80 complex.
Fig. 1.
Dynein/dynactin and the Ndc80 complex additively contribute to microtubule binding at kinetochores of isolated chromosomes. Isolated Xenopus chromosomes in buffer were incubated in the presence or absence of dynamitin protein and anti-Ndc80 complex antibody. After addition of buffer, 10, 15, or 20 μM tubulin, microtubule assembly was initiated, then chromosomes were centrifuged onto coverslips and fixed for immunofluorescence. a Protocol for testing nucleation/binding of microtubules to kinetochores on isolated Xenopus chromosomes. b Immunofluorescence images comparing chromosomes incubated without tubulin (upper panels) to chromosomes incubated with 15 μM tubulin (lower panels). c Quantification of microtubules bound per chromosome. Addition of nonimmune control IgG was used as a control. Dynamitin protein and anti-Ndc80 complex antibodies individually cause a reduction of microtubule association. The combined addition of both dynamitin protein and anti-Ndc80 results in additive reduction in microtubule binding. In comparison to control, for dynamitin, the p value was less than 0.001 at concentrations of tubulin of 10 and 20 μM and less than 0.05 at a concentration of tubulin of 15 μM, n = 50; for the anti-Ndc80 complex antibody, the p value was less than 0.05 for tubulin at 10 μM, was equal to 0.07 at 15 μM, and was less than 0.001 at 20 μM tubulin, n = 50; for dynamitin protein and anti-Ndc80 together, the p value was less than 0.001 at all three concentrations of tubulin, n = 50
Quantification of directional chromosome movements dependent upon dynein/dynactin and the Ndc80 complex
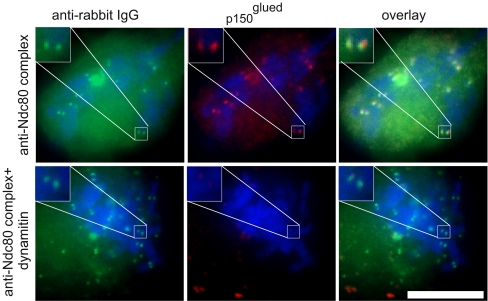
Inhibition of the Ndc80 complex did not block dynein binding at kinetochores in Xenopus egg extracts (McCleland et al. 2003). Consistent with this work, we found that microinjection of the anti-Ndc80 complex antibody did not affect dynein/dynactin association with kinetochores in cells treated with nocodazole. However, microinjection of cells with dynamitin protein significantly decreased the amount of kinetochore-bound dynein/dynactin (Fig. 2). Dynein/dynactin inhibition by overexpression of dynamitin protein from transfected plasmid has also been shown to affect spindle pole organization (Echeverri et al. 1996). However, injection of dynamitin into mitotic cells with assembled spindles does not cause apparent disturbance of spindle poles (Howell et al. 2001). Similarly, when Xenopus S3 cells were injected with dynamitin, we observed no changes in spindle pole organization by GFP–tubulin imaging in normal cells containing bipolar spindles or in cells with unseparated spindle poles that had been preincubated with monastrol.
Fig. 2.
Dynein/dynactin localization in cells injected with antibody to Ndc80 complex antibody and dynamitin. Cells were pretreated with MG132 (25 μM) to block mitotic exit. After injection, all cells were treated with nocodazole (50 ng/ml) for 10 min to disassemble the microtubules and enhance dynein/dynactin association with the kinetochores. The cell in the top row was injected with anti-Ndc80 complex antibodies. The cell in the bottom row was coinjected with anti-Ndc80 complex antibodies and dynamitin. Cells were labeled with anti-rabbit IgG (green) to detect the injected antibody. Cells also were labeled with mouse monoclonal antibody to the p150Glued subunit of dynactin complex (red). Chromosomes labeled with Dapi are depicted in blue in the merged image. In cells injected with only the anti-Ndc80 complex antibodies, p150Glued was found concentrated at kinetochore foci adjacent to the injected antibody signals. Coinjection of dynamitin caused loss of p150Glued concentration at kinetochores. Insets show enlarged views of selected kinetochore regions. Bar = 10 μm
The anti-Ndc80 and anti-Nuf2 antibodies used in this work have been biochemically characterized in previous studies (McCleland et al. 2003). Upon injection of these antibodies as well as antibodies to other two components of the Ndc80 complex, Spc24 and Spc25 into mitotic Xenopus S3 cells, identical responses occurred. Chromosomes were unable to maintain metaphase alignment and failed to move poleward in anaphase (McCleland et al. 2003, 2004). Thus, injection of antibodies to any component of the Ndc80 complex produces an identical response, consistent with the idea that inhibition of the Ndc80 complex leads to the observed phenotypes and is not due to nonspecific effects of the antibodies on other targets.
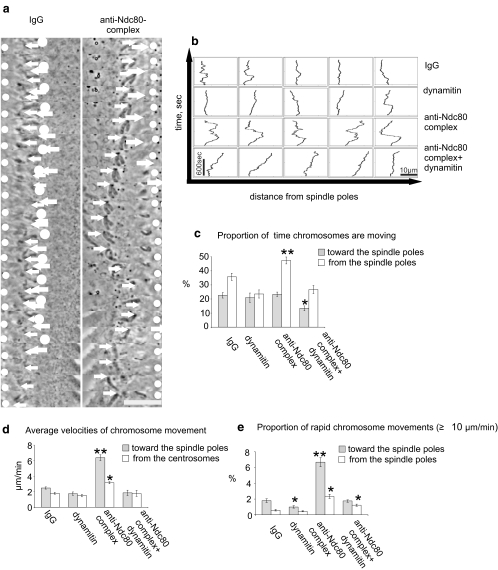
To categorize kinetochore-driven movements as being unambiguously driven by forces acting toward or away from the spindle poles and to quantify these movements, we established a system to analyze chromosome movements in mitotic cells with monopolar spindles induced by inhibition of Eg5 kinesin with monastrol. Because the spindle checkpoint becomes inactivated when the Ndc80 complex is inhibited (McCleland et al. 2003; Meraldi et al. 2004), to maintain cells in M phase, we also treated them with the proteasome inhibitor MG132. MG132 did not significantly affect chromosome movements (not shown). We injected monastrol-treated Xenopus S3 cells with either dynamitin protein, anti-Ndc80 complex antibodies, or a combination of the two (Fig. 3).
Fig. 3.
Chromosomes in prometaphase cells with unseparated spindle poles exhibit distinct modes of movement dependent on dynein/dynactin and the Ndc80 complex. All cells were pretreated with monastrol (35 μM) to prevent spindle pole separation and with MG132 (25 μM) to block premature mitotic exit. a Tracking of chromosomal movements (40–45-s frame intervals). White circles indicate position of the spindle poles, and white arrows indicate position of the kinetochore of a single example chromosome. For each type of injection, the movements of 29 chromosomes were quantified within five to seven injected cells. In a control cell injected with rabbit IgG (left column), the analyzed chromosome exhibits moderate oscillation near the spindle pole. In a cell injected with antibodies to Ndc80 complex (right column), the chromosome undergoes exaggerated movement toward and away from the spindle poles. Bar = 10 μm. b Plots showing the effects of inhibition of dynein/dynactin and the Ndc80 complex in several examples of individual chromosome movements in Xenopus S3 cells. Compared to IgG-injected control cells (first row), chromosomes in cells injected with dynamitin protein (second row) showed little change in overall behavior. Chromosomes in cells injected with antibodies to the Ndc80 complex (third row) exhibited greatly exaggerated movements toward and away from the spindle pole. In cells injected with both dynamitin protein and with antibodies to the Ndc80 complex, the large excursions were suppressed. Instead, most chromosomes eventually drifted away from the spindle pole. Vertical scale bar = 600 s. Horizontal scale bar = 10 μm. c Percent of total time (mean ± sem) the chromosomes spent moving toward or away from the spindle poles. Inhibition of Ndc80 complex increased the proportion of time the chromosomes spend moving away from the spindle poles indicating a loss of stable kinetochore/microtubule attachment. Simultaneous inhibition of dynein/dynactin and the Ndc80 complex and dynein diminished the increase of chromosome movement found upon inhibition of Ndc80 complex alone. d Average velocities of chromosome movement in the injected cells. Chromosomes in cells injected with anti-Ndc80 complex antibodies exhibited a significant increase in the average velocities toward the spindle pole (p < 0.001, n = 29). Cells were observed for 15 min after injection. e Percent of total time (mean ± sem) the chromosomes move rapidly (≥10 μm/min) toward and away from the spindle poles. Inhibition of dynein/dynactin led to a 50% decrease in rapid poleward movements compared with control (p < 0.05). Inhibition of Ndc80 complex led to a significant increase in rapid chromosome movements (p < 0.001). Simultaneous inhibition of Ndc80 complex and dynein/dynactin diminished these rapid movements. Movies showing chromosome behavior in injected cells are available as Movies 1, 2, 3, and 4. Compared with controls, asterisk indicates p < 0.05, and double asterisk indicates p < 0.001
We tracked the movements of individual chromosomes (Fig. 3a) and plotted their excursions toward and away from the unseparated spindle poles (Fig. 3b). The velocities of chromosomes were calculated by measuring chromosome positions at 2–3-s intervals. Movements less than 0.65 μm in 30 s were considered to reflect pauses in movement. We combined the data from all the chromosomes that could be tracked to calculate the percentage of time chromosomes were moving (Fig. 3c), the average velocities (Fig. 3d), and the proportion of rapid movements (≥10 μm/min; Fig. 3e).
In uninjected cells or in control cells injected with rabbit IgG, chromosomes remained arrayed approximately 4 to 6 μm from the spindle poles and exhibited oscillatory movements (Fig. 3a, left column, Fig. 3b, top row, Movie 1). In cells injected with dynamitin protein to inactivate dynein/dynactin, chromosomes showed modest changes in movement, with the only significant change being a decrease in rapid movements and oscillation (Fig. 3e, Movie 2). In contrast, cells injected with anti-Ndc80 complex antibodies exhibited significant changes in several aspects. Initially after injection, chromosomes moved to the periphery of the microtubule array. A few minutes later, chromosomes began rapid movements toward and away from the spindle poles (Fig. 3a,b and Movie 3). By observing GFP–tubulin in the injected cells, we differentiated two modes of poleward movement. One depended on the existing array of microtubules and another, much less common, occurred when a bundle of microtubules grew from the distal kinetochore and then looped back (Khodjakov et al. 2003). Chromosomes that moved because of the latter mechanism were excluded from the quantitative analysis.
Chromosomes in cells injected with anti-Ndc80 complex antibodies moved a greater percentage of time (17.5% increase; p < 0.05, n = 29; Fig. 3e). In addition, increased antipoleward movements were seen in these cells perhaps attributable to the transient nature of attachments in cells with compromised Ndc80 complex, allowing antipoleward forces to operate more frequently.
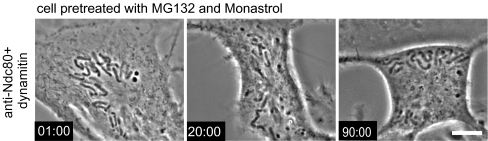
Rapid chromosome movements after inhibition of the Ndc80 complex might reflect persistence of dynein/dynactin at kinetochores. To test this hypothesis, we coinjected dynamitin and anti-Ndc80 complex antibodies into cells. Most chromosomes lost obvious attachment to spindle microtubules, remaining stationary or drifting toward the cell periphery (Fig. 3 b,c and Movie 4). The rapid poleward chromosome movements characteristic of cells injected with the anti-Ndc80 complex antibody were severely reduced (Fig. 3d). We did observe some residual rapid poleward movements in cells injected with both Ndc80 complex antibodies and dynamitin. We attribute these residual movements to remaining activity of dynein/dynactin during observation period selected to be 15 min after injections. It appeared that while effects from the anti-Ndc80 complex antibodies were rapid and fully penetrant within a few minutes, the response to dynamitin injection increased with time. After approximately 30 min to 1 h, all residual rapid poleward movements ceased, and all chromosomes became localized at the cell periphery (Fig. 4).
Fig. 4.
Simultaneous inhibition of Ndc80 complex and dynein/dynactin in cells with monopolar spindles leads to eventual loss of chromosome poleward movement and orientation toward spindle poles. Cells were pretreated with monastrol (3 μM) to prevent spindle pole separation and with MG132 (25 μM) to block premature mitotic exit. Cells were injected with anti-Ndc80 antibody and dynamitin, n = 4 cells. Chromosomes show progressive loss of movement and orientation toward the spindle poles such that by 90 min, most chromosomes become localized near the cell periphery
Chromosome movement in cells with bipolar spindles can occur independently of the Ndc80 complex in prometaphase but not in anaphase
Consistent with our previously published observations (McCleland et al. 2003) as well as those of others in HeLa cells (Deluca et al. 2006), in cells where the Ndc80 complex is compromised, kinetochores of chromosomes continue to associate with spindle microtubules during prometaphase in the absence of stable end-on attachment (Fig. 5a,b, Movies 5 and 6). In our study of cells with bipolar spindles, we found that chromosomes gather near the center of the mitotic spindle but do not undergo full metaphase alignment. Because injection with anti-Ndc80 complex antibodies overrides the mitotic spindle checkpoint, the injected cells proceed to anaphase. However, while sister chromatids separate, no poleward movement of chromatids takes place. Thus, as in cells with monopolar spindles, the chromosomes in cells with bipolar spindles exhibit kinetochore-driven movements in prometaphase that are independent of the Ndc80 complex. However, in anaphase, while the Ndc80 complex function is essential for chromosome movement to the poles, dynein/dynactin-dependent motility is apparently switched off, or if it functions at all, it can do so only in the presence of the active Ndc80 complex. Studies in other systems suggest that dynein/dynactin may play a minor, accessory role in anaphase chromosome movement (Yang et al. 2007; Howell et al. 2001; Sharp et al. 2000).
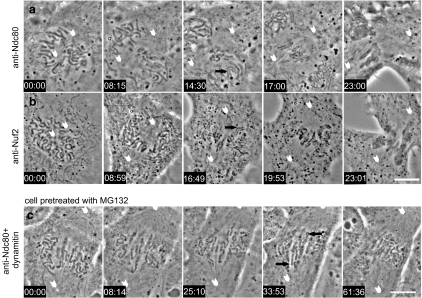
Fig. 5.
Inhibition of the Ndc80 complex in mitotic cells with bipolar spindles induces loss of metaphase alignment and forced exit from M phase. Image sequences from two prometaphase Xenopus S3 cell microinjected with antibodies against Ndc80 protein (a) or Nuf2 protein, n = 4 (b), components of the Ndc80 complex (injection at time point 00:00). Both antibodies cause a similar phenotype. The mitotic spindle continued to mature as the spindle poles (white arrowheads) separated. At a time point 8–9 min after injection, most chromosomes became clustered loosely near the spindle equator. Approximately 15 min after injection, anaphase ensued, indicated by the separation of the two sister chromatids (black arrows). However, sister chromatids remained at the spindle equator without any poleward anaphase movement. Movies (Movies 5 and 6) corresponding to cells in a and b are available in the supplementary material. c Simultaneous inhibition of the Ndc80 complex and dynein/dynactin leads to loss of kinetochore-driven chromosome movement in prometaphase cells. Image sequence from a Xenopus S3 cell treated with 25 μM MG132 for 30 min then coinjected with anti-Ndc80 complex antibodies and dynamitin protein at time point 00:00. Major chromosome movements gradually ceased, and many chromosome became aligned parallel to the spindle axis and often took on an “accordion-like” wrinkled morphology, perhaps because of the interaction of chromosome arms with spindle microtubules (black arrows). During this time, the spindle poles (white arrowheads) moved further apart. The proteasome inhibitor, MG132, was used to inhibit anaphase onset and mitotic exit which ordinarily occurs in cells injected with anti-Ndc80 complex antibodies. Movie 9 corresponding to this cell is available in the supplementary material. Bar = 10 μm
To determine if the behavior of chromosomes in our monopolar spindle model mimicked that of chromosomes in cells with bipolar spindles, we examined chromosome movements in cells with monopolar spindles by simulation of anaphase, induced by treatment with the Cdk1 inhibitor flavopiridol (Potapova et al. 2006). In uninjected, control cells (n = 4), chromosomes moved toward the unseparated spindle poles after flavopiridol treatment and created a tight array around the poles. In cells injected with anti-Ndc80 complex antibodies (n = 4), chromosomes did not move poleward upon Flavopiridol treatment and remained at the periphery until they decondensed and reformed into micronuclei, which were then sometimes propelled toward the cell center. (Supplemental Fig. S1, Movies 7 and 8).
Inhibition of both the Ndc80 complex and dynein/dynactin results in loss of kinetochore-based chromosome movements in prometaphase in cells with bipolar spindles
To test whether the Ndc80-independent chromosome movements in Xenopus S3 cells with bipolar spindles during prometaphase were dependent on dynein/dynactin, we coinjected dynamitin and anti-Ndc80 complex antibodies. To prevent cells from exiting M phase after inhibition of the Ndc80 complex, we again treated cells with the proteasome inhibitor MG132. After coinjection of mitotic Xenopus S3 cells with anti-Ndc80 complex antibodies and dynamitin, chromosomes exhibited altered mobility and position within bipolar spindles. After approximately 10 min, chromosome movements stalled, and the majority of chromosomes aligned parallel to spindle microtubules (Fig. 5c, Movie 9). The chromosomes came to exhibit a wrinkled or “accordion-like” morphology. This morphology could be explained by small residual movements of chromosomes after inhibition of both Ndc80 complex and dynein/dynactin or through interactions of microtubules with chromosome arms, likely mediated by microtubule assembly and disassembly or by chromatin-associated motor molecules such as chromokinesins (Wang and Adler 1995; Brouhard and Hunt 2005).
Discussion
Although rapid poleward chromosome movements in early prometaphase were observed several years ago, it has been difficult to dissect the molecular basis of this chromosome motility. The primary difficulty in analyzing the initial rapid poleward movements of chromosomes on the spindle microtubules is the transient nature of this activity. To prolong and sustain the rapid chromosomal movements, we disrupted the Ndc80 complex, responsible for stable attachment of chromosomes, and kept cells in M phase by the addition of proteasome inhibitor. In this way, we dissociated the transient associations of kinetochores with spindle microtubules at early prometaphase from the mature Ndc80-dependent binding of microtubules to kinetochores. Abrogation of the Ndc80 complex during prometaphase blocks stable attachment resulting in sustained dynein/dynactin-dependent kinetochore motility in prometaphase cells. In such cells, chromosomes exhibited increased rapid poleward movement but could not stably attach to spindle microtubules nor form a compact metaphase plate in cells with bipolar spindles. At anaphase, in cells where the Ndc80 complex is inhibited, chromatids separate but lose all attachment to the anaphase spindle. Based on GFP–tubulin, the anaphase spindle appears normal but lacks kinetochore fiber bundles. Spindle pole separation (anaphase B) occurs normally. These observations show that at anaphase onset, dynein/dynactin activity is either silenced or is of itself incapable of maintaining kinetochore attachment to anaphase spindle microtubules in the absence of the functional Ndc80 complex.
To quantify chromosome movements, we inhibited the kinesin Eg5 by addition of monastrol. Inhibition of Eg5 led to the formation of cells with monopolar spindles and a simplified geometry for measuring poleward and antipoleward movements of chromosomes. Our analysis shows that inhibition of the Ndc80 complex led to a significant increase in overall chromosomal movements with a noticeable increase in rapid (>10 μm/min) poleward movements. To test whether these rapid poleward movements unmasked by inhibition of the Ndc80 complex were dependent on dynein/dynactin, we injected the dynamitin protein. In the presence of dynamitin, the rapid poleward movements were largely repressed.
A recent study postulated a role for dynein in rapid poleward chromosome movements seen in cells during washout of microtubule-disrupting drugs (Yang et al. 2007). In the majority of the results reported by Yang et al., dynein activity was perturbed though a multiple-day small interfering ribonucleic acid (siRNA) treatment against ZW10, a member of the Rod/ZW10/Zwilch complex, a dynein-associated protein assembly thought to be involved in silencing of the mitotic spindle checkpoint. In addition, the ZW10 knockdown was reported to inhibit metaphase alignment and to result in a slower anaphase and an increase in lagging chromatids.
In injections of dynamitin protein in seven X. laevis S3 cells with bipolar spindles, we did not observe defects in metaphase alignment or slowed or lagging chromatids at anaphase (data not shown). Our results are consistent with a study of the effects of dynein/dynactin inhibition with dynamitin reported in mammalian cells by Howell et al. (2001). We suspect that the slightly differing results on metaphase alignment obtained by Yang et al. (2007) may be due to different methods used to compromise dynein/dynactin activity. The more far-reaching effects seen after siRNA knockdown of Zw10 may reflect the fact that the Rod/Zw10/Zwilch complex may participate in functions beyond regulation of dynein at the kinetochore. The Rod/Zw10/Zwilch proteins are required for checkpoint function and have been implicated in Golgi transport in interphase (Chan et al. 2000; Williams et al. 2003; Hirose et al. 2004; Varma et al. 2006). Dynein has also been implicated in chromosome movement in Drosophila. Loss of rapid prometaphase movements, asynchronous chromatid separation, and slowed anaphase were reported in Drosophila spermatocytes of Zw10 and Rod mutants (Savoian et al. 2000). Sharp et al. (2000) reported that dynamitin or anti-dynein injections perturbed metaphase alignment and slowed chromatid movement in Drosophila embryos.
Nevertheless, to corroborate the results we obtained by dynamitin injection in vertebrate cells, we also compared the effects of inhibiting the RZZ complex by microinjection of antibody to the Xenopus Zwilch protein. We found that anti-Zwilch had little effect on chromosome movement when injected alone (n = 5 cells) but led to a significant loss of chromosome movement when injected along with anti-Ndc80 complex antibodies (n = 9 cells; Supplemental Fig. S2 and Movie 10). Similar to the effects of dynamitin, anti-Zwilch antibody abrogated the rapid movements that normally occur after inhibition of the Ndc80 complex and many chromosomes in the coinjected cells eventually came to lie along the cell periphery, a terminal stage very similar to that seen when cells were coinjected with anti-Ndc80 complex antibodies and dynamitin. As in the case of dynamitin, we did not detect perturbations of microtubule association with spindle poles in normal cells with bipolar spindles or in cells treated with monastrol.
Our experiments in Xenopus cells show that when the Ndc80 complex is inhibited, chromosomes in prometaphase continue to attach and move on spindle microtubules. These data are consistent with the idea that initial dynein/dynactin-driven movements mediate lateral attachments that are rapidly converted to Ndc80 complex-dependent end-on attachments. End-on attachments appear to be dominant over lateral. This dominance is likely due to decreased concentration of dynein/dyanctin at kinetochores after stable microtubule attachment. In contrast, levels of Ndc80 complex remain high at kinetochores. In budding yeast, recent studies indicate that kinetochores also associate with microtubules by both lateral attachment and end-on attachment (Tanaka et al. 2007). However, the protein implicated in lateral attachment is not dynein but rather the kinesin-14 member, Kar3. In fission yeast, although the issue of lateral vs end-on attachment was not directly addressed, results suggest that neither the minus-end motors of the kinesin-14 class (Pkl1p and Klp2p) nor dynein are required for chromosome biorientation and congression but contribute to fidelity of chromosome segregation through roles in maintaining the organization of spindle poles (Grischuk et al. 2007).
In summary, in a cell-free system, microtubules appear to associate with kinetochores through two independent mechanisms, one that requires the Ndc80 complex and another that relies on dynein/dynactin. In living cells, abrogation of the Ndc80 complex by antibody microinjection leads to protracted expression of rapid dynein/dynactin-dependent poleward movements of kinetochores on spindle microtubules during prometaphase. These movements closely mimic the rapid poleward movement of chromosomes seen transiently just after nuclear envelope breakdown. However, under normal circumstances, mature attachments via the Ndc80 complex supplant transient attachment. In the absence of functional Ndc80 complex, these dynein/dynactin-based movements persist but can not generate chromosome alignment at the metaphase plate nor any chromatid motion in anaphase. Instead, the Ndc80 complex-dependent attachments are absolutely required for metaphase alignment and anaphase A movement of chromatids to the poles. This study lends important new information concerning the molecular nature of kinetochore motility activities and their precise regulation during the mitotic cell cycle.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Inhibition of the Ndc80 complex prevents chromosome segregation upon anaphase induction. Cells were pretreated with Monastrol (35 μM) to prevent spindle pole separation and with MG132 (25 μM) to block mitotic exit. At time 00:00, cells (n = 4) were treated with flavopiridol (10 μM) to induce a forced mitotic exit that is independent of proteasome activity to mimic anaphase onset. After 5 min of flavopiridol treatment, chromosomes have moved toward the spindle pole in the uninjected control cell (top row). The bottom row shows a cell injected with anti-Ndc80 complex antibodies (n = 4) before flavopiridol treatment. The presence of anti-Ndc80 complex antibodies prevented chromosome movement toward the spindle poles after flavopiridol treatment. Bar = 10 μm (JPG 58 KB)
Inhibition of dynein/dynactin by microinjection of antibody to Zwilch causes a similar phenotype as inhibition of dynein/dynactin by dynamitin injection. a Dynein/dynactin accumulation at kinetochores is impaired by microinjection of anti-Zwilch. Cells were pretreated with MG132 (25 μM) to block mitotic exit. 45 min after injection, cells were treated with nocodazole (50 ng/ml) for 10 min to disassemble the microtubules and enhance dynein/dynactin association with the kinetochores. The cell (n = 5) in the top row was injected with anti-Zwilch antibodies. The cell in the bottom row is a control noninjected cell. Cells were labeled with anti-rabbit IgG to detect injected antibody. Cells were labeled with mouse monoclonal antibody to the p150Glued subunit of the dynactin complex. Chromosomes can be seen in the phase-contrast image. In uninjected cells, p150Glued was found concentrated at kinetochores. Injection of anti-Zwilch antibody caused loss of p150Glued concentration at kinetochores. Images represent one confocal plane. Bar = 10 μm. b Cells (n = 9) were treated with MG132 and monastrol and then injected with anti-Zwilch antibody and anti-Ndc80 complex antibodies. In the depicted example, anti-Zwilch was injected just before the first image at time 00:00, and the anti-Ndc80 complex antibody was injected approximately 10 min later. With time, chromosomes lose attachment and orientation toward the spindle poles and become localized near the cell periphery (arrows). This response is similar to that obtained when anti-Ndc80 complex antibodies and dynamitin are coinjected (see Fig. 4). Bar = 10 μm. The complete video sequence for this cell is available in Movie 10 (JPG 54 KB)
Xenopus S3 cell treated with monastrol and MG132, injected with control IgG. Fluorescence image represents GFP–tubulin. Phase-contrast images were collected every 3 s; fluorescence images were collected every 60 s (MOV 2 MB)
Xenopus S3 cell treated with MG132 and monastrol, injected with dynamitin. GFP image represents tubulin. Fluorescence image shows GFP–tubulin. Phase contrast images were collected every 3 s; fluorescence images were collected every 9 s (MOV 1 MB)
Xenopus S3 cell treated with MG132 and monastrol, injected with anti-Ndc80 complex antibody. Fluorescence image shows GFP–tubulin. Phase-contrast images were collected every 3 s; fluorescence images were collected every 9 s (MOV 3 MB)
Xenopus S3 cell treated with MG132 and monastrol, injected with a combination of anti-Ndc80 complex antibody and dynamitin. Phase-contrast images were collected every 2 s (MOV 4 MB)
Acknowledgments
GJG was supported by the National Institute of General Medical Sciences and the McCasland Foundation. PTS was supported by National Institute of General Medical Sciences, the American Cancer Society, and the Pew Charitable Trust. MJE was supported by National Institutes of Health training grant HD07528.
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s00412-007-0135-3) contains supplementary material, which is available to authorized users.
References
- Brouhard GJ, Hunt AJ (2005) Microtubule movements on the arms of mitotic chromosomes: polar ejection forces quantified in vitro. Proc Natl Acad Sci USA 102:13903–13908 [DOI] [PMC free article] [PubMed]
- Chan GK, Jablonski SA, Starr DA, Goldberg ML, Yen TJ (2000) Human Zw10 and ROD are mitotic checkpoint proteins that bind to kinetochores. Nat Cell Biol 2:944–947 [DOI] [PubMed]
- Cheeseman IM, Chappie JS, Wilson-Kubalek EM, Desai A (2006) The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell 127:983–997 [DOI] [PubMed]
- DeLuca JG, Gall WE, Ciferri C, Cimini D, Musacchio A, Salmon ED (2006) Kinetochore microtubule dynamic and attachment stability are regulated by Hec1. Cell 127:969–982 [DOI] [PubMed]
- Dong Y, Vanden Beldt KJ, Meng X, Khodjakov A, McEwen BF (2007) The outer plate in vertebrate kinetochores is a flexible network with multiple microtubule interactions. Nat Cell Biol 9:516–522 [DOI] [PMC free article] [PubMed]
- Echeverri CJ, Paschal BM, Vaughan KT, Vallee RB (1996) Molecular characterization of the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J Cell Biol 132:617–634 [DOI] [PMC free article] [PubMed]
- Emanuele MJ, Stukenberg PT (2007) Xenopus Cep 57 is a novel kinetochore component involved in microtubule attachment. Cell 130:893–905 [DOI] [PubMed]
- Grishchuk EL, McIntosh JR (2006) Microtubule depolymerization can drive poleward chromosome motion in fission yeast. EMBO J 25:4888–4896 [DOI] [PMC free article] [PubMed]
- Grishchuk EL, Spiridonov IS, McIntosh JR (2007) Mitotic chromosome biorientation in fission yeast is enhanced by dynein and a minus-end-directed, kinesin-like protein. Mol Biol Cell 18:2216–2225 [DOI] [PMC free article] [PubMed]
- Hirose H, Arasaki K, Dohmae N, Takio K, Hatsuzawa K, Nagahama M, Tani K, Yamamoto A, Tohyama M, Tagaya M (2004) Implication of ZW10 in membrane trafficking between the endoplasmic reticulum and Golgi. EMBO J 23:1267–1278 [DOI] [PMC free article] [PubMed]
- Howell BJ, McEwen BF, Canman JC, Hoffman DB, Farrar EM, Rieder CL, Salmon ED (2001) Cytoplasmic dynein/dynactin drives kinetochore protein transport to the spindle poles and has a role in spindle checkpoint inactivation. J Cell Biol 155:1159–1172 [DOI] [PMC free article] [PubMed]
- Khodjakov A, Copenagle L, Gordon MB, Compton DA, Kapoor TM (2003) Minus-end capture of preformed kinetochore fibers contributes to spindle morphogenesis. J Cell Biol 160:671–683 [DOI] [PMC free article] [PubMed]
- King JM, Hays TS, Nicklas RB (2000) Dynein is a transient kinetochore component whose binding is regulated by microtubule attachment, not tension. J Cell Biol 151:739–748 [DOI] [PMC free article] [PubMed]
- McCleland ML, Gardner RD, Kallio MJ Daum J, Gorbsky GJ, Burke DJ, Stukenberg PT (2003) The highly conserved Ndc80 complex is required for kinetochore assembly, chromosome congression, and spindle checkpoint activity. Genes Dev 17:101–114 [DOI] [PMC free article] [PubMed]
- McCleland ML, Kallio MJ, Barret-Wit GA, Kestner CA, Shabanowitz J, Hunt DF, Gorbsky GJ, Stukenberg PT (2004) The vertebrate Ndc80 complex contains Spc24 and Spc24 homologs, which are required to establish and maintain kinetochore-microtubule attachment. Curr Biol 14:131–137 [DOI] [PubMed]
- Meraldi P, Draviam VM, Sorger PK (2004) Timing and checkpoints in the regulation of mitotic progression. Dev Cell 7:45–60 [DOI] [PubMed]
- Merdes A, DeMey J (1990) The mechanism of kinetochore-spindle attachment and polewards movement analyzed in PtK2 cells at the prophase-prometaphase transition. Eur J Cell Biol 53:313–325 [PubMed]
- Mitchison TJ, Kirschner MW (1985) Properties of the kinetochore in vitro. I. Microtubule nucleation and tubulin binding. J Cell Biol 101:755–765 [DOI] [PMC free article] [PubMed]
- Potapova TA, Daum JR, Pittman BD, Hudson JR, Jones TN, Satinover DL, Stukenberg PT, Gorbsky GJ (2006) The reversibility of mitotic exit in vertebrate cells. Nature 440:954–958 [DOI] [PMC free article] [PubMed]
- Rieder CL, Alexander SP (1990) Kinetochores are transported poleward along a single astral microtubule during chromosome attachment to the spindle in newt lung cells. J Cell Biol 110:81–95 [DOI] [PMC free article] [PubMed]
- Savoian MS, Goldberg ML, Reider CL (2000) The rate of poleward chromosome motion is attenuated in Drosophila zw10 and rod mutants. Nat Cell Biol 2:948–952 [DOI] [PubMed]
- Sharp DJ, Rogers GS, Scholey JM (2000) Cytoplasmic dynein is required for poleward chromosome movement during mitosis in Drosophila embryo. Nat Cell Biol 2:922–930 [DOI] [PubMed]
- Tanaka K, Mukae N, Dewar H, van Breugel M, James EK, Prescott AR, Antony C, Tanaka TU (2005) Molecular mechanisms of kinetochore capture by spindle microtubules. Nature 434:987–994 [DOI] [PubMed]
- Tanaka K, Kitamura E, Kitamura Y, Tanaka TU (2007) Molecular mechanism of microtubule-dependent kinetochore transport toward spindle poles. J Cell Biol 178:269–281 [DOI] [PMC free article] [PubMed]
- Varma D, Dujardin DL, Stehman SA, Vallee RB (2006) Role of the kinetochore/cell cycle checkpoint protein ZW10 in interphase cytoplasmic dynein function. J Cell Biol 172:655–662 [DOI] [PMC free article] [PubMed]
- Wang SZ, Adler R (1995) Chromokinesin: a DNA-binding, kinesin-like nuclear protein. J Cell Biol 128:761–768 [DOI] [PMC free article] [PubMed]
- Wei RR, Al-Bassam J, Harrison SC (2007) The Ndc80/HEC1 complex is a contact point for the kinetochore-microtubule attachment. Nat Struct Mol Biol 14:54–59 [DOI] [PubMed]
- Williams BC, Li Z, Liu S, Williams EV, Leung G, Yen TJ, Goldberg ML (2003) Zwilch, a new component of the ZW10/ROD complex required for kinetochore functions. Mol Biol Cell 14:1379–1391 [DOI] [PMC free article] [PubMed]
- Wittmann T, Hyman T (1999) Recombinant p50/dynamitin as a tool to examine the role of dynactin in intracellular processes. Methods Cell Biol 61:137–143 [DOI] [PubMed]
- Wordeman L, Steuer ER, Sheetz MP, Mitchison T (1991) Chemical subdomains within the kinetochore domain of isolated CHO mitotic chromosomes. J Cell Biol 114:285–294 [DOI] [PMC free article] [PubMed]
- Yang Z, Tulu UU, Wadsworth P, Rieder CL (2007) Kinetochore dynein is required for chromosome motion and congression independent of the spindle checkpoint. Curr Biol 17:973–980 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.
Inhibition of the Ndc80 complex prevents chromosome segregation upon anaphase induction. Cells were pretreated with Monastrol (35 μM) to prevent spindle pole separation and with MG132 (25 μM) to block mitotic exit. At time 00:00, cells (n = 4) were treated with flavopiridol (10 μM) to induce a forced mitotic exit that is independent of proteasome activity to mimic anaphase onset. After 5 min of flavopiridol treatment, chromosomes have moved toward the spindle pole in the uninjected control cell (top row). The bottom row shows a cell injected with anti-Ndc80 complex antibodies (n = 4) before flavopiridol treatment. The presence of anti-Ndc80 complex antibodies prevented chromosome movement toward the spindle poles after flavopiridol treatment. Bar = 10 μm (JPG 58 KB)
Inhibition of dynein/dynactin by microinjection of antibody to Zwilch causes a similar phenotype as inhibition of dynein/dynactin by dynamitin injection. a Dynein/dynactin accumulation at kinetochores is impaired by microinjection of anti-Zwilch. Cells were pretreated with MG132 (25 μM) to block mitotic exit. 45 min after injection, cells were treated with nocodazole (50 ng/ml) for 10 min to disassemble the microtubules and enhance dynein/dynactin association with the kinetochores. The cell (n = 5) in the top row was injected with anti-Zwilch antibodies. The cell in the bottom row is a control noninjected cell. Cells were labeled with anti-rabbit IgG to detect injected antibody. Cells were labeled with mouse monoclonal antibody to the p150Glued subunit of the dynactin complex. Chromosomes can be seen in the phase-contrast image. In uninjected cells, p150Glued was found concentrated at kinetochores. Injection of anti-Zwilch antibody caused loss of p150Glued concentration at kinetochores. Images represent one confocal plane. Bar = 10 μm. b Cells (n = 9) were treated with MG132 and monastrol and then injected with anti-Zwilch antibody and anti-Ndc80 complex antibodies. In the depicted example, anti-Zwilch was injected just before the first image at time 00:00, and the anti-Ndc80 complex antibody was injected approximately 10 min later. With time, chromosomes lose attachment and orientation toward the spindle poles and become localized near the cell periphery (arrows). This response is similar to that obtained when anti-Ndc80 complex antibodies and dynamitin are coinjected (see Fig. 4). Bar = 10 μm. The complete video sequence for this cell is available in Movie 10 (JPG 54 KB)
Xenopus S3 cell treated with monastrol and MG132, injected with control IgG. Fluorescence image represents GFP–tubulin. Phase-contrast images were collected every 3 s; fluorescence images were collected every 60 s (MOV 2 MB)
Xenopus S3 cell treated with MG132 and monastrol, injected with dynamitin. GFP image represents tubulin. Fluorescence image shows GFP–tubulin. Phase contrast images were collected every 3 s; fluorescence images were collected every 9 s (MOV 1 MB)
Xenopus S3 cell treated with MG132 and monastrol, injected with anti-Ndc80 complex antibody. Fluorescence image shows GFP–tubulin. Phase-contrast images were collected every 3 s; fluorescence images were collected every 9 s (MOV 3 MB)
Xenopus S3 cell treated with MG132 and monastrol, injected with a combination of anti-Ndc80 complex antibody and dynamitin. Phase-contrast images were collected every 2 s (MOV 4 MB)