Abstract
Orientation with respect to gravity is essential for the survival of complex organisms. The gravity receptor is one of the phylogenetically oldest sensory systems, and special adaptations that enhance sensitivity to gravity are highly conserved. The fish inner ear contains three large extracellular biomineral particles, otoliths, which have evolved to transduce the force of gravity into neuronal signals. Mammalian ears contain thousands of small particles called otoconia that serve a similar function. Loss or displacement of these structures can be lethal for fish and is responsible for benign paroxysmal positional vertigo (BPPV) in humans. The distinct morphologies of otoconial particles and otoliths suggest divergent developmental mechanisms. Mutations in a novel gene Otopetrin 1 (Otop1), encoding multi-transmembrane domain protein, result in nonsyndromic otoconial agenesis and a severe balance disorder in mice. Here we show that the zebrafish, Danio rerio, contains a highly conserved gene, otop1, that is essential for otolith formation. Morpholino-mediated knockdown of zebrafish Otop1 leads to otolith agenesis without affecting the sensory epithelium or other structures within the inner ear. Despite lack of otoliths in early development, otolith formation partially recovers in some fish after 2 days. However, the otoliths are malformed, misplaced, lack an organic matrix, and often consist of inorganic calcite crystals. These studies demonstrate that Otop1 has an essential and conserved role in the timing of formation and the size and shape of the developing otolith.
Keywords: Otolith, Otoconia, Otopetrin 1 (Otop1), Biomineralization, Vestibular systems
Introduction
Otoconia are small (approximately 10 μm) extracellular biomineral particles found in the vestibular portion of the vertebrate inner ear. Otoconia are composed of specific polymorphs of calcium carbonate (CaCO3) crystals precipitated around an organic core of extracellular matrix proteins. These particles are required for normal sensation of linear acceleration and gravity in mammals (Bergstrom et al., 1998; Lim, 1980; Ornitz et al., 1998). In teleost fish, complete loss of the orthologous structure, the otolith, is lethal (Riley and Moorman, 2000). In contrast to the thousands of small otoconial particles in mammals, only three large otoliths form in fish. Few molecules governing the development of otoconia and otoliths have been described and those that have seem to be specific to either structure. It has been proposed that the polymorph of CaCO3 found in otoconia or the otolith is determined by the major matrix proteins that make up the organic core: Otoconin 90 is at the core of calcitic CaCO3 otoconia of birds and mammals; Otoconin 22 is the primary matrix component of aragonitic CaCO3 otoconia in amphibians; Otoconin 54 is the primary matrix constituent of the vateritic CaCO3 otoconia utilized by early jawed fish, such as the garfish (Pote and Ross, 1991); and otolith matrix protein (omp) is the primary matrix protein of the aragonitic fish otolith (Murayama et al., 2000). These major matrix proteins share the ability to bind calcium or other ions and the otoconins share one or two rigid phospholipase A2 structural domains that may mediate their ability to guide the formation of specific CaCO3 crystal polymorphs (Pote et al., 1993; Wang et al., 1998). Starmaker, an ortholog of the mammalian dentin sialoprotein (DSP), is an acidic phosphoprotein recently shown to be required for normal otolith formation in the zebrafish (Söllner et al., 2003). While expression of DSP has been observed in the mouse inner ear, DSP knockout mice do not appear to have significant vestibular dysfunction based on swim testing (T. Sreenath, personal communication), suggesting that the function of starmaker may be specific to the fish otolith.
While there must be important differences in the proteins and pathways required to generate small otoconia particles versus a large otolith, some similarities must also exist. The essential requirement for the formation of both otoliths and otoconia is the availability of Ca2+ and ions. The presence of carbonate ions depends on the activity of carbonic anhydrase. The source of calcium in the endolymph that contributes to the otoconia and the otolith is poorly understood. Organic substances, including acidic proteins, glycosaminoglycans (GAGs) and proteoglycans, are also essential to regulate crystal growth (Addadi et al., 1989; Khan, 1997) and have been identified in both otoliths (Borelli et al., 2003) and otoconia (Tachibana and Morioka, 1992). While it is believed that these proteins and extracellular matrix molecules are required for locally increasing Ca2+ and concentrations and as structural components of the developing otolith or otoconia, the only protein with a known enzymatic function required for otoconial formation is NADPH Oxidase 3 (NOX3). NoX3 is mutated in head-tilt mice that have nonsyndromic otoconial agenesis and may be required for the aggregation of Otoconin 90 proteins in the mouse ear (Paffenholz et al., 2004).
Orchestration of extracellular calcification requires bringing together ionic and proteinaceous components in time and space. The organic matrix components of otoconia are expressed in different regions of the vestibular epithelium; however, all matrix components must associate with an extracellular gelatinous structure called the otolithic membrane in order to localize otoconial development above the sensory epithelium. Otoconial matrix proteins must aggregate into ordered organic cores and Ca2+ and concentrations must be locally increased to allow crystallization. Coordination of these events requires the normal formation of the otocyst (Malicki et al., 1996) and sensory maculae (Haddon et al., 1998), as well as tight regulation of the endolymph ionic environment (Everett et al., 1997; Kozel et al., 1998). This process is temporally restricted, as expression of certain major matrix proteins is dramatically down-regulated after early development (B. Blasiole and E. Ignatova, unpublished data). In mammals, the process of otoconial development is essentially complete by postnatal day 7 (Erway et al., 1986; Lim, 1973; Veenhof, 1969), and little evidence is available for continued otoconial formation or repair. In fish, initial rapid growth of the otolith occurs early in otic development, but the otolith continues to grow throughout the life of the fish, with increments of organic matrix and calcium carbonate added daily to the otolith surface. Disruption of any of these processes can lead to the formation of abnormally shaped or ectopic otoconia or otoconial agenesis.
Ectopic otoconia in humans have been proposed to cause human vestibular dysfunction, in particular benign paroxysmal positional vertigo (BPPV). BPPV is a common cause of dizziness and is associated with dislodged otoconia entering the semicircular canals and causing abnormal vestibular sensation in response to head rotation. It has been estimated that in the elderly population as much as 50% of dizziness can be attributed to BPPV (Oghalai et al., 2000). Otoconial pathology is thus a significant etiology of balance-related falls and accidental deaths in the elderly. While many cases of BPPV have been associated with head trauma, vestibular neuritis, treatment with some pharmacological agents, or age-related degeneration of otoconia, the etiology of approximately half of the cases of BPPV in young and elderly patients is still unknown.
Mutations in a novel protein, Otopetrin 1 (Otop1), cause nonsyndromic otoconial agenesis and a severe balance disorder in tilted and mergulhador mice (Hurle et al., 2003). Mutant mice display near one hundred percent penetrance of the otoconial agenesis phenotype, with no developmental changes in inner ear morphogenesis. Otop1 is predicted to encode a multi-transmembrane domain protein of unknown function with no known homology to any family of receptors, transporters, or channels. Two independent single-base pair mutations have been identified in mutant mice; these mutations are in different regions of the molecule but create identical phenotypes, suggesting that the normal function of Otop1 is necessary for the development of otoconia in the mouse. Homologous genes for Otop1 have been identified in all vertebrate groups examined including zebrafish and Fugu, where they are 41% and 44% identical, respectively, to mouse Otop1 (Hurle et al., 2003), suggesting that they may share a common mechanism of action in both otolith and otoconial morphogenesis.
Here, we show that Otop1 has a conserved and essential role in teleost otolith development. Zebrafish otop1 and mouse Otop1 have similar expression patterns in the developing inner ear. Morpholine oligonucleotide (morpholino)-mediated knockdown of Otop1 expression resulted in otolith agenesis in the majority of treated fish, without affecting morphogenesis of the zebrafish inner ear. In a small percentage of morphant animals, otolith development was greatly delayed and began at 40−50 h postfertilization (hpf), with a variety of otolith phenotypes, including formation of normal otoliths or otoliths lacking an organic matrix and with an atypical crystal polymorph (calcite). These data support a conserved role for Otop1 in the localization and aggregation of otolith matrix proteins and the regulation of the ionic environment of the otolithic membrane during vestibular organ development.
Materials and methods
In situ hybridization
The clone fb76b02.y1 containing the 3′ UTR of otop1 identified from the zebrafish EST project (Washington University) was sequenced to identify orientation and linearized with Not1. A digoxigenin-labeled RNA probe was generated using the Sp6 labeling kit (Roche), following the manufacturer's instructions.
Timed matings of zebrafish were established and embryos isolated at specific time points based on time since fertilization and on developmental milestones (zfin.org). Embryos were dechorionated and fixed overnight in 4% paraformaldehyde in phosphate-buffered saline (PBS) at 48C. Embryos were hybridized overnight with a digoxigenin-labeled probe. Hybridization was detected with anti-digoxigenin–alkaline phosphatase-conjugated IgG and visualized with BM purple AP substrate (Roche) in the presence of 2% levamisole.
For sectioning of in situ hybridized embryos, overstained 3 dpf embryos were embedded in JB-4 plastic (Chan et al., 2001) and sectioned at 4 μm. Similarly prepared embryos were sectioned and stained with Richardson's stain for comparison of histologic structures.
Morpholine oligonucleotide-mediated knockdown of zebrafish otop1
Two independent morpholine oligonucleotides were designed to base pair with the 5′ UTR of otop1. MO-1 covers the region from −91 to −65 bp from the ATG start codon (TTACACCTTCAGGACCCGTTAGTTT) and MO-2 begins at −20 bp and spans the ATG, ending at the +5 nucleotide position (ACCATGCTCGATCGCTGTCGGTAAA). Both morpholinos were purchased from Gene Tools, LLC (Philomath, Oregon) and were 5′ labeled with FITC. Before injection, morpholino oligonucleotides were diluted with Phenol Red tracer and 1× Danieau's buffer (58 mM NaCl, 0.7 mM KCl, 0.4 mM MgSO4, 0.6 mM Ca(NO3)2, 5 mM HEPES, pH 7.6). Timed matings were established. Embryos were collected at the 1-and 2-cell stage and injected with morpholino concentrations from 0.25 to 12 ng. MO-1 was used for all of the following experiments. Dorsal ventral axis defects and pericardial edema were both noted to occur in morpholino-injected fish at high doses (8−12 ng morpholino).
Morphant embryos were maintained at 28°C in charcoal filtered water and were examined and counted under a dissection microscope. To count and photograph otoliths at older stages, embryos were anesthetized with 1% tricaine and immobilized in 3% methyl cellulose.
Histologic preparation
The 4 and 7 dpf wild-type and morphant fish were collected and fixed overnight in cold 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer, pH 7.5. Animals were washed in cold 0.1 M cacodylate buffer and dehydrated by graded series of 25−100% ethanol. Samples were maintained at −20°C until they could be further processed. To prepare samples for thin sections, embryos were cleared in 100% propylene oxide and incubated with an increasing ratio of propylene oxide to unaccelerated Durcupan at room temperature. Fish were then incubated with 100% unaccelerated Durcupan overnight and transferred to accelerated Durcupan, oriented and hardened at 60°C for 48−64 h. Sections were cut with a glass Ralph knife on a rotary microtome at a thickness of 4 or 6 μm. Sections were stained with Richardson stain and coverslipped.
For immunohistochemical labeling, 28 hpf morpholino-treated and control fish were fixed overnight in 4% paraformaldehyde. Following thorough rinsing in PBS, specimens were cryoprotected in 30% sucrose (in PBS), embedded in OCT compound, frozen, and sectioned at 10 μm. Sections were mounted on gelatin/CrK(SO4)2−-coated slides, treated for 2 h in blocking solution (2%NHS, 2% NGS, 1% BSA, in PBS), and incubated overnight in primary antibody against acetylated tubulin (Sigma T6793, 1:100). The next day, sections were incubated for 2 h in Cy3-conjugated anti-mouse secondary antibody and cell nuclei were labeled with bisbenzimide (Sigma).
Scanning electron microscopy
Fixed tissues were dehydrated using a series of graded acetones. Final dehydration was performed by placing the specimens in tetramethylsilane that was sublimated in a dry 60°C oven. The specimens were then mounted on studs and palladium coated.
Single crystal X-ray diffraction
The 7 dpf wild-type and morphant fish from the same clutches were anesthetized and fixed in 100% ethanol. Otoliths were removed by dissection in 100% ethanol and were maintained dry on a covered glass slide until examination. The otoliths were mounted with grease in a random orientation. Preliminary examination and data collection were performed with a Bruker SMART 1 K Charge Coupled Device (CCD) Detector single crystal X-ray diffractometer equipped with a sealed tube X-ray source using graphite monochromated Mo Kα radiation (λ = 0.71073 Å) at −123°C. Typical preliminary unit cell constant determination with a set of 45 narrow frame 0.3° ϖ scans failed due to insufficient harvested reflections. Therefore, a data set was collected with a frame width of 0.3° in ϖ and counting time of 60 s/frame at a crystal to detector distance of 4.835 cm (1301 frames at −27° 2θ and 230 frames at 27° 2θ). The double pass method of scanning was used to exclude any noise. Thresholding the collected frames resulted in 122 reflections. Indexing of the unit cell was carried out with CELL_NOW (Sheldrick, 2002) and the cell was refined using the SMART software package (Bruker Analytical X-ray).
Results
Similar expression of mouse and zebrafish otop1 in the ear during embryonic development
The zebrafish homologue of Otop1 was identified and described previously (Hurle et al., 2003). Using whole-mount in situ hybridization, otop1 mRNA was first identified in the otocyst at 18 h postfertilization (hpf). By 24 hpf, when otolith seeding is nearly complete, otop1 expression was localized in the developing sensory epithelium of the ear (Figs. 1A and B). This pattern is similar to Otop1 expression in the mouse utricle and saccule during otoconial development (Hurle et al., 2003). At later stages, zebrafish otop1 mRNA was restricted to the utricular and saccular maculae (Fig. 1C) in a pattern consistent with expression in precursor and mature sensory hair cells (Figs. 1D and E). By 5 days postfertilization (dpf), otop1 expression was greatly reduced in the otolith organs but was identified in the neuromasts of the lateral line system (Fig. 1F). Expression of otop1 was not detectable in the inner ear at 7 dpf by in situ hybridization but persisted in the anterior and lateral line (data not shown). Reduction or loss of otop1 expression in the inner ear suggests that otop1 has a specific role in the early development and rapid growth of the otolith, but that it may not be required for the daily incremental growth that continues throughout the life of the fish.
Fig. 1.

Whole mount in situ hybridization analysis of otop1 mRNA expression. (A) Lateral view of a 24 hpf embryo showing significant expression of otop1 in the ventral half of the developing otic vesicle. (B) Dorsal view at 24 hpf showing otop1 expression. (C) Lateral view of 3 dpf larva showing otop1 mRNA expression in the sensory epithelium. (D) Four-μm plastic section of the otocyst of a 3 dpf fish (dorsal is up, lateral to the right) stained with Richardson's stain (100×). Pale apical cells with round nuclei (arrow) are hair cells. Cells with elongated, densely staining nuclei (arrowheads) are precursor cells within the macular growth zone. (E) Similar 4 μm plastic section of a 3 dpf fish showing otop1 mRNA localized to the luminal cells of the otocyst and adjacent cells in the monolayer, consistent with expression in the mature and developing hair cells (100×). (F) Lateral view of a 5 dpf larva showing otop1 expression along the entire length of the animal in the anterior and lateral line organs (arrowheads). Scale bars: A, 50 μm; B–C, 250 μm; D–E, 10 μm; and F, 50 μm.
Morpholino-mediated knockdown of otop1
To determine whether the essential function of Otop1 in otoconial/otolith development is conserved in fish, the expression of zebrafish Otop1 protein was knocked down using antisense morpholine oligonucleotides (morpholinos). Morpholinos targeted to 5′ UTR and translation initiation sequences block translation of the message (Nasevicius and Ekker, 2000). Injection of a morpholino designed against otop1 (MO-1) into one-cell stage embryos resulted in complete agenesis of otoliths (Fig. 2). Injection of a second morpholino (MO-2) targeted to an independent region of the otop1 5′ UTR reproduced this defect, confirming that otolith agenesis in zebrafish was specific to loss of Otop1 expression. At 30 hpf, more than 96% of otop1 morphant fish failed to develop both the saccular and utricular otoliths (Table 1), with no other obvious developmental defects (Fig. 2). Expression of pax2a and otx1 at 24 (Figs. 3A–D) and 48 hpf (data not shown) were comparable in uninjected and otop1 morphant fish, an indication that the early steps in otocyst formation progressed normally.
Fig. 2.

Absence of otolith formation in otop1 morphant fish. (A) Lateral view of an uninjected wild-type control at 30 hpf. (B) Lateral view of a 30 hpf morphant fish injected with 10 ng MO-1. Otop1 morphant fish are morphologically normal at all stages of development but lack otolith formation. (C and D) Lateral view of control and morphant 30 hpf otocysts. Otoliths are located at the poles of the developing otocyst of a control fish (arrows) (C) but are absent in the 10-ng MO-1-injected animals (arrow) (D). Scale bars: A–B, 50 μm; and C–D, 250 μm.
Table 1.
Otolith formation in otop1 morphant fish
| Morpholino (ng) | n | Score (%) |
|||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||
| A. Morpholino-mediated knockdown of otop1 30 hpf | |||||
| 0 | 53 | 100 | 0 | 0 | 0 |
| 0.25 | 15 | 67 | 0 | 0 | 33 |
| 0.5 | 46 | 20 | 2 | 0 | 78 |
| 1 | 29 | 7 | 0 | 0 | 93 |
| 2 | 42 | 0 | 0 | 0 | 100 |
| 4 | 24 | 0 | 4 | 0 | 96 |
| 8 | 56 | 0 | 0 | 0 | 100 |
| 10 | 50 | 0 | 0 | 0 | 100 |
| B. Morpholino-mediated knockdown of otop1 7 dpf | |||||
| 0 | 23 | 100 | 0 | 0 | 0 |
| 1 | 33 | 9 | 36 | 31 | 24 |
| 2 | 17 | 0 | 0 | 12 | 88 |
| 4 | 23 | 0 | 0 | 9 | 91 |
Score: 0, 2 otoliths in each ear; 1, otoliths in both ears, but abnormal in number, location, or shape; 2, otoliths in only one ear; 3, otoliths absent in both ears.
Fig. 3.

Normal gene expression and otocyst morphogenesis in morphants. (A) The pax2a expression in wild-type and (B) morphant otocyst. (C) otx1 expression in a wild-type and (D) morphant otocyst. (E) Immunohistochemistry for acetylated tubulin on 10 μm frozen sections through a 28 hpf wild-type otocyst. Tether cell kinocilia (arrow) and otolith (arrowhead) are evident in magnified insert (E′). (F) Morphant otocyst examined for acetylated tubulin. Tether cell kinocilia are evident in magnified insert (F′) (arrow) but no otolith seeding particles were present. (G) DIC image of the otocyst of a morphant fish (100×) at 24 hpf. No aggregated matrix was visible in several ears examined in this manner. (H) omp expression in wild-type and (I) morphant otocyst. (J) starmaker expression in wild-type and (K) morphant otocyst. A–D and H–K are whole mount in situ hybridizations of 24 hpf embryos. All otop1 morphant fish were injected with 4 ng MO-1 at the one-cell stage. Rostral is on the left. Scale bars: A–D, H–K, 250 μm; E–F, 10 μm; G, 25 μm.
Tether cells function at the poles of the otocyst and are required for normal otolith formation (Riley and Grunwald, 1996). Development of this cell type, examined by acetylated tubulin immunohistochemistry of wild-type and injected animals, showed distribution of hair cell kinocilia in a similar pattern to uninjected, age-matched controls (Figs. 3E and F). These data suggest that otop1 knockdown does not disrupt normal patterning or cell differentiation in the developing inner ear, but specifically disrupts otolith formation.
Fish with abnormal otolith development often have visible otolith seeding particles within the otocyst by 24 hpf (Riley and Grunwald, 1996; Riley et al., 1997; Sumanas et al., 2003) even when actual otolith formation is delayed. No otolith matrix material was observed in otop1 morphant fish at 24 hpf by differential interference contrast (DIC) microscopy (Fig. 3G). To determine if the expression of genes known to be required for formation of the otolith matrix was disrupted in otop1 morphant fish, the expression of zebrafish otolith matrix protein (omp) (E. Ignatova, unpublished data) and starmaker (Söllner et al., 2003) mRNA were assessed during early otic development. The expression patterns of omp and starmaker were similar in wild-type and morphant fish at 24 hpf (Figs. 3H–K) and 48 hpf (data not shown), suggesting that loss of Otop1 does not disrupt the normal expression of genes encoding otolith matrix proteins.
Normal formation of the lateral line system in otop1 morphants
Despite expression of otop1 in the developing neuromasts of the lateral line system, morphant animals showed no obvious defects in the morphogenesis of these structures. Examination of the neuromasts of live morphants by DIC at 3 and 5 dpf (data not shown) and scanning electron microscopy of 4 dpf morphants (Figs. 4A and B) revealed normal formation of the cupula, the extracellular fibrous matrix covering the neuromast that transduces movement to the underlying hair cells. Plastic sections through 7 dpf morphants stained with Richardson's stain showed that the distribution and number of anterior, trunk, and posterior lateral line neuromasts was similar to that of wild-type animals. In addition, otop1 morphant neuromasts appeared to have a similar complement of support and hair cells (Figs. 4C and D). Neuromast formation and migration are unlikely to be directly affected by otop1 knockdown, as these structures develop later than the otolith. The normal distribution of the neuromasts in 7 dpf morphant fish suggests that there was no disruption of the normal patterning or migration of the neuromast precursors. Additional examination of these sections showed no histological difference between morphant and wild-type fish in any other structure (data not shown).
Fig. 4.

Normal formation of the lateral line system in otop1 morphant fish. (A–B) SEM image of a 4 dpf WT (A) and morphant (B) posterior lateral line neuromast. The cupula and kinocilia bundle has formed normally in the morphant animal. (C and D) Four-μm plastic section through a 7 dpf uninjected control (C) and 10 ng MO-1-injected morphant anterior neuromast showing a similar complement of hair cells (pale, apical cells with round nuclei) and supporting cells. Scale bars indicate 10 μm. C and D are stained with Richardson's stain.
Delayed otolith formation in otop1 morphant fish
A small percentage of morphant fish that had completely failed to develop otoliths by 30 hpf exhibited otolith formation at 4 dpf. The late formation of otoliths in these fish occurred as a function of the morpholino dose injected (Table 1). Visible otolith particles were first noted between 40 and 50 hpf (data not shown). This timing is consistent with dilution of the morpholino by growth of the fish (Nasevicius and Ekker, 2000) and possible reexpression of Otop1. The process of delayed otolith formation appeared similar to the normal formation of otoliths earlier in development in that multiple small seeding particles agglomerated and attached to the sensory epithelium (Riley et al., 1997). However, in some cases, some seeding particles did not attach to the sensory maculae and were found lodged in the developing semicircular canals or were free floating in the otic cavity (Figs. 6I and J).
Fig. 6.

Delayed otolith formation and dysmorphology in otop1 morphant fish. (A–F) Lateral views of 7 dpf otocysts (rostral to left) of a wild-type (A) and 1 ng MO-1-injected fish (B–F). (A) Wild-type fish develop two ovoid otoliths. (B) Delayed otoliths in morphant fish can appear similar to wild type in shape and location. (C) Otoliths that are identical in location, but with an oblong shape and distinct straight edges. (D) Formation of a single otolith that is larger than a wild-type otolith and attached to the saccular macula. The irregular shape and large size may indicate fusion of the early otolith seeding particles. (E) A single cuboidal otolith located in the saccular sensory macula. (F) Multiple otoliths with polyhedral structures. The posterior-most otolith did not appear to be attached to a sensory macula. (G) Four-μm plastic section through a 7 dpf 1 ng MO-1-injected morphant otocyst showing a wild-type-like otolith attached to the saccular macula. Richardson's stain identifies concentric rings of organic matrix within the otolith structure. (H) Four-μm plastic section through the opposite otocyst showing an angular otolith on the utricular sensory patch with no obvious organic matrix within the crystal. (I) Lateral view of a 4 dpf otocyst (rostral to left) from an 8-ng MO-1-injected fish. At 4 dpf, numerous irregularly shaped otoliths are found throughout the otic cavity and in the semicircular canal (arrow). (J) SEM image of the same 4 dpf morphant otocyst. Note the presence of aggregates of crystals attached to the utricular and saccular otolithic membranes and additional free-floating crystals on the otocyst wall (several otolith particles were lost in preparation). Scale bar for A–F is 250 μm; G–H is 10 μm; I is 250 μm; and J is 25 μm.
By 5 dpf, the development of the zebrafish inner ear is essentially complete, with the formation of the semicircular canals and associated sensory maculae (Whitfield et al., 2002). When compared to wild-type fish, morphant fish injected with 10 ng of MO-1 had normal sensory maculae and canal formation but lacked otoliths (Figs. 5A and B). At 7 dpf, the morphant epithelium had a normal distribution of hair and supporting cells in the saccular sensory macula, as well as normal transitional cells and thin nonsensory epithelium (Figs. 5C and D). Scanning electron microscopy was used to compare the size of the sensory maculae, distribution of hair cells, and the formation of the otolithic membrane. Formation of the gravity organ sensory maculae and the otolithic membrane appeared normal in fish injected with MO-1. In wild-type animals, the otolith had to be removed to examine the underlying macular epithelium; in the instances examined, this led to tearing of this fibrous membrane (Fig. 6E). In morphant animals that did not form an otolith, the membrane remained intact and in contact with each hair cell (Fig. 6F). This arrangement of the fibrous matrix of the otolithic membrane in fish is presumably to simultaneously transduce the motion of the otolith to all hair cells of the macula. In mammals and birds, hair cells do not appear to directly contact the otolithic membrane matrix.
Fig. 5.

Morphogenesis of the otocyst and sensory epithelium. (A and B) Lateral view of a 5 dpf wild-type (A) and morphant (B) otocyst. In the wild-type fish, the utricular otolith is smaller and located in the rostral portion of the otocyst, the saccular otolith is larger and located centrally. The semicircular canals and ampullae (arrowhead) are similar in control and in 10 ng MO-1 morphant fish with otolith agenesis. (C) Plastic section through the utricular macula of a 7 dpf wild-type fish (100×) showing development of the hair cells (pale apical cells, arrow) and supporting cells (darker basal cells, arrowhead). (D) Plastic section of a 7 dpf morphant saccular macula showing a similar distribution of cell types. (E and F) Scanning electron micrographs of a wild-type (E) and morphant (F) utricular otolithic membrane at 7 dpf. Removal of the otolith disrupted the wild-type otolithic membrane. Morphant fish formed an otolithic membrane in the absence of the otolith. The otolithic membrane is fibrous and connects the stereocilia of all hair cells in the macula. O, otolith. Scale bars: A–B, 250 μm; C–D, 10 μm; E–F, 10 μm.
In birds and teleost fish, two other vestibular maculae form during later larval stages: the lagena with an accompanying otolithic/otoconial membrane (8−12 dpf in zebrafish, with otolith formation beginning at 9 dpf; Bever and Fekete, 2002; Riley and Moorman, 2000), and the macula neglecta (17−20 dpf), which lacks an otolith (Whitfield et al., 2002). The formation of the lagenar otolith and the sensory structures of lagena and the macula neglecta occurs too late in development to be affected by otop1 morpholino injection into fertilized eggs (Nasevicius and Ekker, 2000).
By 7 dpf, a variety of otolith phenotypes was noted in otop1 morphant fish with late-forming otoliths (Table 1). Some of the most striking examples were seen in fish exposed to relatively low concentrations of the morpholino. In animals injected with 1 ng of MO-1, observed phenotypes ranged from two near normal otoliths in each ear (Fig. 6B) to a single large rounded otolith (Fig. 6D), to single and multiple polyhedral forms (Figs. 6C, E, and F). In some examples, these crystals resembled mammalian otoconia with polyhedral shapes and sharp edges.
Interestingly, some fish displayed mixed phenotypes. For example, in one otocyst, the morphant fish formed a normally shaped saccular otolith (Fig. 6G) and a small uncalcified utricular otolith that stained strongly with Richardson's stain (data not shown) (Richardson et al., 1960). The organic matrix of the normally shaped otolith stained lightly with Richardson's stain, highlighting the daily growth of the otolith by alternating deposition of organic matrix and inorganic CaCO3. This demonstrates that a normal appearing otolith can develop after a critical window of development proposed to extend from 18 to 24 hpf (Riley et al., 1997). In the opposite ear, both the utricular (Fig. 6H) and saccular otoliths (data not shown) were roughly cuboidal. In these otoliths, no organic matrix could be identified, and multiple histological sections suggested that it was made up of a single inorganic crystal. The structure of the large, polyhedral otolith closely resembles the bgiantQ calcitic otoconia described in several mouse mutants with defects in otoconial synthesis (Erway and Grider, 1984; Lim et al., 1978; Ornitz et al., 1998). Such a change in morphology of the morphant otolith indicated a possible change in the mechanisms of mineralization (see below).
In rare cases, animals injected with higher doses of morpholino recovered otolith formation. Ectopic mineralization was first noted in these animals at approximately 72 hpf. In these instances, otolith particles did not aggregate well and could be identified throughout the otic cavity, including in the developing semicircular canals at 4 dpf (Figs. 6I and J).
Abnormal otoliths in otop1 morphants are composed of calcite
Pure CaCO3 can form crystals with one of three distinct crystalline polymorphs: calcite, aragonite, or vaterite. At 7 dpf, wild-type otoliths (Fig. 7A) are composed of thousands of aragonitic CaCO3 crystallites arranged in multiple orientations over the surface of the growing otolith. In contrast, otoconia contain an organic core and a crystalline casing composed of calcite, the most stable polymorph of CaCO3 (Carlstrom et al., 1953). The crystalline appearance of otoliths that formed in morphant fish at 7 dpf (Fig. 7B) suggested a possible change in crystal polymorph from aragonite to calcite. Single crystal X-ray diffraction of wild-type otoliths yielded a crystalline dust diffraction pattern (Fig. 7C) that is consistent with the disordered arrangement of aragonitic crystallites that has been previously identified by powder X-ray diffraction (Söllner et al., 2003). Notably, a set of unit cell parameters consistent with published values for aragonite (http://ruby.colorado.edu/smyth/min/aragonite.html) was obtained by indexing harvested reflections. Morphant otoliths, with a shape similar to wild-type otoliths (Fig. 6G), gave a similar diffraction pattern (data not shown). The polyhedral otoliths evident in some morphants appeared similar in shape to mammalian calcitic otoconia by scanning electron microscopy (SEM) (Fig. 7B). Single crystal X-ray diffraction analysis of this type of otolith yielded a single crystal diffraction pattern with an identifiable unit cell at −123°C of the following: a = 4.992 (6), b = 4.992 (1), c = 17.012(2) Å, α = 90.00(1), β = 90.01(1), γ = 120.01(1), V = 366.8 (1) (Fig. 7D). These parameters match published values for calcite (http://ruby.colorado.edu/smyth/min/calcite.html).
Fig. 7.
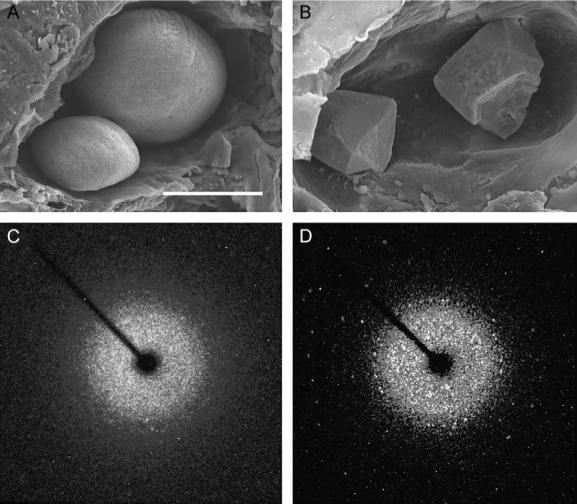
Calcitic otolith formation in otop1 morphant fish. (A) Scanning electron microscopy of the otic cavity of a 7 dpf uninjected age-matched control showing a smaller utricular (left) and larger saccular otolith. Both otoliths are rounded and cover the entire sensory maculae. (B) Eight-ng MO-1-injected morphant otic cavity at 7 dpf. Otoliths are angular. The sensory epithelium is visible below the utricular otolith. The morphant otoliths resembled inorganic crystals instead of organic calcification. (C) The 360° rotation image of a single crystal X-ray diffraction of a wild-type 7 dpf otolith. Little prominent diffraction pattern is present, indicating that calcium carbonate aragonite crystallites are arranged in a dustlike mosaic pattern across the surface of the otolith. (D) Single crystal X-ray diffraction of a morphant otolith showing that otoliths similar to those above (B) behave as a single crystal. The unit cell derived from this diffraction pattern was consistent with the calcitic polymorph of calcium carbonate. Scale bar indicates 50 μm.
Discussion
Otopetrin 1 is essential for formation of both otoconia and otoliths. Thus, Otop1 must function early in the otolith and otoconial developmental pathway, prior to specification of architecture and CaCO3 polymorphs of these divergent structures. The presence of pure calcite crystals in morphant animals that initiated otolith formation outside the critical period of 18−24 hpf proposed by Riley et al. (1997) suggests that the ions required for the biomineralization and the proteins that control crystal growth are not coordinately regulated in otop1 fish. These data also suggest that zebrafish otop1 may regulate the ionic environment of the otolith and that following dilution of the inhibitory effects of the otop1 morpholino between 30 and 96 hpf, crystals form in a purely inorganic manner. This is likely due to temporally restricted expression of proteins that form the initial seeding particles or the organic matrix of the otolith during the initial rapid growth phase of the otolith during early development. Interestingly, the crystalline patterns observed in otop1 morphants are similar to those observed in starmaker morphant fish (Söllner et al., 2003). This suggests that disruption of a variety of components of the otolith developmental pathway can trigger a default mechanism, which leads to formation of inorganic crystals. Under these conditions, formation of calcite, the most stable polymorph of CaCO3, is favored.
Otop1 is the first described molecule that has a comparable knockdown/mutant phenotype in the developing otolith/otoconia of fish and mice. Otolith development appears to be exquisitely sensitive to the concentration of otop1 protein, as doses as low as 0.5 ng of morpholino were sufficient to cause agenesis of the otolith in 78% of injected fish (Table 1). This suggests that Otop1 regulates a critical step in otolith formation and that protein concentration may be tightly regulated. For example, Otop1 may regulate the function or localization of other proteins required for otolith development. During mouse otoconial development, Otop1 is localized to the otolithic membrane (Hurle et al., 2003), an extracellular gelatinous superstructure made up of many proteins that supports otoconial formation and maintenance. Location in the extracellular space is particularly surprising, as Otop1 is predicted to be an integral membrane protein. This may indicate the presence of the protein on membrane bound vesicles called globular substance (Tateda et al., 1998), which are thought to be precursors of otoconia (Erway et al., 1986; Preston et al., 1975; Ross, 1979). In this location, Otop1 could function as a channel or transporter, regulating the contents or function of exocytotic vesicles or may act as a structural protein required for the attachment or nucleation of otoconia.
In mouse mutants for Otop1, loss of gravity sensation results in relatively mild behavioral deficits under normal conditions (Hurle et al., 2003; Ornitz et al., 1998). Animals are unable to swim when dropped in water but are able to walk and rear normally. They do not exhibit circling or head tossing behavior, which has been identified in animals with other types of vestibular defects. This could be due to compensatory mechanisms to maintain balance, including the use of visual cues, semicircular canals, and the proprioceptive system. Zebrafish with abnormal otolith formation have difficulty orienting to gravity and are often unable to swim and feed (Mizuno and Ijiri, 2003; Riley and Grunwald, 1996; Riley and Moorman, 2000; Riley et al., 1997). While the behavioral phenotypes of morphant animals were not specifically examined, several instances were noted in which morphant fish that had developed apparently normal otoliths were unable to orient dorsal side up, even when lit from above. No circling behavior was observed, though most of the fish were raised in relatively shallow water to allow morphant fish to inflate their swim bladders. We propose that the delay in otolith formation in these animals may lead to deficits in the formation of neuronal circuitry between otolith organs and the vestibular nuclei. Morphant fish that did not develop otoliths primarily rested on their side at the bottom of the well, even at 7 dpf. Most fish had an intact startle response indicating normal function of the lateral line organs. Interestingly, some morphants with a single saccular or even semicircular canal-located otolith were able to swim efficiently when lit from above, but would tilt or turn upside down when resting.
Human vestibular dysfunction is an increasing clinical problem (National Institute on Deafness and Other Communication Disorders, 2002). Degeneration or displacement of otoconia is a significant etiology of age-related balance disorders and benign paroxysmal positional vertigo (BPPV) (Lim, 1984; Tusa, 2001). In addition, commonly used pharmacological agents, such as aminoglycoside antibiotics, can also lead to disruption of otoconial structure and function (Johnsson et al., 1980; Takumida et al., 1997). The presence of ectopic calcified particles in late-developing otoliths of morphant fish resembles the pathology associated with human BPPV (Figs. 6I and J). The phenotype may provide a useful model to elucidate the mechanism leading to ectopic otoconia in BPPV. In addition, the studies presented here suggest that reactivating the expression of OTOP1 in the ear of patients with vestibular dysfunction may enhance the mineralization of remaining otoconial particles and reestablish otoconial function. Further understanding of the role of Otop1 and other proteins required for otoconial formation may assist in formulating therapeutic approaches aimed at improving otoconial stability over time and possibly facilitating otoconial regeneration, in addition to adding to our knowledge of mechanisms of calcification in this and other systems.
Acknowledgments
The authors would like to thank Keith C. Cheng at Pennsylvania State University College of Medicine for the use of microscopy and imaging equipment. This work was funded by NIH grant DC02236 (D.M.O., R.T.), DC006283 (M.E.W.), and MH068789 (R.L.). We thank I. Thalmann, I. Boime, and K. Lavine for critically reading the manuscript and for insightful discussion and T. Nicolson for providing the starmaker in situ hybridization probe.
References
- Addadi L, Berman A, Oldak JM, Weiner S. Structural and stereochemical relations between acidic macromolecules of organic matrices and crystals. Connect. Tissue Res. 1989;21:127–134. doi: 10.3109/03008208909050003. [DOI] [PubMed] [Google Scholar]
- Bergstrom RA, You Y, Erway LC, Lyon MF, Schimenti JC. Deletion mapping of the head tilt (het) gene in mice: a vestibular mutation causing specific absence of otoliths. Genetics. 1998;150:815–822. doi: 10.1093/genetics/150.2.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bever MM, Fekete DM. Atlas of the developing inner ear in zebrafish. Dev. Dyn. 2002;223:536–543. doi: 10.1002/dvdy.10062. [DOI] [PubMed] [Google Scholar]
- Borelli G, Mayer-Gostan N, Merle PL, Pontual H, Boeuf G, Allemand D, Payan P. Composition of biomineral organic matrices with special emphasis on turbot (Psetta maxima) otolith and endolymph. Calcif. Tissue Int. 2003;72:717–725. doi: 10.1007/s00223-001-2115-6. [DOI] [PubMed] [Google Scholar]
- Carlstrom D, Engstrom H, Hjorth S. Electron microscopic and X-ray diffraction studies of statoconia. Laryngoscope. 1953;63:1052–1057. doi: 10.1288/00005537-195311000-00002. [DOI] [PubMed] [Google Scholar]
- Chan J, Mably JD, Serluca FC, Chen JN, Goldstein NB, Thomas MC, Cleary JA, Brennan C, Fishman MC, Roberts TM. Morphogenesis of prechordal plate and notochord requires intact Eph/ephrin B signaling. Dev. Biol. 2001;234:470–482. doi: 10.1006/dbio.2001.0281. [DOI] [PubMed] [Google Scholar]
- Erway LC, Grider A., Jr. Zinc metabolism in lethal-milk mice. J. Hered. 1984;75:480–484. doi: 10.1093/oxfordjournals.jhered.a109990. [DOI] [PubMed] [Google Scholar]
- Erway LC, Purichia NA, Netzler ER, D'Amore MA, Esses D, Levine M. Genes, manganese, and zinc in formation of otoconia: labeling, recovery, and maternal effects. Scanning Electron Microsc. 1986:1681–1694. [PubMed] [Google Scholar]
- Everett LA, Glaser B, Beck JC, Idol JR, Buchs A, Heyman M, Adawi F, Hazani E, Nassir E, Baxevanis AD, Sheffield VC, Green ED. Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS). Nat. Genet. 1997;17:411–422. doi: 10.1038/ng1297-411. [DOI] [PubMed] [Google Scholar]
- Haddon C, Jiang YJ, Smithers L, Lewis J. Delta-Notch signalling and the patterning of sensory cell differentiation in the zebrafish ear: evidence from the mind bomb mutant. Development. 1998;125:4637–4644. doi: 10.1242/dev.125.23.4637. [DOI] [PubMed] [Google Scholar]
- Hurle B, Ignatova E, Massironi SM, Mashimo T, Rios X, Thalmann I, Thalmann R, Ornitz DM. Non-syndromic vestibular disorder with otoconial agenesis in tilted/mergulhador mice caused by mutations in Otopetrin 1. Hum. Mol. Genet. 2003;12:777–789. doi: 10.1093/hmg/ddg087. [DOI] [PubMed] [Google Scholar]
- Johnsson LG, Wright CG, Preston RE, Henry PJ. Streptomycin-induced defects of the otoconial membrane. Acta Otolaryngol. (Stockh.) 1980;89:401–406. doi: 10.3109/00016488009127155. [DOI] [PubMed] [Google Scholar]
- Khan SR. Interactions between stone-forming calcific crystals and macromolecules. Urol. Int. 1997;59:59–71. doi: 10.1159/000283025. [DOI] [PubMed] [Google Scholar]
- Kozel PJ, Friedman RA, Erway LC, Yamoah EN, Liu LH, Riddle T, Duffy JJ, Doetschman T, Miller ML, Cardell EL, Shull GE. Balance and hearing deficits in mice with a null mutation in the gene encoding plasma membrane Ca2+-ATPase isoform 2. J. Biol. Chem. 1998;273:18693–18696. doi: 10.1074/jbc.273.30.18693. [DOI] [PubMed] [Google Scholar]
- Lim DJ. Formation and fate of the otoconia. Scanning and transmission electron microscopy. Ann. Otol., Rhinol., Laryngol. 1973;82:23–35. doi: 10.1177/000348947308200109. [DOI] [PubMed] [Google Scholar]
- Lim DJ. Morphogenesis and malformation of otoconia: a review. Birth Defects. 1980;16:111–146. [PubMed] [Google Scholar]
- Lim DJ. Otoconia in health and disease. A review. Ann. Otol., Rhinol., Laryngol., Suppl. 1984;112:17–24. doi: 10.1177/00034894840930s404. [DOI] [PubMed] [Google Scholar]
- Lim DJ, Erway LC, Clark DL. Tilted-head mice with genetic otoconial anomaly. Behavioural and morphological correlates. In: Hood JD, editor. Vestibular Mechanisms in Health and Disease. Academic Press; london: 1978. pp. 195–206. [Google Scholar]
- Malicki J, Schier AF, Solnica-Krezel L, Stemple DL, Neuhauss SC, Stainier DY, Abdelilah S, Rangini Z, Zwartkruis F, Driever W. Mutations affecting development of the zebrafish ear. Development. 1996;123:275–283. doi: 10.1242/dev.123.1.275. [DOI] [PubMed] [Google Scholar]
- Mizuno R, Ijiri K. Otolith formation in a mutant Medaka with a deficiency in gravity sensing. Adv. Space Res. 2003;32:1513–1520. doi: 10.1016/S0273-1177(03)90389-9. [DOI] [PubMed] [Google Scholar]
- Murayama E, Okuno A, Ohira T, Takagi Y, Nagasawa H. Molecular cloning and expression of an otolith matrix protein cDNA from the rainbow trout, Oncorhynchus mykiss. Comp. Biochem. Physiol., B Biochem. Mol. Biol. 2000;126:511–520. doi: 10.1016/s0305-0491(00)00223-6. [DOI] [PubMed] [Google Scholar]
- Nasevicius A, Ekker SC. Effective targeted gene ‘knockdown’ in zebrafish. Nat. Genet. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- National Institute on Deafness and Other Communication Disorders National Institutes of Health (NIH) Balance, Dizziness, and You. 2002 NIH Publication No. 00−4374 (G) [Google Scholar]
- Oghalai JS, Manolidis S, Barth JL, Stewart MG, Jenkins HA. Unrecognized benign paroxysmal positional vertigo in elderly patients. Otolaryngol.-Head Neck Surg. 2000;122:630–634. doi: 10.1016/S0194-5998(00)70187-2. [DOI] [PubMed] [Google Scholar]
- Ornitz DM, Bohne BA, Thalmann I, Harding GW, Thalmann R. Otoconial agenesis in tilted mutant mice. Hear. Res. 1998;122:60–70. doi: 10.1016/s0378-5955(98)00080-x. [DOI] [PubMed] [Google Scholar]
- Paffenholz R, Bergstrom RA, Pasutto F, Wabnitz P, Munroe RJ, Jagla W, Heinzmann U, Marquardt A, Bareiss A, Laufs J, Russ A, Stumm G, Schimenti JC, Bergstrom DE. Vestibular defects in head-tilt mice result from mutations in Nox3, encoding an NADPH oxidase. Genes Dev. 2004;18:486–491. doi: 10.1101/gad.1172504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pote KG, Ross MD. Each otoconia polymorph has a protein unique to that polymorph. Comp. Biochem. Physiol. 1991;98B:287–295. doi: 10.1016/0305-0491(91)90181-c. [DOI] [PubMed] [Google Scholar]
- Pote KG, Hauer CR, Michel H, Shabanowitz J, Hunt DF, Kretsinger RH. Otoconin-22, the major protein of aragonitic frog otoconia, is a homolog of phospholipase A2. Biochemistry. 1993;32:5017–5024. doi: 10.1021/bi00070a007. [DOI] [PubMed] [Google Scholar]
- Preston RE, Johnsson LG, Hill JH, Schacht J. Incorporation of radioactive calcium into otolithic membranes and middle ear ossicles of the gerbil. Acta Otolaryngol. 1975;80:269–275. doi: 10.3109/00016487509121327. [DOI] [PubMed] [Google Scholar]
- Richardson KC, Jarrett L, Finke EH. Embedding in epoxy resin for ultrathin sectioning for electron microscopy. Stain Tech. 1960;35:313–323. doi: 10.3109/10520296009114754. [DOI] [PubMed] [Google Scholar]
- Riley BB, Grunwald DJ. A mutation in zebrafish affecting a localized cellular function required for normal ear development. Dev. Biol. 1996;179:427–435. doi: 10.1006/dbio.1996.0272. [DOI] [PubMed] [Google Scholar]
- Riley BB, Moorman SJ. Development of utricular otoliths, but not saccular otoliths, is necessary for vestibular function and survival in zebrafish. J. Neurobiol. 2000;43:329–337. doi: 10.1002/1097-4695(20000615)43:4<329::aid-neu2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Riley BB, Zhu C, Janetopoulos C, Aufderheide KJ. A critical period of ear development controlled by distinct populations of ciliated cells in the zebrafish. Dev. Biol. 1997;191:191–201. doi: 10.1006/dbio.1997.8736. [DOI] [PubMed] [Google Scholar]
- Ross MD. Calcium ion uptake and exchange in otoconia. Adv. Oto-Rhino-Laryngol. 1979;25:26–33. doi: 10.1159/000402913. [DOI] [PubMed] [Google Scholar]
- Sheldrick GM. TWINABS and CELL_NOW. University of Goettingen; Goettingen, Germany: 2002. [Google Scholar]
- Söllner C, Burghammer M, Busch-Nentwich E, Berger J, Schwarz H, Riekel C, Nicolson T. Control of crystal size and lattice formation by starmaker in otolith biomineralization. Science. 2003;302:282–286. doi: 10.1126/science.1088443. [DOI] [PubMed] [Google Scholar]
- Sumanas S, Larson JD, Miller, Bever M. Zebrafish chaperone protein GP96 is required for otolith formation during ear development. Dev. Biol. 2003;261:443–455. doi: 10.1016/s0012-1606(03)00322-1. [DOI] [PubMed] [Google Scholar]
- Tachibana M, Morioka H. Glucuronic acid-containing glycosaminoglycans occur in otoconia: cytochemical evidence by hyaluronidase-gold labeling. Hear. Res. 1992;62:11–15. doi: 10.1016/0378-5955(92)90198-v. [DOI] [PubMed] [Google Scholar]
- Takumida M, Zhang DM, Yajin K, Harada Y. Effect of streptomycin on the otoconial layer of the guinea pig. ORL J. Otorhinolaryngol. Relat. Spec. 1997;59:263–268. doi: 10.1159/000276950. [DOI] [PubMed] [Google Scholar]
- Tateda M, Suzuki H, Ikeda K, Takasaka T. pH regulation of the globular substance in the otoconial membrane of the guinea-pig inner ear. Hear. Res. 1998;124:91–98. doi: 10.1016/s0378-5955(98)00115-4. [DOI] [PubMed] [Google Scholar]
- Tusa RJ. Benign paroxysmal positional vertigo. Curr. Neurol. Neurosci. Rep. 2001;1:478–485. doi: 10.1007/s11910-001-0110-y. [DOI] [PubMed] [Google Scholar]
- Veenhof VB. The Development of Otoconia in Mice. North Holland; Amsterdam: 1969. [Google Scholar]
- Wang Y, Kowolski PE, Thalmann I, Ornitz DM, Mager DL, Thalmann R. Otoconin-90, the mammalian otoconial matrix protein, contains two domains of homology to secretory phospholipase A2. Proc. Natl. Acad. Sci. USA. 1998;22:15345–15350. doi: 10.1073/pnas.95.26.15345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield TT, Riley BB, Chiang MY, Phillips B. Development of the zebrafish inner ear. Dev. Dyn. 2002;223:427–458. doi: 10.1002/dvdy.10073. [DOI] [PubMed] [Google Scholar]


