Abstract
This article describes pertinent aspects of histochemical and molecular changes of the human pituitary adenomas. The article outlines individual tumor groups with general, specific and molecular findings. The discussion further extends to the unusual adenomas or carcinomas. The description in this article are pertinent not only for the practicing pathologists who are in the position of making proper diagnosis, but also for the pituitary research scientists who engage in solving basic problems in pituitary neoplasms by histochemistry and molecular biology.
Keywords: Pituitary, Adenoma, Pathology, Histochemistry, Cell biology
Introduction
The pituitary adenomas are either clinically functioning adenomas or non-functioning adenomas. The former includes GH secreting (cell) adenomas (abbreviated as GHomas), prolactin (PRL) secreting (cell) adenomas (PRLomas), TSH secreting (cell) adenomas (TSHomas), ACTH secreting (cell) adenomas (ACTHomas), and FSH secreting (cell) adenomas (FSHomas) (Fig. 1). LHomas are rather rare. Frequent adenomas among these are GHomas and PRLomas, the remaining tumors are rather rare. The rest of the adenomas are clinically “non-functioning”. It should be particularly emphasized that majority of clinical nonfunctioning adenomas have been disclosed to be positive of gonadotropin subunits (SUs). The WHO Classification of the Endocrine Tumors follows this concept (Table 1). Except for ACTHomas, the pituitary adenomas are usually macroadenomas (Fig. 2). Sometimes, supra-sellar extension and downward sinus invasion are observed. Very occasionally, the pituitary adenomas can occur ectopically most frequently in the sphenoid sinuses. Pituitary carcinoma is very rare. Metastatic carcinoma to the pituitary is sometimes seen, its autopsy findings are not extremely rare and common primary sites include carcinoma of the lung. The pertinent diagnostic features and characteristics are described pointing out (1) general findings, (2) specific findings, (3) non-surgical therapy and therapy-related changes and (4) molecular findings.
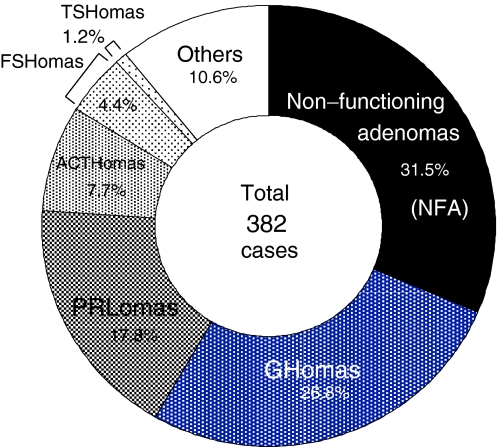
Fig. 1.
Pathological diagnosis in pituitary adenomas. A total of 382 pituitary adenomas are classified with functional classifications using histology, IHC, ultrastructural features as well as biochemical, imaging and surgical findings in surgical resection at Nippon Medical School since 1977. The tumor cells in FNA are positive for gonadotropin SU, and belonging to gonadotropin producing adenoma
Table 1.
Classification of pituitary tumors, 2004 (WHO)
| GH producing adenoma | Gonadotropin producing adenoma |
| Densely granulated adenoma | Unusual plurihormonal adenoma |
| Sparsely granulated adenoma | Silent subtype 3 adenoma |
| Mixed adenoma | |
| Mammosomatotroph adenoma | Null cell adenoma |
| Acidophilic stem cell adenoma | Hormone immuno-negative adenoma |
| PRL producing adenoma | Oncocytoma |
| Densely granulated adenoma | |
| Sparsely granulated adenoma | Others |
| Acidophilic stem cell adenoma | Carcinoma |
| TSH producing adenoma | Atypical adenoma |
| ACTH producing adenoma | |
| Silent ACTH cell adenoma | |
| Subtype 1 | |
| Subtype 2 |
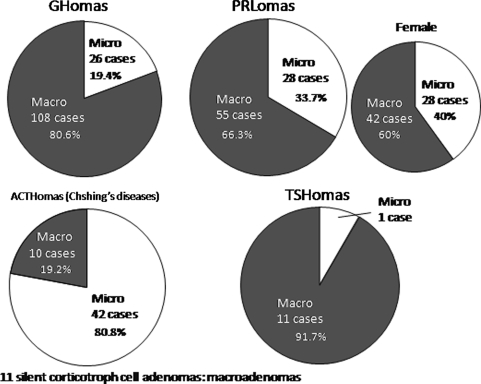
Fig. 2.
A pie chart of the ratio of pituitary macro and micro adenomas. Pituitary adenomas are ordinary macroadenomas except for ACTHomas
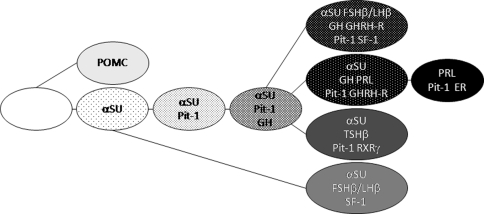
Since Rosenfeld et al. (Nelson et al. 1988; Sornson et al. 1996) and Karin et al. (Bodner et al. 1988) reported the cloning of pituitary-specific transcription factor-1 (Pit-1), or POU1F1 or GHF-1, many transcription factors have been reported and it is generally known that the functional differentiation of the pituitary cells are dependent on the combination of transcription factors and co-factors (Fig. 3). It has been also known that the human pituitary cells show the co-localization of GH and αSU in the normal condition and GHomas (Osamura 1988) (Table 2).
Fig. 3.
Human pituitary cell linage. Pituitary-specific or related transcription factors involved in the development of pituitary gland. αSU is expressed in some of the normal human GH-producing cells and some cases of GH-producing adenomas as it occurs in mice
Table 2.
Immunoprofile of hormones in pituitary adenomas (%)
| GH | PRL | ACTH | FSHβ | LHβ | TSHβ | αSU | |
|---|---|---|---|---|---|---|---|
| GHomas (69 cases) | 100 | 82.6 | 1.5 | 4.3 | 1.5 | 2.9 | 56.5 |
| TSHomas (6 cases) | 83.3 | 83.3 | 16.7 | 0 | 0 | 100 | 83.3 |
| FSHomas (11 cases) | 0 | 27.3 | 18.2 | 100 | 9.1 | 63.6 | 100 |
| PRLomas (27 cases) | 3.7 | 100 | 0 | 0 | 3.7 | 3.7 | 3.7 |
| ACTHomas (15 cases) | 6.7 | 13.3 | 100 | 0 | 6.7 | 0 | 33.3 |
| NFomas* (197 cases) | 4.6 | 10.2 | 5.1 | 42.6 | 19.8 | 2.0 | 43.1 |
| Total 325 cases | |||||||
* Non-functioning adenomas including 37.6% null cell adenomas
GH-producing (cell) adenoma (GHomas)
General findings
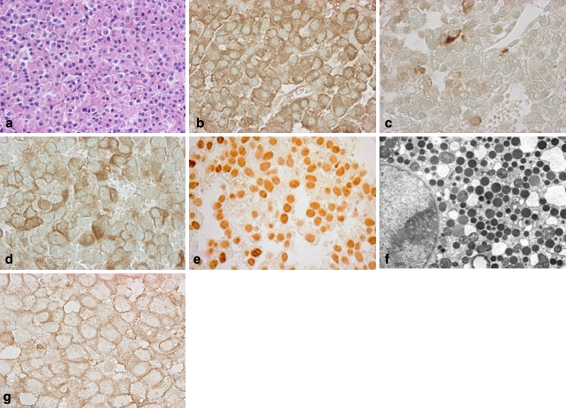
It has been well documented that GHomas, which are secreting growth hormone in excess show two clinically related phenotypes, acromegaly and gigantism, depending on patient age at the onset of disease. In immunohistochemistry (IHC), it shows many GH positive tumor cells (Fig. 4b) and frequently, the tumor cells are also positive for PRL (Fig. 4c) and α-glycoprotein subunit (αSU) (Fig. 4d). The remaining hormones are also occasionally positive but ACTH is very rare. GHomas can classify, into five distinct adenomas (DeLellis et al. 2004; Vidal et al. 2004). The distinctions can affect the medical treatment in the event of surgeries (Bhayana et al. 2005; Ezzat et al. 1995), therefore accurate classification is very important. Of the five types, two are pure GH producing tumors, consisting of monomorphous GH cell are densely granulated, and sparsely granulated somatotroph adenomas. The others are plurihormonal tumors that include mammosomatotroph adenomas, mixed somatotroph–lactotroph adenomas, and acidophil stem cell adenomas (Vidal et al. 2004). By electron microscopy, many tumors are the type of “densely granulated cells” which contain many dense cored numerous secretory granules. Densely granulated adenomas (DGAs) are composed of large or medium size, round or polyhedral acidophilic cells with numerous secretory granules (400–500 nm) in the cytoplasm, and shows strong, uniform and diffuse cytoplasmic immunoreactivity for GH (Fig. 5a). DGA type, <3% of nuclei are MIB-1 positive, and known to be slow growing and reactively non invasive (DeLellis et al. 2004; Hagiwara et al. 2003). Sparsely granulated adenomas (SGAs) composed of small round, partly irregular cell harboring a round nucleus with conspicuous nucleoli. They have relatively a fewer secretory granules (100–250 nm). The cells are chromophobic, and immunoreactivity for GH is weak (Fig. 5b). These types of adenomas are aggressive, and varying degrees of nuclear and cellular pleomorphism are seen (Horvath et al. 1997). Fibrous bodies are feature for this type of adenoma as mentioned below. Mammosomatotroph adenomas are morphologically close to densely granulated somatotroph adenoma and contain abundant and large secretory granules reaching up to 1,500 nm. IHC shows single cell type reactive for both GH and PRL. Mixed somatotroph–lactotroph adenomas are most commonly consisting of densely granulated somatotropes and sparsely granulated lactotropes. Histologically, the adenomas consist of acidophilic cells interspersed with chromophobic. IHC elucidate that GH and PRL are immunostained in different cell populations.
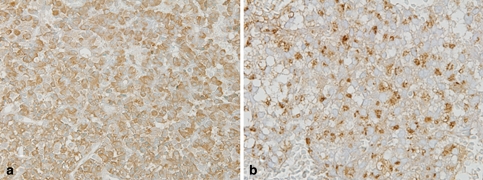
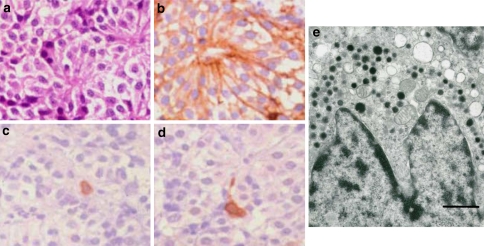
Fig. 4.
GH producing (cell) adenoma (GHoma). The tumor cells of hematoxylin eosin (H&E) staining (a). In IHC, it shows many GH positive tumor cells (b), frequently cells are also positive for PRL (c) and αSU (d). The IHC of pituitary-specific transcription factor 1 (Pit-1), which regulate the functional differentiation of GH–PRL–TSH cell lineage (e). In electron microscopy, many GHoma are type of densely granulated cells, which contain many dense cored numerous secretory granules (f). SSTR2a is immunostained in cell membrane (g), which provides important information for SSAs therapy
Fig. 5.
The IHC of GH of densely and sparsely granulated adenomas. Densely granulated adenoma shows strong and diffuse cytoplasmic immunoreactivity for GH (a). Sparsely granulated adenomas show weak GH immunostaining (b)
Specific findings
Fibrous (keratin) bodies
In some GHomas, the tumor cells contain perinuclear globules of keratin by IHC. These globules have been called as fibrous bodies or recently keratin bodies. Fibrous bodies are strongly reactive for low molecular weight cytokeratins, particularly keratin 8 (DeLellis et al. 2004). Immunohistochemical staining confirms that the globules are composed of an admixture of intermediate filaments and smooth endoplasmic reticulum, are located in the Golgi region, and often indents the nucleus (Neumann et al. 1985; Vidal et al. 2004) (Fig. 6d). Fibrous bodies are the feature of sparsely granulated somatotroph adenoma type. The more granulated adenomas, including mammosomatotrophs and plurihormonal lesions, identifies some perinuclear keratin (Fig. 6c), but the sparsely granulated adenomas clearly decorates globules of keratin by immunostainig using CAM 5.2 keratin (CK8) (Fig. 6a–b).
Fig. 6.
The IHC of CAM 5.2 keratin (CK8). In some GHomas, the tumor cells shows globules of keratin, which are called as fibrous bodies, by IHC (a). b Shows magnified image of dot type fibrous bodies. In granulated adenomas, keratin is immunostained in cytoplasm (c). In electron microscopy, fibrous bodies shows smooth endoplasmic reticulum located in the Golgi region (d)
Stellate amyloid
Very occasionally, GHomas show “stellate” amyloid among the tumor cells. The amyloid is positive for Congo red and is birefringent. By electron microscopy, the amyloid is composed of bundles of fine filaments, which appear to be typical for the amyloid of other origin and nature. Immunohistochemically, the tumor cells are strongly positive for GH and the amyloid is also weakly positive for GH (Osamura et al. 1982).
Changes induced by Octoreotide
In GHoma, treatment with long-acting somtostatin analogs (SSAs) called octreotide (SMS 201–995) results in reducing blood GH level, and tumor shrinkage in 40–50% of patients, especially in those with receptor for somatostatin (Ezzat et al. 1992; Kovacs and Horvath 2005). Octreotide has high affinity for somatostatin receptor (SSTR) 2 and SSTR5. Takei et al. (2007) determined the expression of SSTR2 and SSTR5 by IHC and compared this with the results of octreotide suppression testing before surgery to define whether IHC of SSTR could be associated with the preoperative response to octreotide. They concluded that the percentage of cell membrane immunopositive for SSTR2 is associated with the hormone inhibitory effect, so immunohistochemical analysis of SSTR in GHoma could provide important information for predicting the likelihood of successfully employing SSAs in adjuvant therapy (Fig. 4f).
Silent somatotroph adenomas
These are the pituitary adenomas without clinical symptoms but show positive for specific hormones by IHC in the individual tumor cells. Silent somatotroph adenoma expresses GH and its mRNA without evidence of acromegaly (Naritaka et al. 1999).
Molecular findings
According to the previous studies on the functional development of the pituitary adenomas, it has been clarified that the functioning pituitary adenomas generally follows the combination of transcription factors and co-factors which have been known to function in the physiologic condition (Minematsu et al. 2005; Sanno et al. 2001). For example, GHomas are regulated by Pit–1 (Fig. 4e), which regulate the functional differentiation of GH–PRL–TSH cell lineage (Lee et al. 2005; Miyai et al. 2005) and positive in the nuclei of many tumor cells, and growth hormone-releasing hormone receptor (GHRH-R) (Matsuno et al. 1999; Sanno et al. 1997). Pituitary adenomas sometimes express more than one or two hormones. In our series, about 80% of GHomas are positive for PRL, and 56% of them are positive for αSU. Therefore, GHomas are plurihormonal tumors. Occasionally, GHomas also produce ACTH, which belongs to a different lineage, in the same tumor cells. For the molecular mechanism, aberrant combination of transcription factors has been proposed (Osamura et al. 2004). Some specific genes have been identified that predispose the hereditary pituitary adenomas. The multiple endocrine neoplasia (MEN1) gene, a tumor suppressor, is located on chromosome 11q13 frequently associated with GHoma and PRLoma (Chandrasekharappa et al. 1997). Somatic mutations in the α-subunit of the Gs protein, which led to a constitutive activation of adenylyl cyclase, are reported in about 40% of GH-producing pituitary adenomas (Landis et al. 1990).
PRL producing (cell) adenomas (PRLomas)
General findings
The tumor cells are classically chromophobic and immunohistochemically contain condensed PRL in the Golgi regions near the nuclei (Nebenkern; Golgi pattern) (Fig. 7a–b). PRLomas are usually mono-hormonal. No other hormones are detected by IHC. By electron microscope (EM), the tumor cells contain prominent Golgi saccules and a few small NSGs (Fig. 7c). Clinically, they are associated with manifestation due to the prominent increase in serum PRL levels (hyperprolactinemia) which are usually parallel to the tumor size. They can be electron microscopically classified into sparsely granulated and densely granulated type. The most frequent tumor type is sparsely granulated and they usually show high response to dopamine agonists (Al-Brahim and Asa 2006). Calcospherites or psammoma bodies are sometimes found (DeLellis et al. 2004). Densely granulated adenoma is rare, and is composed of acidophilic to chromophobic cells with abundant and diffuse cytoplasmic PRL granules. Clinically, GH-PRL adenomas with the exception of acidophil stem cell adenomas are also characterized by hyper prolactinemia.
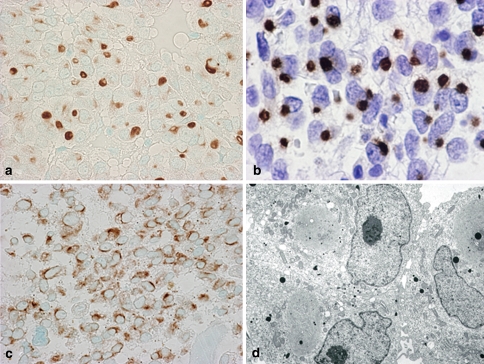
Fig. 7.
PRL producing (cell) adenoma (PRLoma). The tumor cells of hematoxylin eosin (H&E) staining (a) and IHC of PRL, which shows condense in the Golgi regions near the nuclei (b). In electron microscopy, prominent Golgi saccules and a few small NSGs are seen (c). d shows the extrusion of secretory granules, so called “misplaces exocytosis”
Specific findings
Immunohistochemical staining of the “Golgi pattern” is quite unique. Electron microscopically, the extrusion of secretary granules along the lateral cell surfaces into the extracellular space, so-called “misplaced exocytosis”, has been regarded as a specific feature of the sparsely granulated PRLoma (DeLellis et al. 2004; Vidal et al. 2004) .
Changes induced by Bromocriptine
In the tumors from the patients with effective treatment by a dopamine agonist, Bromocriptine (BC), the tumor cells contain accumulated secretory granules. The induction of adenoma cells shrinkage and marked decrease in serum PRL level were observed in BC treatment (Kovacs and Horvath 2005). It has also been reported that BC induces exocytosis of the granules though there was a reduction in serum PRL revels (Mori et al. 2001).
Molecular findings
It has been reported that most PRLomas are immunohistochemcially positive for Pit-1 and estrogen receptor (ER). The binding sites for Pit-1 and ER are close enough to synergize for the production of PRL. PRL cells are of extreme differentiation and most PRLomas are mono-hormonal. Recently, the pituitary tumor transforming gene (PTTG) has been suggested to be expressed in high levels in different pituitary tumor and to play a central role in pituitary tumorigenesis (Yu and Melmed 2004). The role of PTTG in prolactinomas is not clear but PTTG might involve lactotroph angiogenesis in pituitary adenomas in correlation with the increase in estrogen action (Cristina et al. 2007).
ACTH producing (cell) adenomas (ACTHomas)
General findings
ACTH producing adenomas with Cushing’s disease represent 10–15% of pituitary tumors (Trouillas 2002). Most tumors are microadenomas with the cells which are basophilic and strongly PAS-positive (Saeger et al. 2007). The tumor consists of monomorphic round cells arranged diffusely with a sinusoidal pattern (Robert and Hardy 1986). Some tumors are chromophobic and weakly PAS-positive. By IHC, these tumors are immunoreactive for ACTH, β-LPH and/or β-endorphin. The intensity of hormone immunoreactivity varies: basophilic adenomas tend to be strongly positive. In the presence of active ACTH producing adenomas, the non neoplastic coritotropes often show characteristic alterations in Crooke’s cells. These cells are larger than normal ACTH cells and exhibit an interacytoplasmic hyaline ring (Crooke 1935).
Specific findings
Silent ACTH producing adenoma
ACTH producing adenomas without clinical and/or biological signs of hypercorticolism may be discovered by the pathologist, is named as silent ACTH producing adenoma (Horvath et al. 1980; Lloyd et al. 1997). Some of them secrete high molecular weight ACTH (Reincke et al. 1987). The silent densely granulated ACTH adenoma is designated as subtype 1 adenoma (Kovacs and Horvath 1986). The second inactive is the sparsely granulated adenoma and is called subtype 2 adenoma. They are large, frequently invasive into the cavernous sinus and sphenoid sinus.
Crooke’s cell adenoma
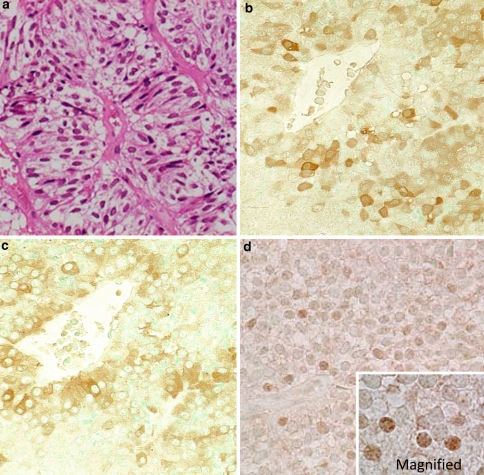
Corticotroph adenomas generally do not undergo Crooke’s hyaline change. Nonetheless, rare adenomas composed of Crooke’s cells are described as the Crooke’s cell adenoma (Franscella et al. 1991). In H&E staining sections, the cells were characterized by densely eosinophilic and hyaline cytoplasm (Fig. 8a). The hyaline material was strongly cytokeratin immunoreactive (Fig. 8c). EM showed the accumulation of intermediate filaments was massive (Fig. 8d). Compared with typical corticotroph adenomas, Crooke’s cell adenomas are clinically aggressive. It typically presents as an invasive macroadenoma. In recent study, invasiveness was found in 72% of this adenoma. They have a higher rate of recurrence than other corticotroph adenomas (George et al. 2003).
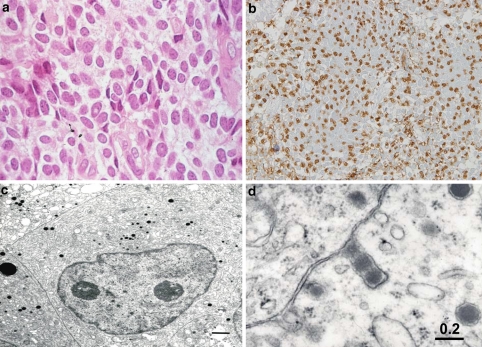
Fig. 8.
Crooke cell adenoma. The Crooke cell adenoma of hematoxylin eosin (H&E) staining, which is characterized by densely eosinophilic and hyaline cytoplasm (a). The IHC of ACTH (b). c Shows the image of CAM 5.2 (keratin) immunostaining. Electron microscopy showed the accumulation of massive intermediate filaments (asterisk) (d)
Molecular findings
In ACTH producing adenoma, the transcription factor NeuroD1 (Oyama et al. 2001) and Tpit (Lamolet et al. 2001) are positive. The tumor cells produce prohormone, proopiomelanocortin (POMC), from which ACTH is derived after proper proteolytic digestion by prohormone convertase (PC1/3). When PC2 is present, α-melanotroph stimulating hormone (αMSH) is positive in the adenoma cells.
TSH producing (cell) adenomas (TSHomas)
General findings
TSH producing adenomas are not frequent (Bertholon-Gregoire et al. 1999). The patients with TSHomas are frequently hyperthyroid presenting Graves’ disease. Sometimes, the patients show high serum GH level or PRL level. The tumor is usually macroadenoma as with extensive invasion. The tumors are composed of choromophobic cells and exhibit sinusoidal growth pattern (Fig. 9a). By IHC, the tumor cells are positive for TSHβ and αSU. The tumors are also sometimes multihormonal with positive staining for GH and PRL (Sanno et al. 1994, 1995). Electron micrographs of the adenoma, show that the tumor cells were elongated and manifested with long cytoplasmic processes and round or ovoid nuclei with prominent nucleoli. There was a moderate development of rough endoplasmic reticulum and Golgi complexes. Small secretory granules measuring between 50 and 200 nm tend to accumulate along the cell membrane (Teramoto et al. 2004).
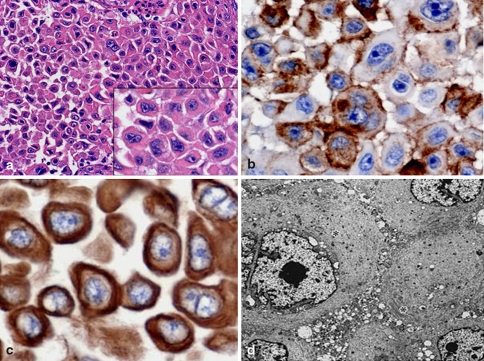
Fig. 9.
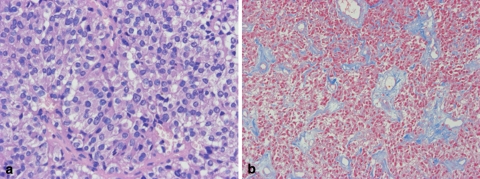
TSH producing (cell) adenoma (TSHoma). The tumor cell of hematoxylin eosin (H&E) staining (a). It is clearly showed by Heidenhain’s azan stain, the stromal fibrosis in tumors (b)
Specific findings
It has been pointed out by neurosurgeons that TSHomas are firm on their operation. By histology, the tumors frequently exhibit stromal fibrosis of various amounts among the tumor cells (Fig. 9b). Ocasional psammoma bodies are also observed.
Molecular findings
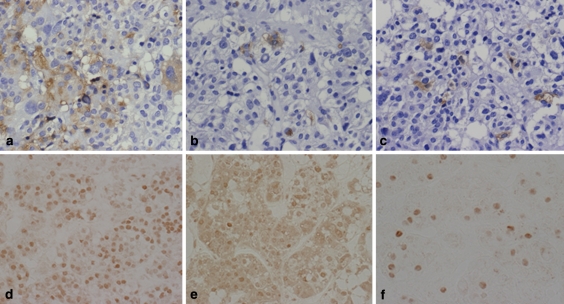
The tumors are multihormonal with the concomitant production of TSH, GH and PRL (Fig. 10a–c). As transcription factors, Pit–1 (Fig. 10d) and GATA-2 (Fig. 10e) have been synergistic for the production of TSH. Pit-1 also regulates GH and PRL.
Fig. 10.
IHC of TSH (TSHoma). It shows many TSH positive tumor cells (a), and multihormonal with the concominant production of PRL (b) and GH (c). For transcription factors, Pit-1 (d) and GATA-2 (e) are positive and sometimes ER (f) is positive
Gonadotropin producing adenomas
FSH producing adenomas (FSHomas)
General findings
Light microscopically, this type of tumors shows frequent unique morphology by H&E staining which is characterized by many chromophobic spindle cells arranged in “pseudorosette” patterns (Fig. 11c). Immunohistochemically, the tumor cells are frequently positive for FSHβSU and αSU (Fig. 11d–e). LHβSU is seldom positive. By EM, the tumor cells show small secretory granules in the cytoplasm along with cell membrane.
Fig. 11.
FSH producing (cell) adenoma (FSHoma). The tumor cells were organized into pseudorosette patterns (a). About 40% of FSHomas are immunopositive for αSU (b), FSHβSU (c) and SF-1 (d). In contrast, LHβSU immunoreactivity is rare
Specific findings
The above pseudorosette pattern is specific for this type of tumors. It is of particular interests that most FSHomas occur in male patients. Activin-signaling in male mouse gonadotropes may be different from the female-type gonadotropes. In male mouse pituitary cells, Smad stimulated expression of gonadotropins includes LHβ and FSHβ through the activin-signaling (Coss et al. 2005).
Gonadotropin subunits (SUs) producing adenomas (Gn-omas)
General findings
The nonfunctional pituitary adenomas have been shown to be derived from gonadotroph cells. And thus, almost all nonfunctional pituitary adenomas are termed Gn-omas. These tumors represent a half of all macroadenomas and present clinically in patients with visual field loss, headaches, hypogonadism, and hypopituitarism (Fig. 12a–b). The tumors are mostly chromophobic and are composed of smaller pituitary cells.
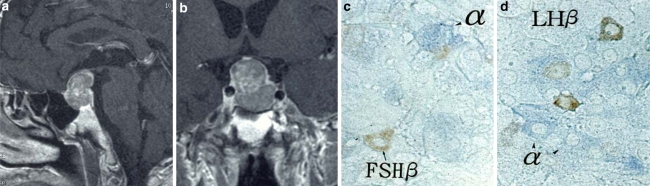
Fig. 12.
Gonadotropin producing (cell) adenoma (Gn-oma). Mid-sagittal (a) and coronal (b) MRI image. The tumor cells present macroadenoma type and no evidence of bioactivities of hormone. αSU (c, d; blue), FSHβSU (c; brown) and LHβSU (d; brown) are localized in different tumor cells
Specific findings
Chromophobe cells without apparent pseudorosette patterns are specifically suggestive of Gn-omas. Focally, pseudorosette pattern is also observed. By IHC, about 40% of the tumors are positive for FSHβSU and αSU. LHβSU is less frequent. It has been claimed that different tumor cells are positive for αSU and βSU which account for the lack of specific function of FSH (Fig. 9c–d). Gn-omas with markedly increased mitochondria in the cytoplasm have been designated as oncocytomas.
Molecular findings
Most gonadotropinoma show FSHβSU and αSU. Frequently, the tumors are also positive for TSHβSU. The transcription factor steroidogenic factor-1 (SF-1) has been specific for gonadotropin production (Fig. 11f) (Asa et al. 1996). GATA-2 is an another transcription factor which synergizes with SF-1 to produce of TSH (Dasen et al. 1999).
Null cell adenomas
General findings
When the tumor cells are immuno-negative for any pituitary hormones, the tumors have been designated as “null cell adenomas”.
Specific findings
These types of adenomas includes chromophobic acidophilic or partly chromophobic, partly acidophilic with a diffuse pattern and elongated small cells forming psuedorosettes around the markedly dilated capillaries. Since some gonadotroph adenomas share the ultrastructural features of null cell adenomas, it is tempting to consider the latter as originating in a gonadotroph. The cells of null cell adenoma show a modest cytoplasm with poorly developed rough endoplasmic reticulum and Golgi apparatus, and rare round secretary granules, measuring up to 250 nm (Horvath and Kovacs 1984).
Plurihormonal adenomas
Silent subtype 3 adenoma
Silent subtype 3 adenomas are rare pituitary tumors that have immunoreactivities for more than one pituitary hormone. They are classified as unusual pulrihormonal adenomas in the World Health Organization (WHO) Classification of Endocrine Tumors (DeLellis et al. 2004). Silent subtype 3 adenomas are described by Horvath et al. (1980) for the first time from the ultrastructural characteristics.
Although they have much variation in terms of histology and IHC, the ultrastructures have been characteristic and diagnostic.
Clinical features
At the time of diagnosis, these tumors are usually macroadenomas. They are usually associated with hyperprolactinemia and often clinically mistaken for prolactinomas and treated especially in women. They sometimes display aggressive behavior with suprasellar or parasellar extension and also have a high recurrence rate. Complete surgical removal is sometimes difficult and the radiotherapy is done in postoperative management (Horvath et al. 1988).
Pathology
The adenoma cells are middle-sized or large, and polar. The euchromatic nuclei contain large prominent nucleolus and often a few fragments of nuclear inclusions (Spheridia) whose function is unknown. Nuclear spheria are not specific for subtype 3 adenoma, but abundant spheria are characteristic of the tumors (Horvath et al. 1988). The ample cytoplasm contains extensively distributed rough-surfaced endoplasmic reticulum, randomly distributed smooth-surfaced endoplasmic reticulum and large tortuous Golgi complex. The secretory granules of about 100–200 nm are numerous and tend to accumulate heavily in cell processes, which is a typical feature of glycoprotein hormone cell differentiation (Horvath et al. 2005). Immunohistochemically, the tumor cells are immunoreactive for mainly GH, PRL and TSH. However, they also have other varied combination of hormones and their cytogenesis remains unresolved.
Pituitary adenomas with “translineage” differentiation
Very occasionally, some human pituitary tumors demonstrate their functional differentiation toward the production of hormones belonging to different cell lineages, i.e., ACTHomas with GH production, GHomas with ACTH production, FSHomas with ACTH production and so on.
We have studied the cases of ACTHomas with GH production and GHomsa with ACTH production. These cases demonstrate both NeuroD1 and Pit-1 transcription factors in the tumor cells could induce “translineage” differentiation. We have postulated that “aberrant” expressions of transcription factors could be the cause of this abnormal differentiation in the tumors (Tahara et al. 2002).
Pituitary adenomas with only early differentiation
Some ACTHomas have been shown to be positive for ACTH and also positive for αSU in the tumor cells. Five of 89 cases of ACTHomas showed this aberrant expression. In human pituitaries, it has been suggested that ACTH appears as a first hormone proceeding to αSU (Osamura, personal observation by IHC). In human, two distinct differentiation pathways exist: (1) ACTH lineage and (2) gonado–GH–PRL–TSH lineage.
These tumors only exhibiting ACTH and αSU are of particular interests in considering their histogenesis. These tumors are considered to be derived from the primitive pituitary ACTH committed progenitor cells with aberrant expression of αSU. Detailed mechanisms remained to be further investigated (Suzuki et al. 2008).
Special types of pituitary adenomas and carcinomas
Even though the incidence is very rare, we see some cases of the very unique and special cases of the pituitary tumors. For this situation, IHC and molecular analysis are expected to play key roles in establishing diagnosis.
Ectopic pituitary adenomas
It has been well documented that the pituitary adenomas can occur outside of sella turcica, most frequently in the sphenoid sinuses. The differential diagnoses contain olfactory neuroblastoma (esthesioneuroblastomas), rhabdomyosaromas and germinomas. The establishment of the proper diagnosis relies on immunohistochemical findings including neuroendocrine markers such as chromogranin A, synaptophysin and SNAP25 as well as some pituitary hormones. Especially, the detection of the pituitary hormones is diagnostic for this lesion (Fig. 13).
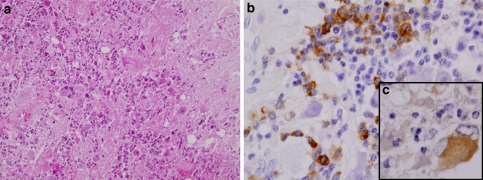
Fig. 13.
H&E staining of the tumor cells (a). Neuroendocrine marker SNAP25 (b) and pituitary hormone FSHβ (c) and ACTH (d) are detected. e Electron microscopy shows dense cored secretory granules
Invasive pituitary adenomas
Some intra-sellar pituitary adenomas can grow downward and invade the sphenoid sinuses. Various types of adenomas can present this growth pattern. A case of acute meningitis induced by invasive pituitary PRLomas has been reported as an autopsy case with a very short clinical history (Onoda et al. 1992).
Pituitary carcinomas
A few cases of pituitary carcinomas have been reported in the literature. Frequently, the pituitary carcinomas demonstrate liver metastasis. The tumor cells are more atypical than usual adenomas and show high MIB-1 proliferative indices. The tumors are frequently functioning, such as PRL producing or ACTH producing.
Gangliocytoma
This is a very rare tumor, which has been reported as a source for GHRH which stimulates hyperplastic GH cells (Kurosaki et al. 2002) (Fig. 14).
Fig. 14.
H&E staining of the tumor cells (a). IHC of GH (b) and GHRH (c). It is suggested that GHRH stimulates the hyperplastic GH cells
Metastatic carcinomas
The metastatic carcinomas in the pituitary region retain the morphologic features of the primary tumors or those of other metastatic sites. Primary lung cancer, especially the small cell carcinoma, has been experienced as a primary source of the metastatic carcinomas to the pituitary.
PRLomas with Germinomas
It is very rare occasion to see germinoma as an intra-sellar tumor. Morphologically, the tumor cells demonstrate typical features similar to testicular seminomas or ovarian dysgerminomas. A reported case of germinoma showed admixed foci of PRLomas (Sugiyama et al. 1999). The morphogenesis of this “mixed” tumor remains to be further investigated.
References
- Al-Brahim NY, Asa SL (2006) My approach to pathology of the pituitary gland. J Clin Pathol 59:1245–1253 [DOI] [PMC free article] [PubMed]
- Asa SL, Bamberger AM, Cao B, Wong M, Parker KL, Ezzat S (1996) The transcription activator steroidogenic factor-1 is preferentially expressed in the human pituitary gonadotroph. J Clin Endocrinol Metab 81:2165–2170 [DOI] [PubMed]
- Bertholon-Gregoire M, Trouillas J, Guigard MP, Loras B, Tourniaire J (1999) Mono- and plurihormonal thyrotropic pituitary adenomas: pathological, hormonal and clinical studies in 12 patients. Eur J Endocrinol 140:519–527 [DOI] [PubMed]
- Bhayana S, Booth GL, Asa SL, Kovacs K, Ezzat S (2005) The implication of somatotroph adenoma phenotype to somatostatin analog responsiveness in acromegaly. J Clin Endocrinol Metab 90:6290–6295 [DOI] [PubMed]
- Bodner M, Castrillo JL, Theill LE, Deerinck T, Ellisman M, Karin M (1988) The pituitary-specific transcription factor GHF-1 is a homeobox-containing protein. Cell 55:505–518 [DOI] [PubMed]
- Chandrasekharappa SC, Guru SC, Manickam P, Olufemi SE, Collins FS, Emmert-Buck MR, Debelenko LV, Zhuang Z, Lubensky IA, Liotta LA, Crabtree JS, Wang Y, Roe BA, Weisemann J, Boguski MS, Agarwal SK, Kester MB, Kim YS, Heppner C, Dong Q, Spiegel AM, Burns AL, Marx SJ (1997) Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science 276:404–407 [DOI] [PubMed]
- Coss D, Thackray VG, Deng CX, Mellon PL (2005) Activin regulates luteinizing hormone beta-subunit gene expression through Smad-binding and homeobox elements. Mol Endocrinol 19:2610–2623 [DOI] [PMC free article] [PubMed]
- Cristina C, Diaz Torga GS, Goya RG, Kakar SS, Perez-Millan MI, Passos VQ, Giannella-Neto D, Bronstein MD, Becu-Villalobos D (2007) PTTG expression in different experimental and human prolactinomas in relation to dopaminergic control of lactotropes. Mol Cancer 6:4 [DOI] [PMC free article] [PubMed]
- Crooke AC (1935) A change in the basophil cells of the pituitary gland common to conditions which exhibit the syndrome attributed to basophil adenoma. J Pathol Bacteriol 41:339–349 [DOI]
- Dasen JS, O’Connell SM, Flynn SE, Treier M, Gleiberman AS, Szeto DP, Hooshmand F, Aggarwal AK, Rosenfeld MG (1999) Reciprocal interactions of Pit1 and GATA2 mediate signaling gradient-induced determination of pituitary cell types. Cell 97:587–598 [DOI] [PubMed]
- DeLellis RA, Lloyd RV, Heitz PU, Eng C (2004) Pathology and genetics of tumors of endocrine organs, World Health Organization Classification of Tumors. IARC Press, Lyon, pp 9–45
- Ezzat S, Snyder PJ, Young WF, Boyajy LD, Newman C, Klibanski A, Molitch ME, Boyd AE, Sheeler L, Cook DM et al (1992) Octreotide treatment of acromegaly. A randomized, multicenter study. Ann Intern Med 117:711–718 [DOI] [PubMed]
- Ezzat S, Kontogeorgos G, Redelmeier DA, Horvath E, Harris AG, Kovacs K (1995) In vivo responsiveness of morphological variants of growth hormone-producing pituitary adenomas to octreotide. Eur J Endocrinol 133:686–690 [DOI] [PubMed]
- Franscella S, Favrod-Coune CA, Pizzolato G (1991) Pituitary corticotroph adenoma with Crooke’s hyalinization. Endocr Pathol 2:111–116 [DOI] [PubMed]
- George DH, Scheithauer BW, Kovacs K, Horvath E, Young WF Jr, Lloyd RV, Meyer FB (2003) Crooke’s cell adenoma of the pituitary: an aggressive variant of corticotroph adenoma. Am J Surg Pathol 27:1330–1336 [DOI] [PubMed]
- Hagiwara A, Inoue Y, Wakasa K, Haba T, Tashiro T, Miyamoto T (2003) Comparison of growth hormone-producing and non-growth hormone-producing pituitary adenomas: imaging characteristics and pathologic correlation. Radiology 228:533–538 [DOI] [PubMed]
- Horvath E, Kovacs K (1984) Gonadotroph adenomas of the human pituitary: sex-related fine-structural dichotomy. A histologic, immunocytochemical, and electron-microscopic study of 30 tumors. Am J Pathol 117:429–440 [PMC free article] [PubMed]
- Horvath E, Kovacs K, Killinger DW, Smyth HS, Platts ME, Singer W (1980) Silent corticotropic adenomas of the human pituitary gland: a histologic, immunocytologic, and ultrastructural study. Am J Pathol 98:617–638 [PMC free article] [PubMed]
- Horvath E, Kovacs K, Smyth HS, Killinger DW, Scheithauer BW, Randall R, Laws ER Jr, Singer W (1988) A novel type of pituitary adenoma: morphological features and clinical correlations. J Clin Endocrinol Metab 66:1111–1118 [DOI] [PubMed]
- Horvath E, Sheithauer BW, Kovacs K, Lloyd RV (1997) Pituitary adenomas. Oxford University Press, New York
- Horvath E, Kovacs K, Smyth HS, Cusimano M, Singer W (2005) Silent adenoma subtype 3 of the pituitary–immunohistochemical and ultrastructural classification: a review of 29 cases. Ultrastruct Pathol 29:511–524 [DOI] [PubMed]
- Kovacs K, Horvath E (1986) Pathology of growth hormone-producing tumors of the human pituitary. Semin Diagn Pathol 3:18–33 [PubMed]
- Kovacs K, Horvath E (2005) Effects of medical therapy on pituitary tumors. Ultrastruct Pathol 29:163–167 [DOI] [PubMed]
- Kurosaki M, Saeger W, Ludecke DK (2002) Intrasellar gangliocytomas associated with acromegaly. Brain Tumor Pathol 19:63–67 [DOI] [PubMed]
- Lamolet B, Pulichino AM, Lamonerie T, Gauthier Y, Brue T, Enjalbert A, Drouin J (2001) A pituitary cell-restricted T box factor, Tpit, activates POMC transcription in cooperation with Pitx homeoproteins. Cell 104:849–859 [DOI] [PubMed]
- Landis CA, Harsh G, Lyons J, Davis RL, McCormick F, Bourne HR (1990) Clinical characteristics of acromegalic patients whose pituitary tumors contain mutant Gs protein. J Clin Endocrinol Metab 71:1416–1420 [DOI] [PubMed]
- Lee EJ, Russell T, Hurley L, Jameson JL (2005) Pituitary transcription factor-1 induces transient differentiation of adult hepatic stem cells into prolactin-producing cells in vivo. Mol Endocrinol 19:964–971 [DOI] [PubMed]
- Lloyd RV, Jin L, Qian X, Kulig E (1997) Aberrant p27kip1 expression in endocrine and other tumors. Am J Pathol 150:401–407 [PMC free article] [PubMed]
- Matsuno A, Katakami H, Sanno N, Ogino Y, Osamura RY, Matsukura S, Shimizu N, Nagashima T (1999) Pituitary somatotroph adenoma producing growth hormone (GH)-releasing hormone (GHRH) with an elevated plasma GHRH concentration: a model case for autocrine and paracrine regulation of GH secretion by GHRH. J Clin Endocrinol Metab 84:3241–3247 [DOI] [PubMed]
- Minematsu T, Miyai S, Kajiya H, Suzuki M, Sanno N, Takekoshi S, Teramoto A, Osamura RY (2005) Recent progress in studies of pituitary tumor pathogenesis. Endocrine 28:37–41 [DOI] [PubMed]
- Miyai S, Yoshimura S, Iwasaki Y, Takekoshi S, Lloyd RV, Osamura RY (2005) Induction of GH, PRL, and TSH beta mRNA by transfection of Pit-1 in a human pituitary adenoma-derived cell line. Cell Tissue Res 322:269–277 [DOI] [PubMed]
- Mori H, Saitoh Y, Maeda T, Okada Y, Hirano H, Tsuji M (2001) Increased exocytosis of secretory granules in contrast to reduced serum hormone levels in pituitary adenomas of humans and rats treated with dopamine agonist. Med Electron Microsc 34:123–133 [DOI] [PubMed]
- Naritaka H, Kameya T, Sato Y, Furuhata S, Otani M, Kawase T (1999) Morphological characterization and subtyping of silent somatotroph adenomas. Pituitary 1:233–241 [DOI] [PubMed]
- Nelson C, Albert VR, Elsholtz HP, Lu LI, Rosenfeld MG (1988) Activation of cell-specific expression of rat growth hormone and prolactin genes by a common transcription factor. Science 239:1400–1405 [DOI] [PubMed]
- Neumann PE, Goldman JE, Horoupian DS, Hess MA (1985) Fibrous bodies in growth hormone-secreting adenomas contain cytokeratin filaments. Arch Pathol Lab Med 109:505–508 [PubMed]
- Onoda N, Kamezu Y, Takagi S, Shinohara Y, Osamura RY (1992) An autopsy case of invasive pituitary adenoma (prolactinoma) with rapid fatal clinical course due to streptococcal meningitis. Acta Pathol Jpn 42:832–836 [DOI] [PubMed]
- Osamura RY (1988) Immunoelectron microscopic studies of GH and alpha subunit in GH secreting pituitary adenomas. Pathol Res Pract 183:569–571 [DOI] [PubMed]
- Osamura RY, Watanabe K, Komatsu N, Ohya M, Kageyama N (1982) Amorphous and stellate amyloid in functioning human pituitary adenomas: histochemical, immunohistochemical and electron microscopic studies. Acta Pathol Jpn 32:605–611 [DOI] [PubMed]
- Osamura RY, Egashira N, Miyai S, Yamazaki M, Takekoshi S, Sanno N, Teramoto A (2004) Molecular pathology of the pituitary. Development and functional differentiation of pituitary adenomas. Front Horm Res 32:20–33 [DOI] [PubMed]
- Oyama K, Sanno N, Teramoto A, Osamura RY (2001) Expression of neuro D1 in human normal pituitaries and pituitary adenomas. Mod Pathol 14:892–899 [DOI] [PubMed]
- Reincke M, Allolio B, Saeger W, Kaulen D, Winkelmann W (1987) A pituitary adenoma secreting high molecular weight adrenocorticotropin without evidence of Cushing’s disease. J Clin Endocrinol Metab 65:1296–1300 [DOI] [PubMed]
- Robert F, Hardy J (1986) Human corticotroph cell adenomas. Semin Diagn Pathol 3:34–41 [PubMed]
- Saeger W, Ludecke DK, Buchfelder M, Fahlbusch R, Quabbe HJ, Petersenn S (2007) Pathohistological classification of pituitary tumors: 10 years of experience with the German Pituitary Tumor Registry. Eur J Endocrinol 156:203–216 [DOI] [PubMed]
- Sanno N, Teramoto A, Matsuno A, Inada K, Itoh J, Osamura RY (1994) Clinical and immunohistochemical studies on TSH-secreting pituitary adenoma: its multihormonality and expression of Pit–1. Mod Pathol 7:893–899 [PubMed]
- Sanno N, Teramoto A, Matsuno A, Takekoshi S, Osamura RY (1995) GH and PRL gene expression by nonradioisotopic in situ hybridization in TSH-secreting pituitary adenomas. J Clin Endocrinol Metab 80:2518–2522 [DOI] [PubMed]
- Sanno N, Teramoto A, Osamura RY, Genka S, Katakami H, Jin L, Lloyd RV, Kovacs K (1997) A growth hormone-releasing hormone-producing pancreatic islet cell tumor metastasized to the pituitary is associated with pituitary somatotroph hyperplasia and acromegaly. J Clin Endocrinol Metab 82:2731–2737 [DOI] [PubMed]
- Sanno N, Tahara S, Kurotani R, Matsuno A, Teramoto A, Osamura RY (2001) Cytochemical and molecular biological aspects of the pituitary and pituitary adenomas–cell differentiation and transcription factors. Prog Histochem Cytochem 36:263–299 [DOI] [PubMed]
- Sornson MW, Wu W, Dasen JS, Flynn SE, Norman DJ, O’Connell SM, Gukovsky I, Carriere C, Ryan AK, Miller AP, Zuo L, Gleiberman AS, Andersen B, Beamer WG, Rosenfeld MG (1996) Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature 384:327–333 [DOI] [PubMed]
- Sugiyama M, Takumi I, Node Y, Sanno N, Teramoto A, Osamura RY (1999) Neurohypophyseal germinoma with prolactinoma. Case illustration. J Neurosurg 90:170 [DOI] [PubMed]
- Suzuki M, Egashira N, Kajiya H, Minematsu T, Takekoshi S, Tahara S, Sanno N, Teramoto A, Osamura RY (2008) ACTH and alpha-subunit are co-expressed in rare human pituitary corticotroph cell adenomas proposed to originate from ACTH-committed early pituitary progenitor cells. Endocr Pathol 19:17–26 [DOI] [PubMed]
- Tahara S, Kurotani R, Ishii Y, Sanno N, Teramoto A, Osamura RY (2002) A case of Cushing’s disease caused by pituitary adenoma producing adrenocorticotropic hormone and growth hormone concomitantly: aberrant expression of transcription factors NeuroD1 and Pit-1 as a proposed mechanism. Mod Pathol 15:1102–1105 [DOI] [PubMed]
- Takei M, Suzuki M, Kajiya H, Ishii Y, Tahara S, Miyakoshi T, Egashira N, Takekoshi S, Sanno N, Teramoto A, Osamura RY (2007) Immunohistochemical detection of somatostatin receptor (SSTR) subtypes 2A and 5 in pituitary adenoma from acromegalic patients: good correlation with preoperative response to octreotide. Endocr Pathol 18:208–216 [DOI] [PubMed]
- Teramoto A, Sanno N, Tahara S, Osamura YR (2004) Pathological study of thyrotropin-secreting pituitary adenoma: plurihormonality and medical treatment. Acta Neuropathol 108:147–153 [DOI] [PubMed]
- Trouillas J (2002) Pathology and pathogenesis of pituitary corticotroph adenoma. Neurochirurgie 48:149–162 [PubMed]
- Vidal S, Horvath E, Kovacs K, Scheithauer BW (2004) Tumors of the Adenohypophysis. Humana Press, Totowa
- Yu R, Melmed S (2004) Pituitary tumor transforming gene: an update. Front Horm Res 32:175–185 [DOI] [PubMed]