Abstract
This study examined self-efficacy for managing pain, symptoms, and function in patients with lung cancer and their caregivers, and associations between self-efficacy and patient and caregiver adjustment. 152 patients with early stage lung cancer completed measures of self-efficacy, pain, fatigue, quality of life, depression, and anxiety. Their caregivers completed a measure assessing their self-efficacy for helping the patient manage symptoms and measures of psychological distress and caregiver strain. Analyses indicated that, overall, patients and caregivers were relatively low in self-efficacy for managing pain, symptoms, and function, and that there were significant associations between self-efficacy and adjustment. Patients low in self-efficacy reported significantly higher levels of pain, fatigue, lung cancer symptoms, depression, and anxiety, and significantly worse physical and functional well being, as did patients whose caregivers were low in self-efficacy. When patients and caregivers both had low self-efficacy, patients reported higher levels of anxiety and poorer quality of life than when both were high in self-efficacy. There were also significant associations between patient and caregiver self-efficacy and caregiver adjustment, with lower levels of self-efficacy associated with higher levels of caregiver strain and psychological distress. These preliminary findings raise the possibility that patient and caregiver self-efficacy for managing pain, symptoms, and function may be important factors affecting adjustment, and that interventions targeted at increasing self-efficacy may be useful in this population.
Self-efficacy, or the confidence in one’s ability to perform a specific behavior or task (Bandura, 1997), is one factor that has been examined in relation to patient’s adjustment to cancer. Self-efficacy for managing pain, symptoms, and function in particular may be critical to a patient’s ability to manage the physical and psychological challenges of cancer. For several reasons, lung cancer provides a particularly good model in which to study self-efficacy for pain and symptom control. First, lung cancer is common, being the second leading site of cancer occurrence among both men and women with over 174,000 new cases projected for 2006 (American Cancer Society, 2006). Second, lung cancer poses significant challenges for patients and their caregivers including aggressive medical treatments and an uncertain prognosis. In addition to pain, patients often must cope with fatigue, shortness of breath, and other troubling symptoms (Degner & Sloan, 1995). Finally, clinical observations suggest that lung cancer patients vary in their confidence that they can manage their symptoms and that those who are more confident show better adjustment to their disease.
Pain and other symptoms of lung cancer also occur in a social context, affecting both patients and their caregivers. Family and other informal caregivers increasingly play a central role in the care of lung cancer patients, providing practical and emotional support, and helping them monitor symptoms, comply with medical treatments, deal with side effects, and communicate with health care professionals (Ferrell et al., 1991; Blanchard et al., 1997). Not surprisingly, caregivers often report high levels of distress, particularly when the patient is experiencing pain (Miakowski et al., 1997). Like patients, caregivers are likely to vary in their ability to cope with the challenges they face (Blanchard et al., 1997). Variations in caregiver self-efficacy can have important implications, not only for the caregiver’s adjustment but also for the patient’s.
One benefit of studying both lung cancer patients and their caregivers is that one can examine the effects of self-efficacy of patient-caregiver dyads. In some dyads, both patients and caregivers may report high self-efficacy, whereas in other dyads only one member or neither member of the dyad may report high self-efficacy. While there is a growing literature regarding couples’ coping with cancer (e.g. Hagedoorn et al., 2000; Manne & Glassman, 2000; Porter et al., 2005), very little research has been focused on dyad self-efficacy. An analysis of dyadic self-efficacy is important because it can shed light on one specific way in which relationships may impact both patients’ and caregivers’ adjustment to lung cancer.
The current study examined the following hypotheses: (1) that lung cancer patients with high levels of self-efficacy for managing pain, symptoms, and function would report lower levels of symptoms, better quality of life, and less psychological distress; (2) that caregivers high in self-efficacy for helping the patient manage pain, symptoms, and function would report lower levels of caregiver strain and mood disturbance; and (3) that in dyads where the patient and caregiver are both high in self-efficacy both would report better adjustment.
Method
Participants
The current study reports on baseline data of 152 patients with early stage lung cancer and their caregivers who were enrolled in a larger program of research evaluating the efficacy of a partner-assisted coping skills training intervention. Participants were recruited between December, 2002 and March, 2005. The entry criteria for patients included having (a) a diagnosis of early stage lung cancer (non-small cell lung cancer, Stages I-IIIa and IIIb without pleural effusion, or limited stage small cell lung cancer), (b) no other cancers in the past 5 years, (c) ability to read and speak English, (d) access to a telephone and willing to participate in a psycheducational intervention, and (e) a caregiver who was also willing to participate. As is common in the cancer literature, “caregiver” was broadly defined in this study as any friend or family member who provided practical and/or emotional support to the patient. To identify the patient’s primary caregiver, the patient was asked to list the people they relied on for support with things like getting to the doctor and taking medication. They were then asked to review this list and identify the main person they relied on for support. This person was identified as the primary caregiver and was invited to participate in the study.
643 patients were screened for inclusion. Of these, 143 were deemed ineligible and 500 were approached about participation. Of those approached, 316 declined and 184 consented. The most common reasons for declining included lack of interest (42%), “too much trouble” (6%), and lack of time (6%). Of the 184 who consented, 32 dropped out prior to completing baseline questionnaires primarily because of death/declining health (47%), or loss of time or interest (41%). This left a sample of 152 dyads (30% of eligible patients) who were included in the current analyses.
Procedures
Participants were recruited from the Duke University Thoracic Oncology Program as well as several community oncology clinics in the Durham, NC area. Patients completed a telephone survey assessing self-efficacy, pain, fatigue, quality of life, and psychological distress. Caregivers completed a telephone survey assessing their self-efficacy for helping the patient manage symptoms, and their levels of caregiver strain and mood disturbance. Medical information for the patient, including cancer stage, treatments, and date of diagnosis, were extracted from the patient’s medical record. All procedures were approved by the Duke University Medical Center Institutional Review Board and the Duke Comprehensive Cancer Center Protocol Review Committee.
Measures
Self-efficacy
Self-efficacy for managing pain, symptoms, and function was assessed with a modified version of a standard self-efficacy scale (Lorig et al., 1989). The original scale was modified by removing 9 items relevant to patients with arthritis but not cancer (e.g. “How certain are you that you can button and unbutton 3 medium-size buttons in a row in 12 seconds?”) and adding 7 items regarding management of pain (from the Chronic Pain Self-Efficacy scale; Anderson et al. 1995) and other common cancer symptoms such as nausea and shortness of breath. Patients rated 16 items regarding their perceived ability to manage a variety of symptoms on a scale of 10 (not at all certain) to 100 (completely certain). The caregiver version of the instrument is identical to that used with patients except that caregivers are asked to rate how confident they are that they can help the patient manage symptoms (e.g. “How certain are you that you can help the patient decrease his/her pain quite a bit?”, “How certain are you that you can do something to help the patient feel better if he/she is feeling blue?”). The scale contains three subscales: self-efficacy for managing pain, self-efficacy for managing other symptoms (e.g. fatigue, nausea, depression), and self-efficacy for function. Because the three subscales were highly correlated with each other (r’s=.71-.86 for patients; .80-.86 for caregivers) the total score was utilized for this report. The possible range for the total score is 0 to 100 with higher scores indicating greater self-efficacy. Cronbach alphas for the total scale score were .95 for patients and .96 for caregivers. Prior studies using this instrument to assess self-efficacy in lung cancer patients (Porter et al., 2002), prostate cancer patients and their caregivers (Campbell et al., 2004), and caregivers of cancer patients at the end of life (Keefe et al., 2003) have demonstrated evidence of internal consistency and construct validity.
Pain
Pain was assessed using two items from the Brief Pain Inventory (BPI; Cleeland & Ryan, 1994) in which participants rate their usual pain in the past week and their worst pain in the past week on a scale from 1 (“no pain”) to 10 (“pain as bad as you can imagine”). The worst and usual BPI pain intensity ratings have demonstrated good test-retest reliability in a sample of cancer inpatients who completed the second pain ratings 1-7 days later (worst, r=.93; usual, r=.78) (Daut et al., 1983). The validity of the BPI has also been supported by studies that have shown a significant relationship between higher pain ratings and increased analgesic and narcotic use (Daut et al., 1983). Because ratings of usual and worst pain were highly correlated (r=.89) in the present study, they were combined into a single summary score with a possible range of 1 to 10 with higher scores indicating more severe pain.
Fatigue was measured using the Brief Fatigue Inventory (BFI; Mendoza et al., 1999). The BFI has a total of 9 items that measure (a) levels of current fatigue, usual fatigue over the past 24 hours, and worst fatigue over the past 24 hours on a scale from 1 (“no fatigue”) to 10 (“fatigue as bad as you can imagine”); and (b) how fatigue has interfered in the past 24 hours with general activity, mood, walking ability, normal work (including both work inside and outside the home and daily chores), relations with other people, and enjoyment of life on a scale of 1 (“does not interfere”) to 10 (“completely interferes”). Factor analysis has shown that 75% of the variance on the BFI can be explained by a single factor suggesting that the BFI measures a single construct (Mendoza et al., 1999). In a study using the BFI to assess fatigue in 305 cancer patients and 290 healthy control subjects, the patients reported significantly higher levels of fatigue than the controls, evidence for the sensitivity of the BFI with cancer patients. In addition, the BFI demonstrated evidence of internal consistency and was correlated with performance status and with physiological markers of anemia and nutritional status, indicating evidence of construct validity (Mendoza et al., 1999). Scores on the BFI can range from 1 to 10 with higher scores indicating more symptoms of fatigue. Cronbach alpha in the current study was .95.
Quality of life was measured using the Functional Assessment of Cancer Therapy - Lung Cancer (FACT-L; Cella et al., 1995). The FACT-L consists of four general and one lung cancer symptom-specific subscale. The present study utilized two general subscales (physical well being and functional well being) along with the lung cancer specific subscale which includes items assessing shortness of breath, coughing, weight loss, and loss of appetite. These subscales were chosen as they assess constructs that are conceptually distinct from those assessed by the other measures used in this study (with the exception of one item of the physical well being subscale assessing pain). In each subscale, items are rated on a 5-point scale (0=not at all; 4=very much). The possible range on each subscale is 0 to 28 with higher scores indicating better functioning. The FACT is widely used in cancer studies and both the general measure and the lung cancer specific measure have been shown to possess adequate psychometric properties including internal consistency, convergent validity, and sensitivity to change in performance status ratings (an indicator of patient functional status; Cella et al., 1993, 1995). Cronbach’s alphas in the present study were .83 (physical well being), .84 (functional well being), and .73 (lung cancer symptoms).
Depressive symptoms
Depressive symptoms were assessed using the Beck Depression Inventory (BDI). The BDI is a 21-item self-report inventory assessing current degree of depression through items pertaining to affective, cognitive, motivational, and physiologic areas of depressive symptomatology. Total scores can range from 0 to 63, with higher scores indicating higher levels of depressive symptoms reported (Beck et al., 1961). The BDI has high internal consistency in clinical and nonclinical populations, and good discriminant, construct, and concurrent validity (Beck & Beamesderfer, 1974). Cronbach alpha in the current study was .86.
Anxiety
Anxiety was measured using the trait anxiety version of the State Trait Anxiety Inventory (STAI; Spielberger, 1983). The STAI was developed as a tool for investigating anxiety in normal (non-psychiatric) adults, but has been used in assessing anxiety in neuropsychiatric, medical, and surgical patients. The scale has demonstrated good psychometric properties (Spielberger, 1983). Cronbach alpha in the current study was .92. Scores on the trait anxiety scale can range from 20 to 80 with higher scores indicating more symptoms of anxiety.
Caregiver mood
Caregiver mood was assessed using a brief version of the Profile of Mood States-B (POMS-B; Lorr & McNair, 1982). Eighteen adjectives -- three each assessing negative mood states of depression, anxiety, and hostility and three each their positive mood counterparts of elation, composure, and agreeableness -- were used to rate average mood on scales from 0=very much unlike this to 3=very much like this. These items were selected from the larger number appearing on the POMS-B because of their high item-total correlations for their respective subscales (Affleck et al., 1988). Scores for the total mood disturbance scale of the POMS-B can range from -24 to 177 with higher scores indicating greater mood disturbance. Cronbach’s alpha for the total mood disturbance score was .89.
Caregiver strain
Caregiver strain was assessed with the Caregiver Strain Index (Robinson, 1983), a 13-item scale that assesses a variety of stressors commonly experienced by caregivers. Sample items include “My sleep has been disturbed because ___ is out of bed, restless, or sick at night”, and “It is inconvenient (e.g. because helping takes so much time, to prepare meals, or because appointments take so long”). For each item, the respondent indicates in a yes/no fashion whether that item applies to him/her, thus yielding a total score ranging from 0 to 13 with higher scores indicative of more burden associated with caregiving. Prior studies have reported internal consistency scores ranging from .79 (Miaskowski et al., 1997) to .86 (Robinson, 1983). In addition, construct validity of the scale has been determined in three areas: patient characteristics, caregivers’ subjective perceptions of the caregiving relationship, and physical and emotional status of the caregiver (Robinson, 1983). In the current study, Cronbach’s alpha was .84.
Statistical analyses
First, means and standard deviations on the self-efficacy scale were calculated in order to describe the level of self-efficacy for symptom management reported by lung cancer patients and their caregivers, and correlation analyses were conducted to determine the degree of association between patient and caregiver self-efficacy. Associations between self-efficacy and medical and demographic variables for both patients and caregivers were also examined. Second, general linear regression analyses were used to examine associations between patient and caregiver self-efficacy and measures of patient and caregiver adjustment. Demographic variables (age, gender, and education) and medical status variables (patient’s cancer stage, time since diagnosis, and current or prior treatment with chemotherapy and radiation) were entered first as covariates, followed by either patient or caregiver self-efficacy. A separate regression analysis was conducted for each of the measures of patient and caregiver adjustment. Analyses of patient adjustment included patient demographics as covariates, while analyses of caregiver adjustment included caregiver demographics. Third, to examine associations between the level of self-efficacy of both members of the dyad (the patient and the caregiver) on outcomes, patients and caregivers were classified as high or low in self-efficacy based on a median split of their self-efficacy scores and dyads were placed into one of four categories: (1) Both patient and caregiver are high in self-efficacy; (2) the patient is high in self-efficacy and the caregiver is low; (3) the patient is low in self-efficacy and the caregiver is high; (4) both patient and caregiver are low in self-efficacy. Analyses of variance were then used to predict patient and caregiver adjustment from the dyad self-efficacy category.
Results
Participant Characteristics
Participant characteristics are presented in Table 1. Slightly more than half of the patients (52.6%) were men, and the majority of caregivers (67%) were women. Both patients and caregivers were predominantly Caucasian and well-educated. 76% of the caregivers were spouses of the patient, 14% were sons or daughters, and 8% were sisters, brothers, and friends. In most cases (73%), the patients and caregivers lived in the same household.
Table 1.
Participant characteristics
| Patients (N=152) | Caregivers (N=152) | |
|---|---|---|
| Mean age (SD) | 65.1 (9.8) | 60.0 (13.2) |
| Gender (% male) | 52.6 | 33.6 |
| Race | ||
| Caucasian | 86.8% | 85.7% |
| African American | 11.2% | 10.9% |
| Native American | 1.3% | 0.7% |
| Other | 0.7% | 2.7% |
| Education | ||
| <12 years | 15.1% | 7.9% |
| High school graduate | 30.3% | 34.2% |
| Some college | 27.6% | 27.6% |
| College graduate | 15.8% | 12.5% |
| Post-graduate | 11.2% | 17.8% |
| Median days since diagnosis (IQR) | 232 (710) | |
| 25th percentile | 120 | |
| 75th percentile | 830 | |
| Treatment | ||
| Surgery | 80.5% | |
| Chemotherapy - current | 12.7% | |
| Chemotherapy - in past | 33.1% | |
| Radiation - current | 6.0% | |
| Radiation - in past | 27.2% | |
| Cancer stage* | ||
| 1 | N=81 (53.3%) | |
| 2 | N=22 (14.5%) | |
| 3 | N=44 (28.9%) | |
| Limited stage | N=4 (2.6%) | |
Note: Staging information was not available for one participant.
Descriptive analyses
Mean scores on self-efficacy to manage symptoms were 64.4 (SD=21.1, range=14.7-100.0) for patients and 61.6 (SD=20.9, range=10.0-100.0) for caregivers. These scores are somewhat lower than those reported in a study of prostate cancer patients (range=71.1-82.9; Campbell et al., 2004) but slightly higher than those we found in a previous study of lung cancer patients (range=56.1-59.3; Porter et al., 2002). The correlation between patient and caregiver self-efficacy scores was r=.38 (p<.0001).
Regarding pain, 54% of the patients reported they experienced no pain in the past week (BPI summary score=1), 25% reported mild pain (BPI summary score between 1.5 and 4.5), and 21% reported high levels of pain (BPI summary score of 5 and above).
Analyses were performed to determine whether patients’ self-efficacy scores were related to any demographic or medical variables using t-tests [for gender, marital status, race (white versus other), surgery, chemotherapy, and radiation], correlations (for age, education, and time since diagnosis), and analyses of variance (for type of relationship with the caregiver and cancer stage). Patient self-efficacy was significantly associated with age (r=.24, p<.01), cancer stage [F(2,147)=6.79, p<.01], and prior/current treatment with chemotherapy [t (150)=2.50, p<.01] and radiation [t (150)=3.26, p<.001]. Older patients, patients with Stage 1 and 2 versus Stage 3 disease, and patients who had never received chemotherapy and radiation reported higher levels of self-efficacy.
Similar analyses were conducted to examine whether caregivers’ self-efficacy scores were related to demographic or medical variables. Caregiver self-efficacy was significantly associated with caregiver age (r=.19, p<.05), and with the patient’s prior/current treatment with chemotherapy [t (150)=2.09, p<.05] and radiation [t (150)=2.11, p<.05] therapy. Older caregivers and caregivers of patients who had never received chemotherapy and radiation reported higher levels of self-efficacy.
In order to examine associations between self-efficacy and measures of patient and caregiver adjustment independent of demographic and medical variables, the following analyses controlled for age, gender, education, cancer stage, time since diagnosis, and prior/current treatment with chemotherapy and radiation.
Patient self-efficacy
General linear regression analyses were used to examine associations between patient self-efficacy and measures of patient and caregiver adjustment. Demographic variables (age, gender, and education) and medical status variables (patient’s cancer stage, time since diagnosis, and prior/current treatment with chemotherapy and radiation) were entered first as covariates, followed by patient self-efficacy. Table 2 summarizes the results of these analyses. Patient self-efficacy explained significant proportions of the variance in all of the patient and caregiver measures of adjustment that were examined. Patients with high levels of self-efficacy reported much lower levels of pain, fatigue, lung cancer symptoms, anxiety, and depression, and much higher levels of physical well being and functional well being. In addition, when patients reported high self-efficacy their caregivers reported lower levels of caregiver strain and mood disturbance.
Table 2.
Summary of regression analyses predicting patient adjustment and caregiver adjustment from patient self-efficacy
| Measure | F* | p* | Total Model R2* | Change in R2 with self-efficacy added |
|---|---|---|---|---|
| Pain | 26.53 | <.0001 | .31 | .13 |
| Fatigue | 56.46 | <.0001 | .48 | .33 |
| Physical well being | 39.45 | <.0001 | .40 | .18 |
| Functional well being | 107.60 | <.0001 | .53 | .37 |
| Lung cancer QOL | 67.37 | <.0001 | .43 | .28 |
| Depressive symptoms | 74.63 | <.0001 | .47 | .30 |
| Anxiety | 69.19 | <.0001 | .48 | .26 |
| Caregiver strain | 5.28 | .02 | .37 | .16 |
| Caregiver mood disturbance | 10.63 | .001 | .34 | .12 |
Note: For patient outcomes, the total model included patient age, patient gender, patient education, cancer stage, time since diagnosis, and treatment with chemotherapy and radiation in addition to self-efficacy. For caregiver outcomes, the total model included caregiver age, caregiver gender, caregiver education, and the patient’s medical variables (cancer stage, time since diagnosis, and treatment with chemotherapy and radiation) in addition to self-efficacy. F and p values refer to the contribution of patient self-efficacy in the total model.
Caregiver self-efficacy
General linear regression analyses were used to examine associations between caregiver self-efficacy and measures of patient and caregiver adjustment. Caregiver demographics (age, gender, and education) and patient medical status variables (patient’s cancer stage, time since diagnosis, and prior/current treatment with chemotherapy and radiation) were entered first as covariates, followed by caregiver self-efficacy. Table 3 summarizes the results of these analyses. Caregiver self-efficacy explained significant proportions of the variance in the caregiver’s level of mood disturbance, and in patient levels of pain, fatigue, physical and functional well being, lung cancer specific symptoms, depressive symptoms, and anxiety. Caregivers who were high in their self-efficacy for helping the patient manage symptoms reported lower levels of mood disturbance, and patients whose caregivers were high in self-efficacy reported much lower levels of pain, fatigue, anxiety, and depression and much higher levels of quality of life.
Table 3.
Summary of regression analyses predicting patient adjustment and caregiver adjustment from caregiver self-efficacy
| Measure | F* | p* | Total Model R2* | Change in R2 with self-efficacy added |
|---|---|---|---|---|
| Pain | 6.81 | .01 | .22 | .04 |
| Fatigue | 9.00 | .004 | .23 | .08 |
| Physical well being | 15.95 | .0001 | .31 | .09 |
| Functional well being | 25.20 | <.0001 | .29 | .13 |
| Lung cancer QOL | 15.02 | .0002 | .23 | .08 |
| Depressive symptoms | 23.44 | <.0001 | .30 | .13 |
| Anxiety | 16.73 | <.0001 | .30 | .08 |
| Caregiver strain | 3.72 | .06 | .37 | .16 |
| Caregiver mood disturbance | 5.71 | .02 | .31 | .09 |
Note: For patient outcomes, the total model included patient age, patient gender, patient education, cancer stage, time since diagnosis, and treatment with chemotherapy and radiation in addition to self-efficacy. For caregiver outcomes, the total model included caregiver age, caregiver gender, caregiver education, and the patient’s medical variables (cancer stage, time since diagnosis, and treatment with chemotherapy and radiation) in addition to self-efficacy. F and p values refer to the contribution of caregiver self-efficacy in the total model.
In order to determine whether the caregiver’s level of self-efficacy was associated with patient outcomes independent of the patient’s level of self-efficacy, a second set of regression analyses was conducted predicting patient adjustment from caregiver self-efficacy with demographic and medical variables and patient self-efficacy entered first as covariates. Caregiver self-efficacy continued to show significant associations with patient physical well being [F(1,149)=4.81, p=.03], functional well being [F(1,149)=7.03, p=.009], and depressive symptoms [F(1,149)=7.14, p=.008] after controlling for the patient’s level of self-efficacy. The associations between caregiver self-efficacy and the patient’s lung cancer symptoms and anxiety approached significance (p’s<.10) while the associations between caregiver self-efficacy and patient pain and fatigue were no longer significant (p’s>.34).
Dyad Efficacy
We were interested in examining associations between the level of self-efficacy of both members of the dyad (the patient and the caregiver) and measures of adjustment. To do this, patients and caregivers were first categorized as high or low in self-efficacy based on a median split of their self-efficacy scores (which were performed separately for patients and caregivers). Next, dyads were placed into one of four categories: (1) Both patient and caregiver are high in self-efficacy (“both high”; n=46, 30.1%); (2) The patient is high in self-efficacy and the caregiver is low (“patient high-caregiver low”; n=28, 18.3%); (3) The patient is low in self-efficacy and the caregiver is high (“patient low-caregiver high”; n=30, 19.6%); (4) Both patient and caregiver are low in self-efficacy (“both low”; n=49, 32.0%).
Bartlett’s test of homogeneity of variances indicated that there were unequal variances across dyad self-efficacy groups for all of the variables except functional well being and caregiver strain, violating an assumption of analysis of variance. Thus, weighted least squares analyses were used to compare the dyad self-efficacy groups on patient pain, fatigue, physical well being, lung cancer symptoms, depression, anxiety, and caregiver mood, while analyses of covariance were used to compare the dyad self-efficacy groups on patient functional well being and caregiver strain. Patient and caregiver age and education, cancer stage, and prior/current chemotherapy and radiation were entered first as covariates. Results indicated that the dyad self-efficacy groups differed significantly on the following patient measures: pain [F(10,150)=9.12, p<.0001], fatigue [F(10,106)=15.52, p<.0001], physical well being [F(10,149)=13.12, p<.0001], functional well being [F(10,149)=17.33, p<.0001], lung cancer symptoms, [F(10,149)=13.50, p<.0001], depression [F(10,149)=22.41, p<.0001], and anxiety [F(10,149)=28.44, p<.0001]. The differences between dyad self-efficacy groups in caregiver strain and caregiver mood approached significance (p’s<.10).
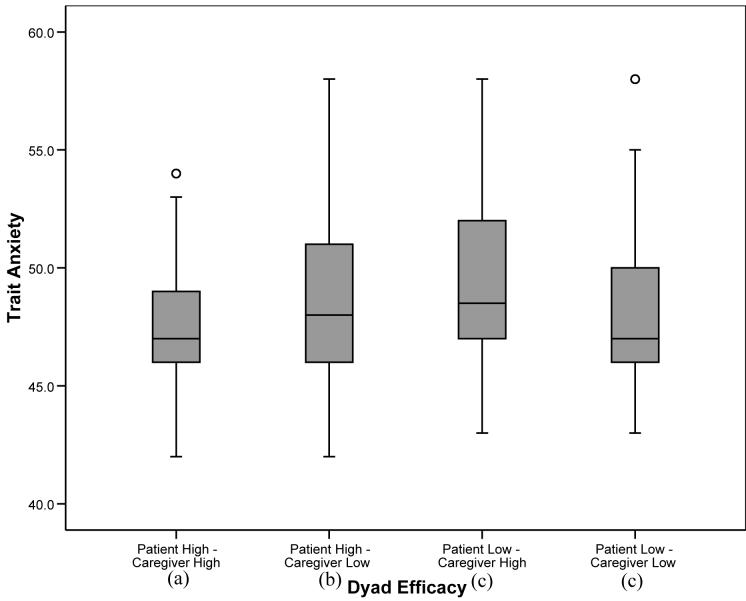
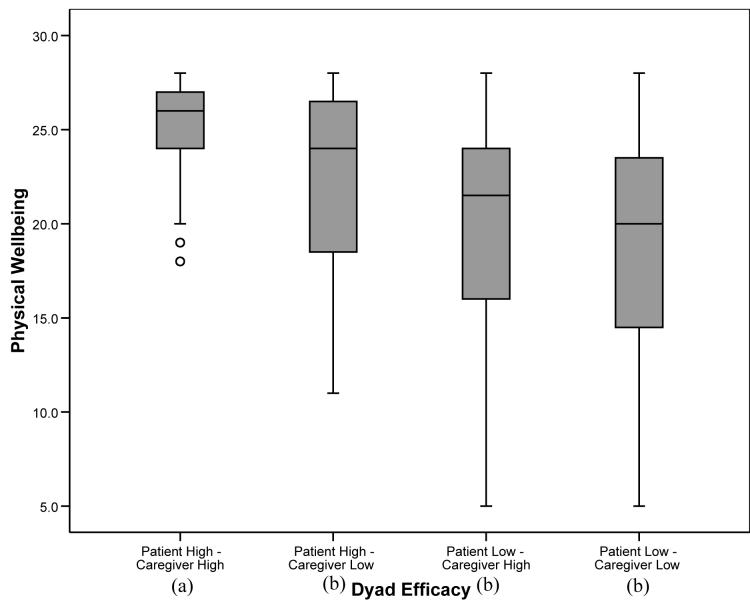
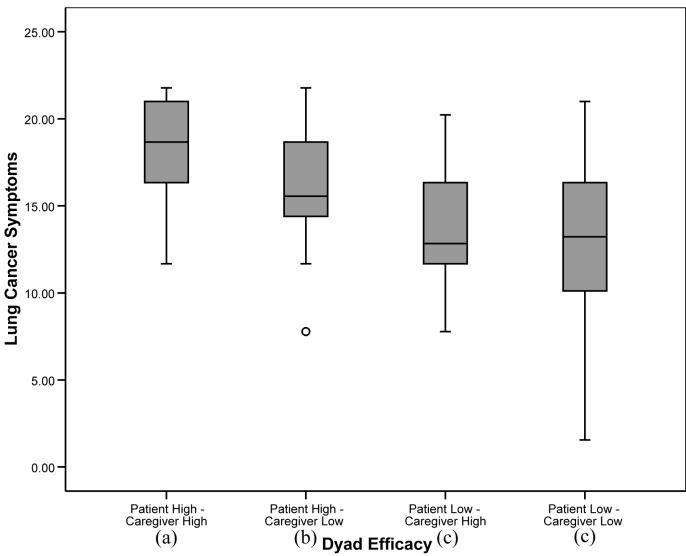
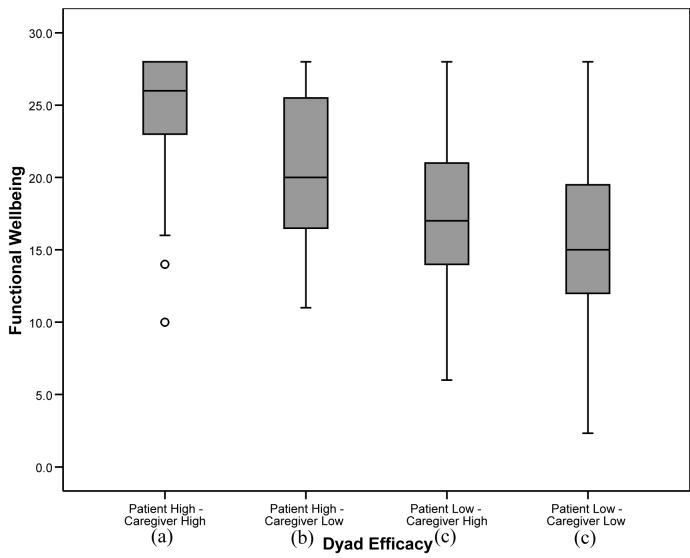
For those outcome variables in which the overall analysis was significant, post-hoc tests were conducted to determine significant differences between the four dyad self-efficacy categories. To balance the concerns of avoiding Type 1 error while attempting to avoid dismissing potentially important relationships, alpha was set at .01 for the post-hoc analyses. For pain, fatigue, and depression, patients in “patient high-caregiver high” and “patient high-caregiver low” dyads reported significantly lower levels of symptoms than patients in “patient low-caregiver high” and “patient low-caregiver low” dyads (p’s<.01), suggesting a main effect for patient self-efficacy similar to that found with the regression analyses. For anxiety, patients in “patient high-caregiver high” dyads reported significantly lower levels than patients in the other three categories, including patients in “patient high-caregiver low” dyads. In addition, patients in “patient high-caregiver low” dyads reported lower levels of anxiety than patients in “patient low-caregiver high” and “patient low-caregiver low” dyads (see Figure 1). For the QOL scales (physical well being, functional well being, and lung cancer symptoms), patients in “patient high-caregiver high” dyads reported significantly higher levels of QOL than patients in the three other categories. In addition, patients in “patient high-caregiver low” dyads reported higher QOL in the functional and lung cancer symptom domains than patients in “patient low-caregiver low” dyads (see Figures 2-4).
Figure 1.
Box plots displaying patient anxiety by dyad self-efficacy category. Medians are indicated by the horizontal line within each box, the 25th and 75th percentiles by the upper and lower boxes, nearest values not beyond 1.5 times the interquartile range with whiskers, and outliers with dots. The overall weighted least squares analysis was significant (p<.0001). Groups with differing subscripts differed significantly from each other (p’s<.01).
Figure 2.
Box plots displaying patient physical well being by dyad self-efficacy category. Medians are indicated by the horizontal line within each box, the 25th and 75th percentiles by the upper and lower boxes, nearest values not beyond 1.5 times the interquartile range with whiskers, and outliers with dots. The overall weighted least squares analysis was significant (p’s<.0001). Groups with differing subscripts differed significantly from each other (p’s<.01).
Figure 4.
Box plots patient lung cancer symptoms by dyad self-efficacy category. Medians are indicated by the horizontal line within each box, the 25th and 75th percentiles by the upper and lower boxes, nearest values not beyond 1.5 times the interquartile range with whiskers, and outliers with dots. The overall weighted least squares analysis was significant (p’s<.0001). Groups with differing subscripts differed significantly from each other (p’s<.01).
Discussion
Findings from this study build on an expanding body of evidence indicating the importance of self-efficacy in the adjustment of cancer patients. This study adds uniquely to this literature by (a) using a measure of self-efficacy focused on one particular area of concern for cancer patients and their caregivers, namely management of pain, symptoms, and function; (b) studying lung cancer patients, a population that has been understudied despite its prevalence and the high levels of symptoms and distress associated with the disease; and (c) examining the role of caregiver self-efficacy in patient and caregiver adjustment.
To our knowledge, this is the first study to directly examine how self-efficacy for managing pain, symptoms, and function relates to pain and other important outcomes in lung cancer patients. The results showed that patients who rated their self-efficacy as high experienced lower levels of pain, fatigue, and psychological distress and higher quality of life. Importantly, these findings were obtained even after controlling for demographic and medical variables that could influence pain and other outcomes. It is important to note that the cross-sectional nature of the data preclude any conclusions regarding the direction of causality. It is possible, for instance, that lower levels of symptoms and distress resulted in higher perceived self-efficacy, or that other unmeasured factors led to both higher self-efficacy and lower levels of symptoms and distress. However, clinical observations suggest that feelings of helplessness and loss of control are a frequent problem for patients coping with cancer (Akechi et al., 1998; Uchitomi et al., 2003). The findings of this study suggest that clinicians interested in understanding how lung cancer patients adapt to their illness may benefit by attending to patients’ perceptions of self-efficacy. For example, clinicians could listen for expressions of doubt regarding the ability to manage symptoms and provide those patients with education and resources for self-management strategies.
Lung cancer not only poses challenges to patients but also to their caregivers. One of the most interesting findings of this study was that caregivers’ ratings of their own self-efficacy for helping the patient manage symptoms were significantly related to patients’ ratings of symptoms and distress. Even after controlling for patients’ self-efficacy ratings, caregivers’ ratings of self-efficacy were meaningfully related to important indices of patient adjustment such as functional well being, physical well being, and depressive symptoms. Patients with caregivers high in self-efficacy scored approximately 0.70 standard deviations higher on functional and physical well being and 0.60 standard deviations lower on depressive symptoms than patients with caregivers low in self-efficacy, differences that may be clinically significant. In addition, caregivers’ self-efficacy was meaningfully related to their own mood and level of caregiver strain. As noted above, the direction of causality of these associations cannot be determined from this study. However, given the negative impact that a disease such as lung cancer can have on caregivers, understanding factors such as self-efficacy that may be associated with improved mood and reduced caregiver strain is particularly important. Overall, these findings suggest that caregiver self-efficacy ratings may provide unique information that is useful in understanding patient and caregiver adjustment to lung cancer.
The findings regarding caregiver self-efficacy in lung cancer patients add to a growing body of literature indicating the important role that spouses and other informal caregivers play in the adjustment of cancer patients (e.g. Hagedoorn et al, 2000; Manne et al, 2000). While preliminary, these findings also raise that possibility that one specific way in which caregivers can be supportive is by demonstrating confidence in their ability to help the patient manage their symptoms. Having a caregiver who is low in self-efficacy may compound the patient’s feelings of helplessness, while having a caregiver who is high in self-efficacy may help bolster the patient’s resilience. Overall, these findings suggest that the patient’s social environment is important both in understanding patients’ adjustment and in developing interventions to help patients manage symptoms and adapt to the challenges associated with their disease (Porter et al., 2005).
From a clinical perspective, the overall pattern of findings raises the possibility that interventions targeted at improving self-efficacy may help alleviate patient and caregiver suffering. There have been several recent intervention studies targeting patient self-efficacy that have found beneficial effects (Lev et al., 2001; Stiegelis et al., 2004). However, the interventions tested in these studies were primarily educational, e.g. providing patients with information about cancer symptoms and possible coping strategies. Findings from the current study suggest several ways to potentially enhance such interventions. First, techniques based on self-efficacy theory (e.g. therapist modeling of coping skills, role playing with therapist feedback, and applying learned skills to challenging situations; Bandura, 1997) could be more fully integrated into such interventions. This could lead to stronger and more enduring treatment effects on self-efficacy. Second, given the importance of caregiver self-efficacy for the patient’s adjustment, including caregivers in interventions may be quite beneficial for both themselves and the patient. Finally, intervention efforts could target patients who are low in self-efficacy. These individuals are likely to show the greatest benefits from such interventions, particularly if their caregivers also have low levels of self-efficacy.
The pattern of findings obtained suggested that self-efficacy was associated with the various measures of adjustment in similar ways. This is not surprising given that pain, fatigue, quality of life, and psychological distress tend to be inter-related (and were highly correlated with each other in this sample with r’s ranging from .46 to .73). While this may reflect shared method variance, it is also consistent with the observation that symptoms and psychological distress tend to cluster together in lung cancer patients (e.g. Fox & Lyon, 2006; Wang et al., 2006). One implication of this phenomenon is the possibility that interventions designed to enhance self-efficacy may improve patient adjustment across a variety of domains, rather than having to target specific symptoms with specific interventions.
While it may be possible to target multiple symptoms with an intervention to enhance self-efficacy, it may be necessary to define self-efficacy as it relates to adjustment to cancer more precisely than has been done to date. Previous studies in this area have utilized a variety of self-efficacy measures, many of which assess patients’ confidence in their ability to cope with a wide range of challenges (e.g. obtaining information, maintaining a positive attitude, seeking social support; Merluzzi et al., 2001; Wolfe et al., 2005). The use of such global measures makes it difficult to identify the ways in which self-efficacy might be related to specific problems in adjustment. From both a research and clinical perspective, it may be helpful to identify and measure specific domains of self-efficacy. An intervention targeted at self-efficacy for seeking social support, for instance, would look quite different from one targeting symptom management, and would likely produce disparate results.
The findings from this study also suggest that further research on the impact of dyad self-efficacy is warranted. Longitudinal studies involving multiple measures of both patient and caregiver self-efficacy would be particularly valuable in understanding how patient and caregiver self-efficacy covary across time, and the influence this has on each person’s adjustment. Patient and caregiver responses to interventions designed to enhance self-efficacy could also help determine when and with whom it is most helpful to focus intervention efforts. For instance, it may be possible to identify circumstances (e.g. at the end-of-life) or patient characteristics (e.g. extremely high levels of psychological distress) that make it more effective to intervene with a caregiver as opposed to a patient. Alternatively, it may be possible to identify patient-caregiver dyads (e.g. those in which both individuals are low in self-efficacy) who are most likely to benefit from a joint intervention.
This study has a number of limitations. First, the study design was correlational. Thus, it is not possible to determine whether low levels of patient/caregiver self-efficacy leads to increases in patient symptoms and decreases in patient QOL or vice versa. However, there has been at least one longitudinal study indicating that cancer patients’ self-efficacy at one time point is associated with subsequent levels of psychological distress (Lev et al., 1999). In addition, findings from recent intervention studies targeting cancer patients’ self-efficacy suggest that these interventions can indeed result in reductions in psychological distress (Stiegelis et al., 2004) and symptom distress (Lev et al., 2001). Second, the generalizability of the findings may be compromised by the high refusal rate for the study, and the predominance of white, well educated participants. Additional studies with larger, more representative, and more ethnically diverse samples are warranted. A third limitation is that the findings were based on self-report measures of inter-related constructs, raising the possibility that shared method variance may partially account for the associations. However, as noted above, the pattern of results is consistent with the observation that symptoms and psychological distress tend to cluster in lung cancer patients (e.g. Fox & Lyon, 2006; Wang et al., 2006). Future studies should consider including objective measures of disease severity and clinical ratings of symptoms and distress in order to increase the validity of their results.
Figure 3.
Box plots displaying patient functional well being by dyad self-efficacy category. Medians are indicated by the horizontal line within each box, the 25th and 75th percentiles by the upper and lower boxes, nearest values not beyond 1.5 times the interquartile range with whiskers, and outliers with dots. The overall weighted least squares analysis was significant (p’s<.0001). Groups with differing subscripts differed significantly from each other (p’s<.01).
Acknowledgments
This study was supported by National Cancer Institute grant R01 CA91947. The authors thank Kimberly Carson, MPH, Heidi Suarez, BS, RN, and the physicians and staff of the Duke Thoracic Oncology clinic for assistance with participant recruitment, and all of the study participants for their time and effort.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Affleck G, Tennen H, Pfeiffer C, Fifel J. Appraisals of control and predictability in adapting to a chronic diseases. J Pers Soc Psychol. 1988;53:273–279. doi: 10.1037//0022-3514.53.2.273. [DOI] [PubMed] [Google Scholar]
- Akechi T, Kugaya A, Okamura H, Nishiwaki Y, Yamawaki S, Uchitomi Y. Predictice factors for psychological distress in ambulatory lung cancer patients. Support Care Cancer. 1998;6(3):281–6. doi: 10.1007/s005200050167. [DOI] [PubMed] [Google Scholar]
- American Cancer Society . Cancer Facts and Figures 2006. American Cancer Society; Atlanta: 2006. [Google Scholar]
- Anderson KO, Dowds BN, Pelletz RE, Edwards WT, Peeters-Asdourian C. Development and initial validation of a scale to measure self-efficacy beliefs in patients with chronic pain. Pain. 1995;63:77–84. doi: 10.1016/0304-3959(95)00021-J. [DOI] [PubMed] [Google Scholar]
- Bandura A. Self-efficacy: The exercise of control. W.H. Freeman; New York: 1997. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Beck AT, Beamesderfer KA. Assessment of depression: the depression inventory. In: Pichot P, editor. Modern problems in pharmacopsychiatry. Karger; Basel, Switzerland: 1974. pp. 15–169. [DOI] [PubMed] [Google Scholar]
- Blanchard CG, Albrecht TL, Ruckdeschel JC. The crisis of cancer: psychological impact on family caregivers. Oncology. 1997;11:189–94. [PubMed] [Google Scholar]
- Campbell LC, Keefe FJ, McKee DC, Edwards CL, Herman SH, Johnson LE, Colvin M, McBride CM, Donattuci CF. Prostate cancer in African Americans: Relationship of patient and partner self-efficacy to quality of life. J Pain Symptom Manage. 2004;28:433–444. doi: 10.1016/j.jpainsymman.2004.02.020. 2004. [DOI] [PubMed] [Google Scholar]
- Cella DF, Bonomi AE, Lloyd SR, Tulsky DS, Kaplan E, Bonomi P. Reliability and validity of the Functional Assessment of Cancer Therapy-Lung (FACT-L) quality of life instrument. Lung Cancer. 1995;12(3):199–220. doi: 10.1016/0169-5002(95)00450-f. [DOI] [PubMed] [Google Scholar]
- Cella DF, Tulsky DS, et al. The functional assessment of cancer therapy scale: Development and validation of the general measure. J Clin Oncol. 1993;11:570–9. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- Cleeland CS, Ryan KM. Pain assessment: Global use of the Brief Pain Inventory. Annals Acad Med Singapore. 1994;23:129–138. [PubMed] [Google Scholar]
- Daut RL, Cleeland CS, Flanery RC. Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain. 1983;17:197–210. doi: 10.1016/0304-3959(83)90143-4. [DOI] [PubMed] [Google Scholar]
- Degner LF, Sloan JA. Symptom distress in newly diagnosed ambulatory cancer patients and as a predictor of survival in lung cancer. J Pain Symptom Manage. 1995;10(6):423–431. doi: 10.1016/0885-3924(95)00056-5. [DOI] [PubMed] [Google Scholar]
- Ferrell BR, Rhiner M, Cohen M, Grant M. Pain as a metaphor for illness. Part I: impact of cancer pain on family caregivers. Oncol Nurs Forum. 1991;18:1303–1309. [PubMed] [Google Scholar]
- Fox SW, Lyon DE. Symptom clusters and quality of life in survivors of lung cancer. Oncol Nurs Forum. 2006;33(5):931–6. doi: 10.1188/06.ONF.931-936. [DOI] [PubMed] [Google Scholar]
- Hagedoorn M, Kuijer RG, Buunk BP, DeJong GM, Wobbes T, Sanderman R. Marital satisfaction in patients with cancer: does support from intimate partners benefit those who need it the most? Health Psychol. 2000;19(3):274–82. [PubMed] [Google Scholar]
- Keefe FJ, Ahles TA, Porter LS, Sutton LM, McBride CM, Pope MS, McKinstry ET, Furstenberg CP, Dalton J, Baucom DH. The self-efficacy of family caregivers for helping cancer patients manage pain at end-of-life. Pain. 2003;1-2:157–162. doi: 10.1016/s0304-3959(02)00448-7. [DOI] [PubMed] [Google Scholar]
- Lev EL, Daley KM, Conner NE, Reith M, Fernandez C, Owen SV. An intervention to increase quality of life and self-care self-efficacy and decrease symptoms in breast cancer patients. Sch Inq Nurs Pract. 2001;15:277–94. [PubMed] [Google Scholar]
- Lev EL, Paul D, Owen SV. Age, self-efficacy, and change in patients’ adjustment to cancer. Cancer Practic. 1999;7:170–176. doi: 10.1046/j.1523-5394.1999.74004.x. [DOI] [PubMed] [Google Scholar]
- Lin C. Comparison of the effects of perceived self-efficacy on coping with chronic cancer pain and coping with chronic low back pain. Clin J Pain. 1998;14(4):303–310. doi: 10.1097/00002508-199812000-00006. [DOI] [PubMed] [Google Scholar]
- Lorig K, Chastain RL, Ung E, Shoor S, Holman HR. Development and evaluation of a scale to measure perceived self-efficacy in people with arthritis. Arthritis Rheum. 1989;32:37–44. doi: 10.1002/anr.1780320107. [DOI] [PubMed] [Google Scholar]
- Lorr M, McNair D. Profile of Mood States-B. Educational and Industrial Testing Service; San Diego: 1982. [Google Scholar]
- Manne S. Cancer in the marital context: a review of the literature. Cancer Invest. 1998;16(3):188–202. doi: 10.3109/07357909809050036. [DOI] [PubMed] [Google Scholar]
- Manne S, Glassman M. Perceived control, coping efficacy, and avoidance coping as mediators between spouses’ unsupportive behaviors and cancer patients’ psychological distress. Health Psychol. 2000;19(2):155–64. doi: 10.1037//0278-6133.19.2.155. [DOI] [PubMed] [Google Scholar]
- McCorkle R, Benoliel JQ, Donaldson G, Georgiadou F, Moinpour C, Goodell B. A randomized clinical trial of home nursing care for lung cancer patients. Cancer. 1989;64(6):1375–82. doi: 10.1002/1097-0142(19890915)64:6<1375::aid-cncr2820640634>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Mendoza TR, Wang SX, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, Huber SL. The rapid assessment of fatigue severity in cancer patients: Use of the Brief Fatigue Inventory. Cancer. 1999;85:1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Miaskowski C, Zimmer EF, Barrett KM, Dibble SL, Wallhagen M. Differences in patients’ and family caregivers’ perceptions of the pain experience influence patient and caregiver outcomes. Pain. 1997;72:217–226. doi: 10.1016/s0304-3959(97)00037-7. [DOI] [PubMed] [Google Scholar]
- Porter LS, Keefe FJ, Hurwitz H, Faber M. Disclosure between patients with gastrointestinal cancer and their spouses. Psychooncology. 2005;14(12):1030–42. doi: 10.1002/pon.915. [DOI] [PubMed] [Google Scholar]
- Porter LS, Keefe FJ, McBride CM, Pollack K, Fish L, Garst J. Perceptions of patients’ self-efficacy for managing pain and lung cancer symptoms: Correspondence between patients and family caregivers. Pain. 2002;98:169–178. doi: 10.1016/s0304-3959(02)00042-8. [DOI] [PubMed] [Google Scholar]
- Porter LS, Keefe FJ, Lipkus I, Hurwitz H. Ambivalence over emotional expression in patients with gastrointestinal cancer and their caregivers: Associations with patient pain and quality of life. Pain. 2005;117:340–348. doi: 10.1016/j.pain.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Robinson B. Validation of a Caregiver Strain Index. J Gerontology. 1983;38:344–348. doi: 10.1093/geronj/38.3.344. [DOI] [PubMed] [Google Scholar]
- Speilberger CD. Manual for the State-Trait Anxiety Inventory (Form Y) Consulting Psychologists; Palo Alto, CA: 1983. [Google Scholar]
- Stiegelis HE, Hagedoorn M, Sanderman R, Bennenbroek FTC, Buunk BP, Van den Bergh ACM, Botke G, Ranchor AV. The impact of an informational self-management intervention on the association between control and illness uncertainty before and psychological distress after radiotherapy. Psychooncology. 2004;13:248–259. doi: 10.1002/pon.738. [DOI] [PubMed] [Google Scholar]
- Syrjala KL, Chapko ME. Evidence for a biopsychosocial model of cancer treatment-related pain. Pain. 1995;61(1):69–79. doi: 10.1016/0304-3959(94)00153-6. [DOI] [PubMed] [Google Scholar]
- Uchitomi Y, Akechi T, Fujimori M, Okamura M, Ooba A. Mental adjustment after surgery for non-small cell lung cancer. Palliat Support Care. 2003;1(1):61–70. doi: 10.1017/s1478951503030050. [DOI] [PubMed] [Google Scholar]
- Wang XS, Fairclough DL, Liao Z, Komaki R, Chang JY, Mobley GM, Cleeland CS. Longitudinal study of the relationship between chemoradiation therapy for non-small-cell lung cancer and patient symptoms. J Clin Oncol. 2006;24(27):4485–91. doi: 10.1200/JCO.2006.07.1126. [DOI] [PubMed] [Google Scholar]