Abstract
Obstructive sleep apnea (OSA) is associated with several pathophysiological conditions, including hypertension, obesity, insulin resistance, hypothalamic-pituitary-adrenal (HPA) dysregulation, and other endocrine and metabolic disturbances comprising the “metabolic syndrome”. Repeated episodes of hypoxia in OSA may represent a chronic intermittent stress, leading to HPA dysregulation. Alterations in HPA reactivity could then contribute to or exacerbate other pathophysiological processes. We showed previously that another metabolic stressor, chronic intermittent cold stress, enhanced noradrenergic facilitation of acute HPA stress reactivity. In this study, we investigated whether chronic intermittent hypoxia (CIH), a rat model for the arterial hypoxemia that accompanies OSA, similarly sensitizes the HPA response to novel acute stress. Rats were exposed to CIH (alternating cycles of normoxia [3 min at 21% O2] and hypoxia [3 min at 10% O2], repeated continuously for 8 hr/day during the light portion of the cycle for 7 days). On the day after the final CIH exposure, there were no differences in baseline plasma ACTH, but the peak ACTH response to 30 min acute immobilization stress was greater in CIH-stressed rats than in controls. Induction of Fos expression by acute immobilization stress was comparable following CIH in several HPA-modulatory brain regions, including the paraventricular nucleus, bed nucleus of the stria terminalis, and amygdala. Fos induction was attenuated in lateral hypothalamus, an HPA-inhibitory region. By contrast, acute Fos induction was enhanced in noradrenergic neurons in the locus coeruleus following CIH exposure. Thus, similar to chronic cold stress, CIH sensitized acute HPA- and noradrenergic stress reactivity. Plasticity in the acute stress response is important for long-term adaptation, but may also contribute to pathophysiological conditions associated with states of chronic or repeated stress, such as OSA. Determining the neural mechanisms underlying these adaptations may help us better understand the etiology of such disorders, and inform the development of more effective treatments.
Keywords: adrenocorticotropic hormone, metabolic syndrome, norepinephrine, obstructive sleep apnea, paraventricular nucleus, stress
Obstructive sleep apnea (OSA) is a complex pathophysiological state defined by a disordered breathing pattern that induces repeated episodes of hypoxia during sleep, as frequently as 100–600 times per night (Sullivan and Issa, 1985, Ohayon et al., 2000, Vgontzas et al., 2003, Coughlin et al., 2004). So defined, OSA afflicts up to 7% of adult males, but a more liberal definition of “mild” OSA, characterized by >5 hypoxic events per hour may include as many as 17–24% of adult males (see Vgontzas et al., 2003). In addition to disordered breathing and sleep disruption, the OSA syndrome is also characterized by other physiologic and metabolic disturbances, including obesity, insulin resistance, glucose intolerance, dyslipidemia, endocrine dysregulation and arterial hypertension (Smith et al., 1998, Ohayon et al., 2000, Pankow et al., 2000, Vgontzas et al., 2003, Coughlin et al., 2004, Lanfranco et al., 2004, Buckley and Schatzberg, 2005). The increased cardiovascular morbidity of OSA has been associated independently with sleep-disordered breathing, i.e., it is not attributable secondarily to other factors such as obesity (Ohayon et al., 2000, Coughlin et al., 2004). Likewise, dysregulation of the neuroendocrine hypothalamic-pituitary-adrenal (HPA) stress axis has also been shown to be associated independently with sleep apnea (Bratel et al., 1999, Lanfranco et al., 2004, Buckley and Schatzberg, 2005).
Hypoxia, as a metabolic stressor, activates the HPA axis, both acutely and chronically (Raff et al., 1986, Jacobson and Dallman, 1989, Raff, 1996, Mueller et al., 2004). Activation of the HPA axis represents the major neuroendocrine response to acute stress, whereby neurons in the hypothalamic paraventricular nucleus (PVN) release corticotropin-releasing hormone (CRH) into the pituitary portal system to stimulate secretion of adrenocorticotropic hormone (ACTH) from the anterior pituitary, which in turn elicits release of adrenal corticosteroids (reviewed in Herman and Cullinan, 1997). Secondary regions in the limbic forebrain, including the lateral bed nucleus of the stria terminalis (BSTL), central and medial amygdala (CeA and MeA), and lateral hypothalamus (LH) regulate this response in various contexts (Dunn, 1987, Feldman et al., 1994, Herman and Cullinan, 1997, Cecchi et al., 2002, Ma and Morilak, 2005b). In addition to these primary circuits that mediate or regulate activation of the HPA axis, other brain systems also play a more widespread modulatory role in coordinating, integrating and modifying the systemic stress response. One such system is the brain noradrenergic system, also activated by hypoxia (Elam et al., 1981, Smith et al., 1995, McDonald et al., 2000). Stress-induced release of norepinephrine (NE) plays an important modulatory function in many brain regions, facilitating synaptic transmission in circuits that mediate or regulate specific physiological responses evoked by stress (Berridge and Waterhouse, 2003, Morilak et al., 2005a). We have shown previously that stress-induced release of NE in PVN, BSTL and MeA facilitates activation of the HPA axis elicited in response to acute immobilization stress (Cecchi et al., 2002, Ma and Morilak, 2005b).
Chronic intermittent stress, such as the repeated hypoxia that occurs in OSA, can invoke long-term regulatory plasticity in the HPA axis. For example, we and others have shown that another metabolic stressor, chronic intermittent cold stress, can sensitize the acute reactivity of the HPA axis, exaggerating the response evoked upon subsequent exposure to a novel acute heterotypic stimulus such as immobilization stress (Bhatnagar and Dallman, 1998, Pardon et al., 2003). Sensitization of the HPA axis has been postulated to arise from regulatory alterations in the activity or efficacy of afferent systems that modulate stress response circuits in the forebrain (Hauger et al., 1990, Dallman et al., 1992, Melia et al., 1994, Stam et al., 2000, Pardon et al., 2003). After exposure to chronic intermittent cold stress, the acute induction of Fos expression in many brain regions was enhanced, suggesting neural substrates that may possibly be responsible for the HPA sensitization, including the noradrenergic nucleus locus coeruleus (LC), the PVN, amygdala and to a lesser extent the BSTL (Bhatnagar and Dallman, 1998, Bhatnagar et al., 2000). Similar, albeit more limited observations have been made after chronic intermittent hypoxia (Sica et al., 2000). Consistent with the greater induction of Fos seen in noradrenergic cell groups, chronic intermittent cold stress has also been shown to sensitize the acute excitability of brain noradrenergic neurons (Mana and Grace, 1997, Jedema et al., 2001), amplify the acute stress-induced release of NE in forebrain limbic areas such as hippocampus, medial prefrontal cortex and BSTL (Nisenbaum et al., 1991, Finlay et al., 1995, Pardon et al., 2003), enhance noradrenergic facilitation of the HPA response to acute stress in the BSTL (Pardon et al., 2003), and enhance the sensitivity of the HPA axis to direct activation by α1-adrenergic receptors in the PVN (Ma and Morilak, 2005a).
Such neuroendocrine adaptations evoked by chronic or repeated stress can serve to maintain homeostasis and enhance survival in the face of an unmitigated threat or challenge, but prolonged or excessive activation of the stress response can itself become maladaptive, contributing to the variety of negative health consequences associated with chronic stress (McEwen, 2000). Chronic stress and long-term dysregulation of the stress response are well-known predisposing factors in many pathophysiologic conditions, including cardiovascular disease (Cossette et al., 2001) and the metabolic syndrome (Chrousos, 2000). The repeated episodes of hypoxia experienced by OSA patients may thus represent a chronic intermittent stressor, and as such may induce dysregulation of the HPA axis, contributing to the pathology associated with OSA. In this study we investigated the potential sensitizing effects of CIH exposure on acute HPA stress reactivity in rats. To then begin to address possible neural substrates underlying any such plasticity induced by CIH in the acute HPA response to a novel heterotypic stressor, we also investigated the effects of CIH exposure on the induction by acute immobilization stress of Fos expression in the PVN, BST, MeA and CeA, and in brainstem noradrenergic neurons. Portions of this work have been presented in abstract form (Morilak et al., 2005b).
Experimental Procedures
Animals
A total of 59 male Sprague-Dawley rats (Harlan, Indianapolis, IN), weighing 250–275 g upon arrival, were used in these experiments. They were allowed to acclimatize to the animal facility for at least one week prior to use in any experimental procedures. The housing facility was maintained on a 12/12 hr light cycle, with lights on at 07:00 hr. Animals were housed initially three per cage, with ad libitum access to food and water, then singly housed after any surgical procedure and during the conduct of the experiments. All animal procedures were conducted according to NIH guidelines, and were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio.
Chronic intermittent hypoxia (CIH)
In each experiment, rats were randomly assigned to either a control or CIH condition. Rats in both conditions were singly housed, and their home cages, with food and water, were relocated into custom-built plexiglas chambers one day before beginning the one-week treatment period. All rats had unlimited 24-hr access to food and water throughout the experiment. The O2 level in each chamber was monitored and regulated by timer-controlled valves connected to room air and to an N2 source, which entered the chamber via separate flow meters (Hinojosa-Laborde and Mifflin, 2005). The CIH treatment was applied for 7 days, 8 hrs/day during the light phase, beginning 1 hour after lights on (08:00–16:00 hr). During this time, O2 was reduced from 21% to 10% over 1.5 min, held at 10% for 1.5 min, returned to 21% over 1 min, and held at 21% for 2 min. This cycle repeated continuously for 8 hr. Control animals were housed in identical chambers for an equivalent amount of time, and were exposed to the same timer- and valve-controlled changes in air flow as the CIH rats. However, the only source of gas in the control chambers was room air, so they remained at normoxic levels throughout the protocol. Immediately after the final hypoxia session, the cages were returned to the main housing room. All experiments were conducted on day 8, i.e., the day after the last exposure to intermittent hypoxia. This protocol has been shown to increase mean arterial pressure in male rats by approximately 6 mm Hg, and to increase heart rate by approximately 20 beats per minute (Hinojosa-Laborde and Mifflin, 2005).
Experiment 1: Effects of CIH exposure on HPA reactivity to acute immobilization stress
A total of 14 rats were used in this experiment. Surgery was conducted 3 days before beginning the CIH treatment. Animals were anesthetized with a cocktail of ketamine 43 mg/kg, acepromazine 1.4 mg/kg, and xylazine 8.6 mg/kg, given in a volume of 1.0 ml/kg, i.m. An indwelling silastic catheter was implanted into the jugular vein, then exteriorized at the back of the neck and loaded with heparinized saline (50 U/ml) to maintain patency. Rats were housed singly for 2 days before relocating them to the hypoxia chambers, one day before beginning the CIH treatment as described above. Control rats were treated identically, but were exposed only to room air.
On the day following the last CIH session, the experiment was conducted between 09:00–12:00. Rats were transported to the testing room, and the venous catheter connected via PE tubing to a 1 ml syringe filled with heparin-saline (50 U/ml) for repeated blood sampling. After allowing 2 hr acclimitization to the testing room, a baseline blood sample (0.4 ml) was drawn. Rats were then subjected to 30 min acute immobilization stress by holding them prone on a flat, plastic rack large enough to support their body securely (26 cm × 13 cm) while their limbs were taped gently but securely to the rack with medical adhesive tape. Strips of tape were placed across the body and back of the head to prevent excessive head movements. This procedure took approximately 1 min, and the duration of the stress period was 30 min from that point. A blood sample was taken after 5 min stress, and another after 30 min, immediately before the animals were released at the end of the stress period. At the termination of the stress period, the animal was held in place gently while the tape was removed with scissors, and then returned to its home cage. Additional blood samples were taken after 15, 30 and 60 min of post-stress recovery. All blood samples were replaced immediately by infusing an equivalent volume of sterile saline. We have determined in previous experiments that this repeated sampling and replacement procedure does not affect plasma ACTH measures (see Cecchi et al., 2002, Ma and Morilak, 2005b). Blood was collected into tubes containing 10 μl of 1.0 M EDTA. Plasma was separated by centrifugation at 12,000 rpm for 5 min at 4° C, and stored at −80°C until assayed. Plasma ACTH levels were determined in 200 μl samples by radioimmunoassay (Nichols Institute Diagnostics, San Juan Capistrano, CA). The detection limit of the assay was 15 pg/ml. Intra-assay and inter-assay coefficients of variation were 9% and 11%, respectively.
Baseline ACTH levels were first compared in rats exposed to CIH and control rats using a t-test. Responses to acute stress were then analyzed by 2-way ANOVA with repeated measures, with CIH Treatment as the between-subjects factor and Time as the within-subject factor. Post hoc comparisons were conducted using the Newman Keuls test to identify effects of CIH on ACTH secretion at each time point, including the response to stress. Significance was determined at p < 0.05 in all analyses.
Experiment 2: Effects of CIH exposure on Fos induction by acute immobilization stress
A total of 40 unoperated rats were used in this experiment. After one-day habituation to the CIH chambers, the one-week CIH and control treatments were applied as above. On day 8, rats in each chronic treatment condition were further subdivided into 3 groups (n=5–9 rats/group). Unstressed rats from each treatment condition were sacrificed with no further treatment (Control n=8; CIH n=5) and the other two groups were subjected to acute immobilization stress as described above, with the exception that the duration of immobilization was 60 min, determined in previous experiments to be optimal for induction of Fos expression (Ma and Morilak, 2004). One group of stressed rats from each treatment condition were sacrificed immediately at the termination of the 60 min stress (Control n=8; CIH n=5). The remaining groups were sacrificed 1 hr after termination of the stress (i.e., 2 hr after the onset of 60 min stress), during which they recovered in their home cages (Control n=9; CIH n=5).
At the designated time point, rats were deeply anesthetized and perfused transcardially with 50 ml of 100 mM phosphate-buffered saline (PBS), pH 7.4 containing 1000 IU/ml heparin, followed by 350 ml of 4% paraformaldehyde in PBS. Brains were post-fixed for 1 hr in the same fixative, rinsed and then cryoprotected for 48 h in 20% sucrose-PBS at 4 °C. After the brains had sunk, they were frozen in isopentane on dry ice and stored at −70 °C until sectioned and processed (< 1 month).
Three series of 40 μm sections were collected from each brain. Sections were stored in cryoprotectant (Hoffman et al., 1992) at −20°C until they were processed for immunohistochemistry. Forebrain and hindbrain sections were processed separately. Fos immunohistochemistry was conducted as previously described (Gottlieb et al., 2006) in sets that included at least one member from each treatment group. Forebrain and hindbrain sections were stained for Fos using a commercially available antibody directed at the amino acid residues 4–17 in human c-Fos (Rabbit anti-c-Fos Ab5, Calbiochem, San Diego, CA). After the sections were treated with 3% heat-inactivated horse serum and hydrogen peroxide, they were incubated in the primary antibody (1:30,000) for 72 h at 4°C. The sections were incubated in a biotyinlated horse anti-rabbit secondary antibody (Vector Laboratories, Burlingame, CA) that was diluted 1:200 in PBS for 2 h at room temperature. After an additional 60-min rinse in PBS, sections were reacted with an avidin-peroxidase conjugate (Vectastain ABC Kit; Vector Laboratories) and PBS containing 0.04% 3,3′–diaminobenzidine hydrochloride and 0.04% nickel ammonium sulfate. Hindbrain sections were also stained for dopamine-β-hydroxylase (DBH) using a commercially available antibody (mouse anti-dopamine-β-hydroxylase, MAB308 Chemicon Int., Temecula, CA; diluted 1:1,000). After a 60-min PBS rinse, these sections were incubated in a Cy3 labeled anti-mouse secondary antibody (Jackson ImmunoResearch, West Grove, PA) diluted 1:250 for 3h at room temperature. After staining was complete, sections were mounted on gelatin coated slides, air dried for 1–2 days, and coverslipped with Permount.
Tissue sections containing regions of interest were analyzed using an Olympus microscope (IX 50) equipped for epifluorescence. Digital images were acquired using a Spot camera (SPOT RT Slider, Diagnostic Instruments, Sterling Heights, MI) connected to a Pentium computer running Spot imaging software (v.3.24). Areas were identified using the atlas of Paxinos and Watson (Paxinos and Watson, 1998). For analysis, three to six images were obtained from each region.
Forebrain regions examined included the amygdala (medial and central nuclei together), bed nucleus of the stria terminalis (medial, ventral and lateral subdivisions), lateral hypothalamus and PVN. The magnocellular and parvocellular portions of the PVN were analyzed separately. Hindbrain regions included locus coeruleus, rostral ventrolateral medulla, caudal ventrolateral medulla and the nucleus tractus solitarius. In the brainstem, the portion of the NTS used for the analysis included images from 300 μm caudal to obex and 300–400 μm past the rostral edge of the area postrema (Randolph et al., 1998, Curtis et al., 1999). The noradrenergic A1 region was defined at its posterior extent by the pyramidal decussation, and at its anterior extent by the appearance of the principle nucleus of the inferior olive (Randolph et al., 1998, Curtis et al., 1999). The number of DβH and Fos positive cells were recorded for the A2 region of the NTS, A1, and the locus coeruleus.
Cells counts were made from the same set of digital images by three observers who were blind to the identity of the samples. Counts from each observer were averaged for each image. The data from the cell counts were analyzed by two-way analysis of variance with Student-Newman-Keuls t-test for post hoc analysis of significant main effects (SigmaStat, v. 2.03, Systat Software Inc., Point Richmond, CA). Significance was set at P < 0.05. All values are presented as mean ± SEM.
Results
Experiment 1: Effects of CIH exposure on HPA reactivity to acute immobilization stress
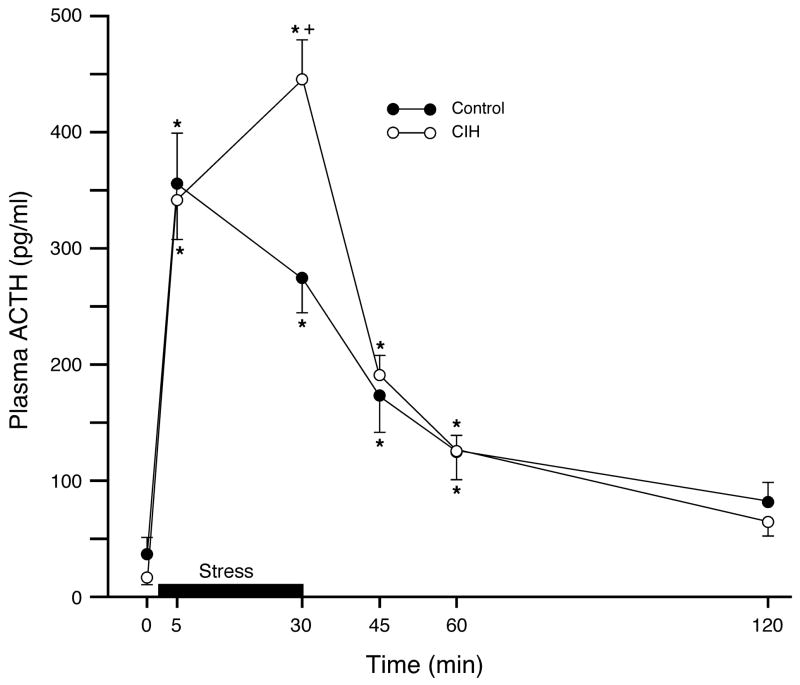
There were no differences detected in baseline plasma ACTH levels between animals exposed to CIH and control rats (Control: 36.4 ± 15.1 pg/sample; CIH: 12.2 ± 2.6 pg/sample; mean ± SEM; n=7/group; t12 = 1.47, p = 0.17). Analysis of the response to subsequent acute immobilization stress revealed a significant main effect of Time (F5,60 = 80.146, p < 0.0001). There was not an overall main effect of chronic treatment (F1,12 = 0.688, p = 0.42), but a highly significant Time × Treatment interaction (F5,60 = 5.634, p < 0.0003). Post hoc analysis indicated that stress significantly increased plasma ACTH concentrations in both treatment groups, returning to baseline after 60 min post-stress recovery (Figure 1), and that the acute ACTH response to immobilization stress was greater in the CIH-exposed rats than in controls (Figure 1). Whereas the response in control animals peaked at 5 min and then decreased slightly by 30 min, the plasma ACTH response in rats exposed to CIH continued to increase at the 30 min time point, at which time it was significantly higher than in controls.
Figure 1.
Sensitizing effect of chronic intermittent hypoxia on subsequent activation of the HPA axis in response to a novel heterotypic challenge, acute immobilization stress. There were no baseline differences in plasma ACTH between groups. Acute immobilization stress (bar) significantly increased plasma ACTH concentrations in both groups. However, the response at the 30 min time point was significantly elevated in CIH-exposed rats compared to controls, indicating sensitization. Data expressed as mean ± SEM; n = 7/group; *p < 0.05 for each group compared to their respective baseline; +p < 0.05 for CIH-exposed rats compared to controls at the same time point.
Experiment 2: Effects of CIH exposure on Fos induction to acute immobilization stress
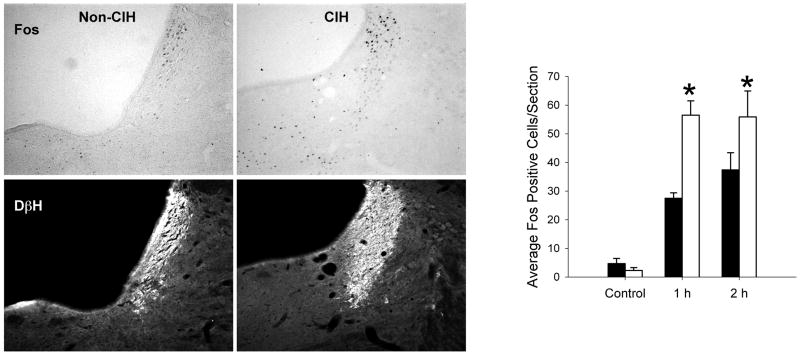
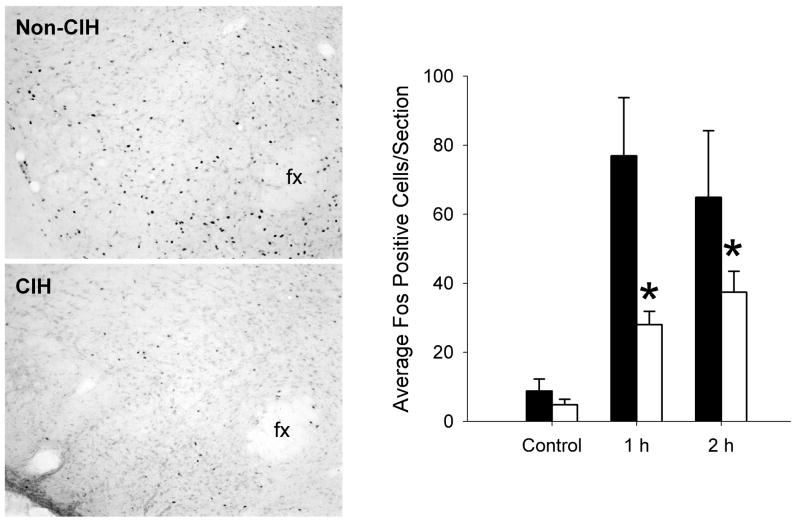
In the brain regions studied, basal levels of Fos staining were very low in both control and CIH rats that were not exposed to acute immobilization stress. In the LC, prior exposure to CIH significantly influenced the induction of Fos expression by acute immobilization stress (main effect of Time: F2,27 = 51.01, p < 0.001; Chronic Treatment: F1,27 = 14.96, p < 0.001; Time × Treatment interaction: F2,27 = 5.88, p < 0.01). There was significantly greater Fos staining in rats previously exposed to CIH at 1 hr and 2 hr following immobilization stress (Figure 2). By contrast, prior exposure to CIH was associated with decreased Fos staining following immobilization stress in the LH. Analysis of the perifornical region of the LH indicated significant main effects of Time (F2,28 = 5.23, p < 0.01) and Chronic Treatment (F1,28 = 4.29, p < 0.05), but not a significant interaction (F2,28 = 0.96, p = 0.40). Post hoc analysis of the significant main effect of chronic treatment indicated that the rats exposed to CIH had significantly lower Fos staining in the LH compared to controls (Figure 3).
Figure 2.
Acute immobilization stress-induced Fos expression in the locus coeruleus (LC) was sensitized following chronic intermittent hypoxia. Left: Representative examples of dual-immunohistochemical staining for Fos immunoreactivity (DAB immunoperoxidase labeling, top panels) and DβH (Cy3 immunofluorescence, bottom panels) in a single section cut through the LC of a rat given no chronic pretreatment (non-CIH) and in a rat exposed to 1 week chronic intermittent hypoxia (CIH). Both rats were sacrificed after 60 min of recovery following 60 min acute immobilization stress (i.e., 2 hours after acute stress onset). Right: Summary data showing the effects of CIH on the induction of Fos immunoreactvity in the LC by acute immobilization stress. Black bars represent rats with no chronic treatment prior to immobilization stress, and white bars represent rats exposed to chronic intermittent hypoxia for 7 day before receiving acute immobilization stress. Control indicates rats with no exposure to immobilization stress; 1 h indicates rats perfused immediately after one hour of immobilization stress; 2 h indicates rats exposed to 1 hour of immobilization stress then 1 hour of recovery prior to perfusion. *p < 0.05 compared to the group with no CIH treatment. n = 5–9 rats per group. Data expressed as mean ± SEM.
Figure 3.
Acute immobilization stress-induced Fos expression in the lateral hypothalamus (LH) was attenuated following chronic intermittent hypoxia. Left: Representative examples of Fos immunoreactivity in the perifornical region of the LH of a rat given no chronic pretreatment (non-CIH) and in a rat exposed to 1 week chronic intermittent hypoxia (CIH). Both rats were sacrificed immediately after 60 min acute immobilization stress. fx, fornix; medial is to the right. Right: Summary data showing the effects of CIH on the induction of Fos immunoreactvity in the LH by acute immobilization stress. Black bars represent rats with no chronic treatment prior to immobilization stress, and white bars represent rats exposed to chronic intermittent hypoxia for 7 day before receiving acute immobilization stress. Control indicates rats with no exposure to immobilization stress; 1 h indicates rats perfused immediately after one hour of immobilization stress; 2 h indicates rats exposed to 1 hour of immobilization stress then 1 hour of recovery prior to perfusion. *p < 0.05 compared to the group with no CIH treatment. n = 5–9 rats per group. Data expressed as mean ± SEM.
In the parvocellular and magnocellular portions of the PVN, there were significant main effects of Time (parvocellular PVN: F2,33 = 11.2, p < 0.001; magnocellular PVN: F2,33 = 51.0, p < 0.001), but no main effect of Chronic Treatment, nor a Time × Treatment interaction. Post hoc analyses indicated that Fos staining in the PVN was significantly increased at both the 1 hr and 2 hr time points compared to non-stressed controls, while the difference between 1 hr and 2 hr was not statistically significant (Table 1). Similar results were observed in the majority of other brain regions analyzed (see Table 1).
Table 1.
Average number of Fos-positive cells per section induced by acute immobilization stress (60 min). Each of these regions showed significant increases in Fos staining following acute immobilization stress that were not affected by prior exposure to CIH. “Control” indicates rats perfused with no exposure to immobilization stress, 1 hr indicates rats perfused immediately after one hour of immobilization stress, 2 hr indicates rats perfused two hours after the start of 1 hour immobilization stress.
| Non-CIH | CIH | |||||
|---|---|---|---|---|---|---|
| Region | Control | 1 hr | 2 hr | Control | 1 hr | 2 hr |
| NTS | 9.0 ± 3 | 59.0 ± 11 | 68.0 ± 9 | 8.9 ± 3 | 71.5 ± 6 | 69.3 ± 5 |
| A2 | 2.2 ± 1 | 19.1 ± 5 | 23.1 ± 5 | 2.6 ± 1 | 22.5 ± 1 | 19.1 ± 1 |
| CVL | 1.8 ± 0.6 | 19.4 ± 4 | 22.5 ± 4 | 1.3 ± 1 | 19.7 ± 1 | 18.8 ± 1 |
| A1 | 1.0 ± 0.3 | 11.5 ± 3 | 11.4 ± 2 | 1.0 ± 0.1 | 11.4 ± 1 | 10.2 ± 1 |
| RVLM | 2.1 ± 1 | 31.1 ± 2 | 41.0 ± 1 | 3.6 ± 1 | 38.2 ± 3 | 34.8 ± 2 |
| PVNp | 11.3 ± 2 | 141.1 ± 28 | 173.5 ± 40 | 10.1 ± 2 | 137.2 ± 11 | 148.8 ± 14 |
| PVNm | 6.9 ± 1 | 69.0 ± 15 | 56.4 ± 4 | 4.1 ± 0.5 | 38.1 ± 3 | 66.5 ± 11 |
| BSTm | 5.8 ± 1 | 17.2 ± 3 | 18.3 ± 2 | 6.6 ± 1 | 15.1 ± 2 | 16.2 ± 1 |
| BSTl | 11.1 ± 1.5 | 38.7 ± 5 | 32.3 ± 5 | 14.7 ± 1 | 26.8 ± 2 | 31.4 ± 2 |
| BSTv | 12.2 ± 1.5 | 41.2 ± 4 | 36.0 ± 3 | 21.2 ± 2 | 36.6 ± 4 | 40.4 ± 3 |
| Amyg | 11.6 ± 2 | 58.8 ± 8 | 64.1 ± 5 | 10.0 ± 2 | 57.7 ± 10 | 61.4 ± 10 |
Discussion
In this study, activation of the rat HPA axis by an acute stressor was enhanced following a period of chronic intermittent hypoxia, presented during the portion of the circadian cycle when the rats were asleep, as a model of the arterial hypoxemia that accompanies obstructive sleep apnea (OSA) in humans. The mechanisms whereby chronic intermittent hypoxia may induce such regulatory alterations in the acute physiological response to a subsequent acute stress may contribute to the detrimental consequences of chronic stress in general, or to certain pathophysiological conditions associated specifically with OSA.
OSA is characterized by a disordered breathing pattern that results in repeated episodes of hypoxia occurring during sleep (Ohayon et al., 2000, Vgontzas et al., 2003, Coughlin et al., 2004), typically up to 100–600 times per night (Sullivan and Issa, 1985). The severity of OSA is quantified as the number of apneic and hypopneic episodes that occur in an hour. An “apnea:hypopnea index” of 5–15 indicates a mild case of OSA and is estimated to occur in 20% of the adult U.S. population (Shamsuzzaman et al., 2003). According to this classification scheme, the parameters used in our study (10 episodes per hour) mimic a mild case of OSA. In rodents, similar exposures to CIH induce many of the pathological findings observed in OSA patients and in patients with the metabolic syndrome: a persistent increase in blood pressure (Lesske et al., 1997, Hinojosa-Laborde and Mifflin, 2005); elevated sympathetic outflow and reactivity (Greenberg et al., 1999); insulin resistance (Iiyori et al., 2007); and hyperlipidemias (Li et al., 2005).
It is well-established that hypoxia activates the HPA axis in humans and experimental animals, both acutely and chronically (Raff et al., 1986, Jacobson and Dallman, 1989, Raff, 1996, Mueller et al., 2004). Thus, the repeated induction of intermittent hypoxia in OSA potentially represents a chronic metabolic stressor, and OSA has been associated with elevated reactivity of the HPA stress axis (Bratel et al., 1999, Buckley and Schatzberg, 2005). Sensitization of the HPA axis in response to a heterotypic acute stressor has been well characterized after exposure to chronic intermittent cold stress, another type of chronic metabolic stressor (Dallman et al., 1992, Bhatnagar and Dallman, 1998, Pardon et al., 2003, Ma and Morilak, 2005a). It is thus possible that some of the pathological conditions associated with OSA, such as hypertension and sympathetic hyperactivity, may be related to HPA sensitization and to the detrimental consequences of chronic repeated stress.
The brain noradrenergic system is activated by, and plays a role in the response to a variety of physiologic and homeostatic challenges (e.g., Morilak et al., 1987, Svensson, 1987, Valentino et al., 1991), including hypoxia (Elam et al., 1981, Smith et al., 1995, McDonald et al., 2000). Originating primarily in the LC and in the A1 and A2 cell groups of the caudal ventrolateral medulla and NTS, the noradrenergic system projects widely throughout the brain, modulating the integrated response to a variety of acutely stressful challenges. At a cellular level, the effect exerted by NE on target neurons is modulatory in nature. Rather than simple inhibition or activation, NE alters the “signal to noise ratio” of evoked activity, making synaptic transmission in target circuits more effective (Woodward et al., 1991). Thus, stress-induced release of NE facilitates evoked responses in many CNS circuits, enhancing the acute physiological response capabilities of the organism.
The results of the current study suggest that alterations in the stress-activated modulatory function of NE may play a role in sensitizing the HPA response to acute stress following a period of chronic intermittent hypoxia. Noradrenergic afferents from A1, A2 and LC innervate the PVN (Sawchenko and Swanson, 1982, Liposits et al., 1986, Cunningham et al., 1990), where noradrenergic terminals make synaptic contact with neurons that synthesize CRH (Liposits et al., 1986), the final common output for HPA activation. Acute stress elicits robust NE release in PVN (Pacak et al., 1992, Ma and Morilak, 2005a), which acts via α1-receptors to facilitate activation of the HPA axis (Gibson et al., 1986, Plotsky, 1987, Szafarczyk et al., 1987, Kiss and Aguilera, 1992, al-Damluji, 1993, Ma and Morilak, 2005a). Along with the increase in Fos expression induced by acute immobilization stress in noradrenergic neurons in the LC, as defined by co-expression of DβH in the present study, we have reported previously that exposure to chronic stress can sensitize the response of post-synaptic α1-adrenergic receptors in PVN (Ma and Morilak, 2005a), which may also contribute to sensitization of HPA reactivity. Further, in addition to the primary output neurons located in PVN, the HPA axis is also regulated secondarily by several extrahypothalamic forebrain regions, including the BSTL and amygdala, through both direct and indirect connections to PVN (Canteras et al., 1995, Cullinan et al., 1995, Herman and Cullinan, 1997, Dayas et al., 2001, Dayas and Day, 2002). We have shown previously that the functions of the BSTL and medial amygdala in facilitating reactivity of the HPA axis are also modulated by stress-induced release of NE acting via α1-adrenergic receptors (Cecchi et al., 2002, Ma and Morilak, 2005b). Indeed, we have found that the modulatory influence of NE in both BSTL and PVN is an important factor in sensitization of the HPA axis following chronic intermittent cold exposure (Pardon et al., 2003, Ma and Morilak, 2005b).
By contrast with the enhanced Fos induction seen in noradrenergic LC neurons following CIH, acute immobilization stress-induced Fos expression was attenuated in the perifornical region of the lateral hypothalamus after CIH treatment. This region of the hypothalamus, situated immediately adjacent to the PVN, contains GABAergic neurons that innervate PVN and serve to inhibit activation of the HPA axis. In turn, the LH receives inputs from several extra-hypothalamic brain regions that exert both inhibitory and excitatory (i.e., disinhibitory) influences on the HPA axis (see reviews in Herman et al., 2003, Herman et al., 2005). Thus, attenuation of the acute stress-induced activation of this HPA-inhibitory region could also have contributed to the enhanced HPA stress reactivity following CIH treatment.
Conclusion
To conclude, the results of the present study indicate that the HPA axis was sensitized following chronic intermittent hypoxia, similar to the sensitization seen after chronic intermittent cold stress. Specifically, after exposure to CIH, subsequent HPA activation in response to a novel, acute immobilization stress was enhanced. More generally, as an animal model of the arterial hypoxemia that accompanies sleep apnea, CIH induces many of the same pathophysiological consequences observed in OSA. Therefore, these findings may have implications for understanding the mechanisms underlying such pathology in OSA patients. For instance, there is a well-established relationship between OSA and the metabolic syndrome (Coughlin and Calverley, 2001, Vgontzas et al., 2003, Coughlin et al., 2004). The relationships between glucocorticoid levels and both cardiovascular disease (Walker, 2007), and metabolic syndrome (Wang, 2005, Witchel and DeFranco, 2006), suggest that enhanced stress activation of the HPA axis could contribute, at least in part, to the pathology of OSA. Further, as the present results indicated that activation of the ascending noradrenergic system by an acute heterotypic immobilization stress was also enhanced following chronic intermittent hypoxia, it is possible that chronic stress-induced plasticity in the noradrenergic system may have contributed to the HPA sensitization seen following chronic intermittent hypoxia. Increased noradrenergic stress reactivity may also, therefore, play a role in enhancing acute HPA reactivity in individuals with OSA, thus representing a potential central mechanism contributing to stress-related pathophysiology associated with OSA.
Acknowledgments
Expert technical assistance was provided by Tiffany M. Fleming and Myrna Herrera-Rosales. This work was supported by a Presidential Research Enhancement Fund (PREF) award from the University of Texas Health Science Center at San Antonio, and by research grants from the National Institutes of Health (MH053851 and MH072672 to DAM; HL062579 to JTC; HL086804 to SWM). The authors have no conflicts of interest to report, financial or otherwise, that may have influenced the conduct, interpretation or presentation of these studies.
Abbreviations
- ACTHA
Adrenocorticotropic hormone
- ANOVA
Analysis of variance
- BSTm
Bed nucleus of the stria terminalis, medial subdivision
- BSTL
Bed nucleus of the stria terminalis, lateral subdivision
- BSTv
Bed nucleus of the stria terminalis, ventral subdivision
- CeA
Central nucleus of the amygdala
- CIH
Chronic intermittent hypoxia
- CNS
Central nervous system
- CRH
Corticotropin-releasing hormone
- CVL
Caudal ventrolateral medulla
- DAB
3,3′–diaminobenzidine
- DBH
Dopamine-β-hydroxylase
- EDTA
Ethylenediaminetetraacetic acid
- fx
Fornix
- HPA
Hypothalamic-pituitary-adrenal
- LC
Locus coeruleus
- LH
Lateral hypothalamus
- MeA
Medial nucleus of the amygdala
- NE
Norepinephrine
- NTS
Nucleus tractus solitarius
- OSA
Obstructive sleep apnea
- PBS
Phosphate-buffered saline
- PVN
Paraventricular nucleus
- RVLM
Rostral ventrolateral medulla
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- al-Damluji S. Adrenergic control of the secretion of anterior pituitary hormones. Bailliere’s Clin Endocrinol Metabol. 1993;7:355–392. doi: 10.1016/s0950-351x(05)80180-6. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Revs. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84:1025–1039. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Viau V, Chu A, Soriano L, Maijer OC, Dallman MF. A cholecystokinin-mediated pathway to the paraventricular thalamus is recruited in chronically stressed rats and regulates hypothalamic-pituitary-adrenal function. J Neurosci. 2000;20:5564–5573. doi: 10.1523/JNEUROSCI.20-14-05564.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratel T, Wennlund A, Carlstrom K. Pituitary reactivity, androgens and catecholamines in obstructive sleep apnoea. Effects of continuous positive airway pressure treatment (CPAP. Resp Med. 1999;93:1–7. doi: 10.1016/s0954-6111(99)90068-9. [DOI] [PubMed] [Google Scholar]
- Buckley TM, Schatzberg AF. On the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: Normal HPA axis activity and circadian rhythm, exemplary sleep disorders. J Clin Endocrin Metab. 2005;90:3106–3114. doi: 10.1210/jc.2004-1056. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Organization of projections from the medial nucleus of the amygdala: A PHAL study in the rat. J Comp Neurol. 1995;360:213–245. doi: 10.1002/cne.903600203. [DOI] [PubMed] [Google Scholar]
- Cecchi M, Khoshbouei H, Javors M, Morilak DA. Modulatory effects of norepinephrine in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuroscience. 2002;112:13–21. doi: 10.1016/s0306-4522(02)00062-3. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. The role of stress and the hypothalamic-pituitary-adrenal axis in the pathogenesis of the metabolic syndrome: Neuro-endocrine and target tissue-related causes. Int J Obes. 2000;24(Suppl 2):S50–S55. doi: 10.1038/sj.ijo.0801278. [DOI] [PubMed] [Google Scholar]
- Cossette S, Frasure-Smith N, Lesperancé F. Clinical implications of a reduction in psychological distress on cardiac prognosis in patients participating in a psychosocial intervention program. Psychosom Med. 2001;63:257–266. doi: 10.1097/00006842-200103000-00009. [DOI] [PubMed] [Google Scholar]
- Coughlin SR, Calverley P. Sleep disordered breathing - a new component of syndrome X? Obesity Rev. 2001;2:267–274. doi: 10.1046/j.1467-789x.2001.00045.x. [DOI] [PubMed] [Google Scholar]
- Coughlin SR, Mawdsley L, Mugarza JA, Calverley PMA, Wilding JPH. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J. 2004;25:735–741. doi: 10.1016/j.ehj.2004.02.021. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Helmreich DL, Watson SJ. A neuroanatomy of stress. In: Friedman MJ, et al., editors. Neurobiological and clinical consequences of stress: From normal adaptation to PTSD. Lippincott-Raven Publishers; Philadelphia: 1995. pp. 135–147. [Google Scholar]
- Cunningham ET, Bohn MC, Sawchenko PE. Organization of adrenergic inputs to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J Comp Neurol. 1990;292:651–667. doi: 10.1002/cne.902920413. [DOI] [PubMed] [Google Scholar]
- Curtis KS, Cunningham JT, Heesch CM. Fos expression in brain stem nuclei of pregnant rats after hydralazine-induced hypotension. Am J Physiol. 1999;277:R532–R540. doi: 10.1152/ajpregu.1999.277.2.R532. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Scribner KA, Bradbury MJ, Walker C-D, Strack AM, Cascio CS. Stress, feedback and facilitation in the hypothalamo-pituitary-adrenal axis. J Neuroendocrinol. 1992;4:517–526. doi: 10.1111/j.1365-2826.1992.tb00200.x. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Day TA. Medullary neurones regulate hypothalamic corticotropin-releasing factor cell responses to an emotional stressor. Neuroscience. 2001;105:707–719. doi: 10.1016/s0306-4522(01)00213-5. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Day TA. Opposing roles for medial and central amygdala in the initiation of noradrenergic cell responses to a psychological stressor. Eur J Neurosci. 2002;15:1712–1718. doi: 10.1046/j.1460-9568.2001.02011.x. [DOI] [PubMed] [Google Scholar]
- Dunn JD. Plasma corticosterone responses to electrical stimulation of the bed nucleus of the stria terminalis. Brain Res. 1987;407:327–331. doi: 10.1016/0006-8993(87)91111-5. [DOI] [PubMed] [Google Scholar]
- Elam M, Yao T, Thoren P, Svensson TH. Hypercapnia and hypoxia: Chemoreceptor-mediated control of locus coeruleus neurons and splanchnic, sympathetic nerves. Brain Res. 1981;222:373–381. doi: 10.1016/0006-8993(81)91040-4. [DOI] [PubMed] [Google Scholar]
- Feldman S, Conforti N, Itzak A, Weidenfeld J. Differential effect of amygdaloid lesions on CRF-41, ACTH and corticosterone responses following neural stimuli. Brain Res. 1994;658:21–26. doi: 10.1016/s0006-8993(09)90005-1. [DOI] [PubMed] [Google Scholar]
- Finlay JM, Zigmond MJ, Abercrombie ED. Increased dopamine and norepinephrine release in medial prefrontal cortex induced by acute and chronic stress: Effects of diazepam. Neuroscience. 1995;64:619–628. doi: 10.1016/0306-4522(94)00331-x. [DOI] [PubMed] [Google Scholar]
- Gibson A, Hart SL, Patel S. Effects of 6-hydroxydopamine-induced lesions of the paraventricular nucleus, and of prazosin, on the corticosterone response to restraint in rats. Neuropharmacology. 1986;25:257–260. doi: 10.1016/0028-3908(86)90248-0. [DOI] [PubMed] [Google Scholar]
- Gottlieb HB, Ji LL, Jones H, Penny ML, Fleming T, Cunningham JT. Differential effects of water and saline intake on water deprivation-induced c-Fos staining in the rat. Am J Physiol. 2006;290:R1251–R1261. doi: 10.1152/ajpregu.00727.2005. [DOI] [PubMed] [Google Scholar]
- Greenberg HE, Sica A, Batson D, Scharf SM. Chronic intermittent hypoxia increases sympathetic responsiveness to hypoxia and hypercapnia. J Appl Physiol. 1999;86:298–305. doi: 10.1152/jappl.1999.86.1.298. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Lorang M, Irwin M, Aguilera G. CRF receptor regulation and sensitization of ACTH responses to acute ether stress during chronic intermittent immobilization stress. Brain Res. 1990;532:34–40. doi: 10.1016/0006-8993(90)91738-3. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: Hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: Hypothalamo-pituitary-adrenocortical axis. Prog Neuro-Psychopharmacol Biol Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Hinojosa-Laborde C, Mifflin SW. Sex differences in blood pressure response to intermittent hypoxia in rats. Hypertension. 2005;46:1016–1021. doi: 10.1161/01.HYP.0000175477.33816.f3. [DOI] [PubMed] [Google Scholar]
- Hoffman GE, Smith MS, Fitzsimmons MD. Detecting steroidal effects on immediate early gene expression in the hypothalamus. Neuroprotocols. 1992;1:55–66. [Google Scholar]
- Iiyori N, Alonso LC, Li J, Sanders MH, Garcia-Ocana A, O’Doherty RM, Polotsky VY, O’Donnell CP. Intermittent hypoxia causes insulin resistance in lean mice independent of autonomic activity. Am J Respir Crit Care Med. 2007;175:851–857. doi: 10.1164/rccm.200610-1527OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson L, Dallman MF. ACTH secretion and ventilation increase at similar arterial PO2 in conscious rats. J Appl Physiol. 1989;66:2245–2250. doi: 10.1152/jappl.1989.66.5.2245. [DOI] [PubMed] [Google Scholar]
- Jedema HP, Finlay JM, Sved AF, Grace AA. Chronic cold exposure potentiates CRH-evoked increases in electrophysiologic activity of locus coeruleus neurons. Biol Psychiatry. 2001;49:351–359. doi: 10.1016/s0006-3223(00)01057-x. [DOI] [PubMed] [Google Scholar]
- Kiss A, Aguilera G. Participation of α1-adrenergic receptors in the secretion of hypothalamic corticotropin-releasing hormone during stress. Neuroendocrinol. 1992;56:153–160. doi: 10.1159/000126223. [DOI] [PubMed] [Google Scholar]
- Lanfranco F, Gianotti L, Pivetti S, Navone F, Rossetto R, Tassone F, Gai V, Ghigo E, Maccario M. Obese patients with obstructive sleep apnoea syndrom show a peculiar alteration of the corticotroph but not of the thyrotroph and lactotroph function. Clin Endocrinol. 2004;60:41–48. doi: 10.1111/j.1365-2265.2004.01938.x. [DOI] [PubMed] [Google Scholar]
- Lesske J, Fletcher EC, Bao G, Unger T. Hypertension caused by chronic intermittent hypoxia--influence of chemoreceptors and sympathetic nervous system. J Hypertens. 1997;15:1593–1603. doi: 10.1097/00004872-199715120-00060. [DOI] [PubMed] [Google Scholar]
- Li J, Thorne LN, Punjabi NM, Sun C-K, Schwartz AR, Smith PL, Marino RL, Rodriguez A, Hubbard WC, O’Donnell CP, Polotsky VY. Intermittent hypoxia induces hyperlipidemia in lean mice. Circ Res. 2005;97:698–706. doi: 10.1161/01.RES.0000183879.60089.a9. [DOI] [PubMed] [Google Scholar]
- Liposits Z, Sherman D, Phelix C, Paull WK. A combined light and electron microscopic immunocytochemical method for simultaneous localization of multiple tissue antigens; Tyrosine hydroxylase immunoreactive innervation of corticotropin releasing factor synthesizing neurons in the paraventricular nucleus of the rat. Histochem. 1986;85:95–106. doi: 10.1007/BF00491754. [DOI] [PubMed] [Google Scholar]
- Ma S, Morilak DA. Induction of Fos expression by acute immobilization stress is reduced in locus coeruleus and medial amygdala of Wistar-Kyoto rats compared to Sprague-Dawley rats. Neuroscience. 2004;124:963–972. doi: 10.1016/j.neuroscience.2003.12.028. [DOI] [PubMed] [Google Scholar]
- Ma S, Morilak DA. Chronic intermittent cold stress sensitizes the HPA response to a novel acute stress by enhancing noradrenergic influence in the rat paraventricular nucleus. J Neuroendocrinol. 2005a;17:761–769. doi: 10.1111/j.1365-2826.2005.01372.x. [DOI] [PubMed] [Google Scholar]
- Ma S, Morilak DA. Norepinephrine release in medial amygdala facilitates activation of the HPA axis in response to acute immobilisation stress. J Neuroendocrinol. 2005b;17:22–28. doi: 10.1111/j.1365-2826.2005.01279.x. [DOI] [PubMed] [Google Scholar]
- Mana MJ, Grace AA. Chronic cold stress alters the basal and evoked electrophysiological activity of rat locus coeruleus neurons. Neuroscience. 1997;81:1055–1064. doi: 10.1016/s0306-4522(97)00225-x. [DOI] [PubMed] [Google Scholar]
- McDonald TJ, Le WW, Hoffman GE. Brainstem catecholaminergic neurons activated by hypoxemia express GR and are coordinately activated with fetal sheep hypothalamic paraventricular neurons. Brain Res. 2000;885:70–78. doi: 10.1016/s0006-8993(00)02936-x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Allostasis and allostatic load: Implications for neuropsychopharmacology. Neuropsychopharmacology. 2000;22:108–124. doi: 10.1016/S0893-133X(99)00129-3. [DOI] [PubMed] [Google Scholar]
- Melia KR, Ryabinin AE, Schroeder R, Bloom FE, Wilson MC. Induction and habituation of immediate early gene expression in rat brain by acute and repeated restraint stress. J Neurosci. 1994;14:5929–5938. doi: 10.1523/JNEUROSCI.14-10-05929.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morilak DA, Barrera G, Echevarria DJ, Garcia AS, Hernandez A, Ma S, Petre CO. Role of brain norepinephrine in the behavioral response to stress. Prog Neuropsychopharmacol Biol Psychiatr. 2005a;29:1214–1224. doi: 10.1016/j.pnpbp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Fornal CA, Jacobs BL. Effects of physiological manipulations on locus coeruleus neuronal activity in freely moving cats. II. Cardiovascular challenge. Brain Res. 1987;422:24–31. doi: 10.1016/0006-8993(87)90536-1. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Ma S, Fleming T, Ji LL, Mifflin SW, Cunningham JT. Chronic cold stress and chronic intermittent hypoxia sensitize acute stress-induced ACTH secretion and Fos staining in LC and forebrain of rats. Soc Neurosci Abstr. 31 Online, Program no. 526.7 2005b [Google Scholar]
- Mueller NK, Dolgas CM, Herman JP. Stressor-selective role of the ventral subiculum in regulation of neuroendocrine stress responses. Endocrinology. 2004;145:3763–3768. doi: 10.1210/en.2004-0097. [DOI] [PubMed] [Google Scholar]
- Nisenbaum LK, Zigmond MJ, Sved AJ, Abercrombie ED. Prior exposure to chronic stress results in enhanced synthesis and release of hippocampal norepinephrine in response to a novel stressor. J Neurosci. 1991;11:1478–1484. doi: 10.1523/JNEUROSCI.11-05-01478.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon MM, Guilleminault C, Priest RG, Zulley J, Smirne S. Is sleep-disordered breathing an independent risk factor for hypertension in the general population (13,057 subjects)? J Psychosomatic Res. 2000;48:593–601. doi: 10.1016/s0022-3999(00)00142-2. [DOI] [PubMed] [Google Scholar]
- Pacak K, Armando I, Fukuhara K, Kvetnansky R, Palkovits M, Kopin IJ, Goldstein DS. Noradrenergic activation in the paraventricular nucleus during acute and chronic immobilization stress in rats. Brain Res. 1992;589:91–96. doi: 10.1016/0006-8993(92)91165-b. [DOI] [PubMed] [Google Scholar]
- Pankow W, Lies A, Lohmann FW. Sleep-disordered breathing and hypertension. N Engl J Med. 2000;343:966–967. doi: 10.1056/NEJM200009283431310. [DOI] [PubMed] [Google Scholar]
- Pardon M-C, Ma S, Morilak DA. Chronic cold stress sensitizes brain noradrenergic reactivity and noradrenergic facilitation of the HPA stress response in Wistar Kyoto rats. Brain Res. 2003;971:55–65. doi: 10.1016/s0006-8993(03)02355-2. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. Academic Press; San Diego: 1998. [Google Scholar]
- Plotsky PM. Facilitation of immunoreactive corticotropin-releasing factor secretion into the hypophysial-portal circulation after activation of catecholaminergic pathways or central norepinephrine injection. Endocrinol. 1987;121:924–930. doi: 10.1210/endo-121-3-924. [DOI] [PubMed] [Google Scholar]
- Raff H. Endocrine adaptation to hypoxia. In: Fregly MJ, Blatteis CM, editors. Handbook of Physiology, Section 4: Environmental Physiology. Oxford University Press; 1996. pp. 1259–1275. [Google Scholar]
- Raff H, Sandri RB, Segerson TP. Renin, ACTH, and adrenocortical function during hypoxia and hemorrhage in conscious rats. Am J Physiol. 1986;250:R240–R244. doi: 10.1152/ajpregu.1986.250.2.R240. [DOI] [PubMed] [Google Scholar]
- Randolph RR, Li Q, Curtis KS, Sullivan MJ, Cunningham JT. Fos expression following isotonic volume expansion of the unanesthetized male rat. Am J Physiol. 1998;274:R1345–1352. doi: 10.1152/ajpregu.1998.274.5.R1345. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW. The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Res Rev. 1982;4:275–325. doi: 10.1016/0165-0173(82)90010-8. [DOI] [PubMed] [Google Scholar]
- Shamsuzzaman ASM, Gersh BJ, Somers VK. Obstructive sleep apnea: Implications for cardiac and vascular disease. J Amer Medical Assoc. 2003;290:1906–1914. doi: 10.1001/jama.290.14.1906. [DOI] [PubMed] [Google Scholar]
- Sica AL, Greenberg HE, Scharf SM, Ruggiero DA. Chronic-intermittent hypoxia induces immediate early gene expression in the midline thalamus and epithalamus. Brain Res. 2000;883:224–228. doi: 10.1016/s0006-8993(00)02800-6. [DOI] [PubMed] [Google Scholar]
- Smith DW, Buller KM, Day TA. Role of ventrolateral medulla catecholamine cells in hypothalamic neuroendocrine cell responses to systemic hypoxia. J Neurosci. 1995;15:7979–7988. doi: 10.1523/JNEUROSCI.15-12-07979.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RP, Veale D, Pépin J-L, Lévy PA. Obstructive sleep apnoea and the autonomic nervous system. Sleep Med Revs. 1998;2:69–92. doi: 10.1016/s1087-0792(98)90001-6. [DOI] [PubMed] [Google Scholar]
- Stam R, Bruijnzeel AW, Wiegant VM. Long-lasting stress sensitisation. Eur J Pharmacol. 2000;405:217–224. doi: 10.1016/s0014-2999(00)00555-0. [DOI] [PubMed] [Google Scholar]
- Sullivan CE, Issa FG. Obstructive sleep apnea. Clin Chest Med. 1985;6:633–650. [PubMed] [Google Scholar]
- Svensson TH. Peripheral, autonomic regulation of locus coeruleus noradrenergic neurons in brain: Putative implications for psychiatry and psychopharmacology. Psychopharmacology. 1987;92:1–7. doi: 10.1007/BF00215471. [DOI] [PubMed] [Google Scholar]
- Szafarczyk A, Malaval F, Laurent A, Gibaud R, Assenmacher I. Further evidence for a central stimulatory action of catecholamines on adrenocorticotropin release in the rat. Endocrinol. 1987;121:883–892. doi: 10.1210/endo-121-3-883. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Page ME, Curtis AL. Activation of noradrenergic locus coeruleus neurons by hemodynamic stress is due to local release of corticotropin-releasing factor. Brain Res. 1991;555:25–34. doi: 10.1016/0006-8993(91)90855-p. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Bixler EO, Chrousos GP. Metabolic disturbances in obesity versus sleep apnoea: The importance of visceral obesity and insulin resistance. J Int Med. 2003;254:32–44. doi: 10.1046/j.1365-2796.2003.01177.x. [DOI] [PubMed] [Google Scholar]
- Walker B. Glucocorticoids and cardiovascular disease. Eur J Endocrinol. 2007;157:545–559. doi: 10.1530/EJE-07-0455. [DOI] [PubMed] [Google Scholar]
- Wang M. The role of glucocorticoid action in the pathophysiology of the Metabolic Syndrome. Nutr Metab (London) 2005 doi: 10.1186/1743–7075-2–3. open access. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witchel SF, DeFranco DB. Mechanisms of disease: regulation of glucocorticoid and receptor levels - impact on metabolic syndrome. Nature Clin Prac (Endocrinol and Metab) 2006;2:621–631. doi: 10.1038/ncpendmet0323. [DOI] [PubMed] [Google Scholar]
- Woodward DJ, Moises HC, Waterhouse BD, Yeh HH, Cheun JE. Modulatory actions of norepinephrine on neural circuits. Adv Exper Med Biol. 1991;287:193–208. doi: 10.1007/978-1-4684-5907-4_16. [DOI] [PubMed] [Google Scholar]