Abstract
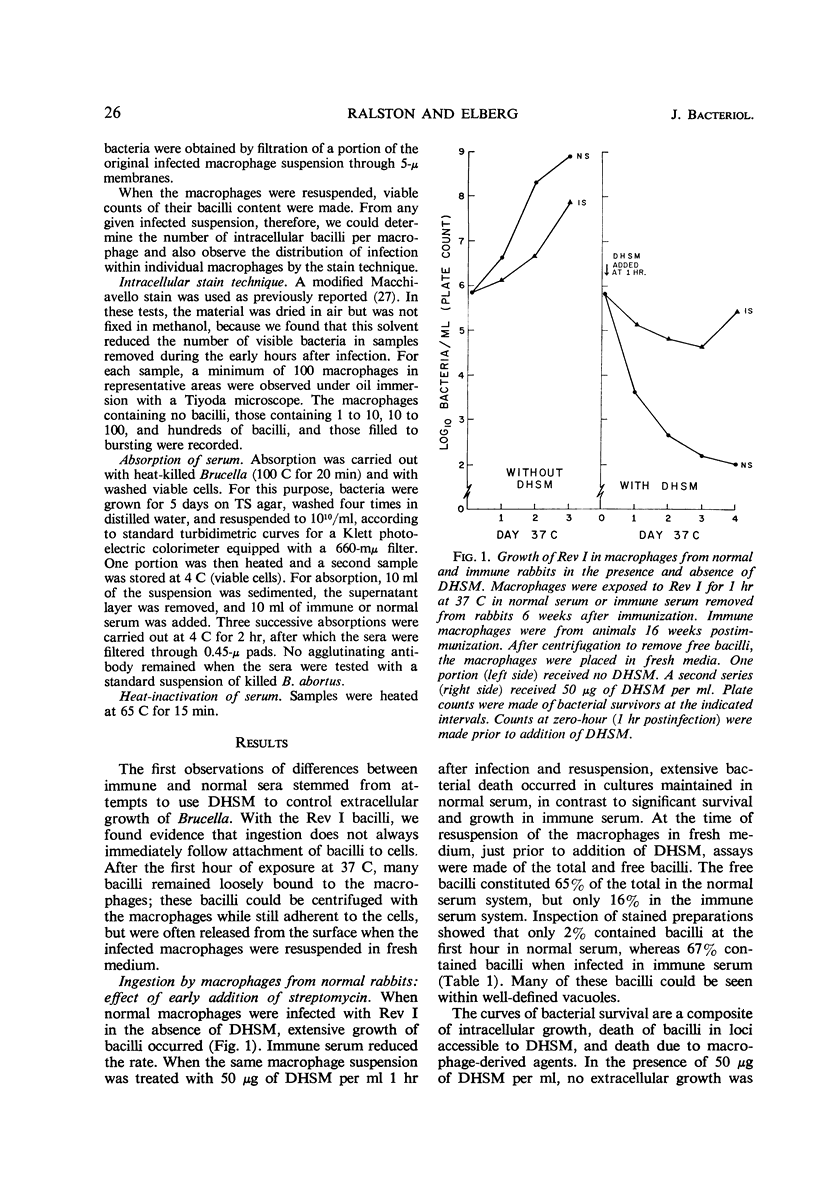
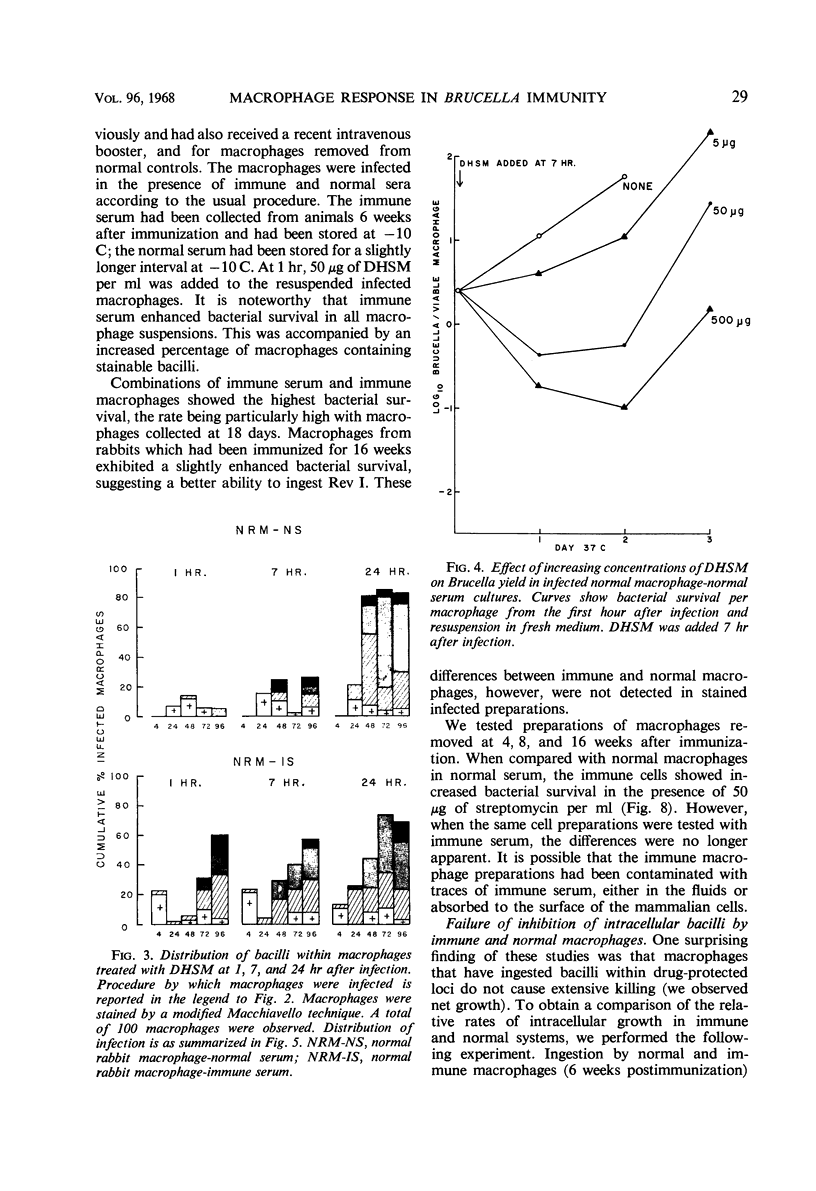
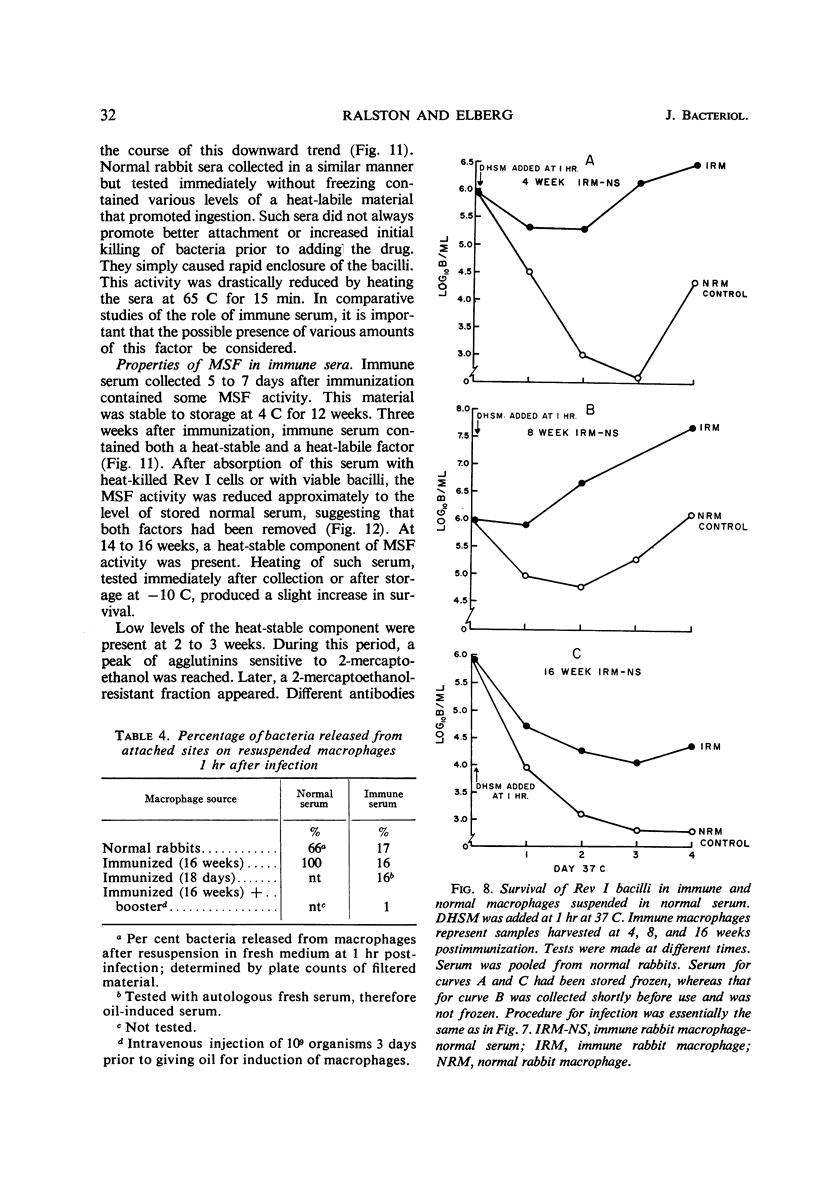
Injection of rabbits with living Brucella melitensis Rev I induced the appearance of a macrophage-stimulating-factor (MSF) in the sera of these animals. MSF was involved in ingestion of bacilli, hastening the formation of protected loci as measured by the addition of lethal amounts of dihydrostreptomycin. When sufficient time had been allowed for effective ingestion, streptomycin had little effect. This in turn allowed for multiplication of bacilli intracellularly in the presence of 5 to 250 μg of drug per ml. MSF mediated more effective ingestion by both immune and normal macrophages. Under such conditions, there was little, if any, intracellular growth restriction by macrophages from immune animals. The activity appeared within the first 5 days after injection with 109 organisms and was present for several months. Three weeks after injection, the activity of serum was partially heat-labile. All activity was removed by absorption with heat-killed or living Rev I cells, suggesting that a specific globulin is concerned.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRUMFITT W., GLYNN A. A., PERCIVAL A. FACTORS INFLUENCING THE PHAGOCYTOSIS OF ESCHERICHIA COLI. Br J Exp Pathol. 1965 Apr;46:215–226. [PMC free article] [PubMed] [Google Scholar]
- BRUMFITT W., PERCIVAL A. Adjustment of urine pH in the chemotherapy of urinary-tract infections. A laboratory and clinical assessment. Lancet. 1962 Jan 27;1(7222):186–190. doi: 10.1016/s0140-6736(62)91077-2. [DOI] [PubMed] [Google Scholar]
- Bonventre P. F., Oxman E. Phagocytosis and intracellular disposition of viable bacteria by the isolated perfused rat liver. J Reticuloendothel Soc. 1965 Nov;2(4):313–325. [PubMed] [Google Scholar]
- ELBERG S. S. Cellular immunity. Bacteriol Rev. 1960 Mar;24(1):67–95. doi: 10.1128/br.24.1.67-95.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELBERG S. S., FONG J., SCHNEIDER P. Studies on tubercle bacillus-monocyte relationship. I. Quantitative analysis of effect of serum of animals vaccinated with BCG upon bacterium-monocyte system. J Exp Med. 1956 Oct 1;104(4):455–465. doi: 10.1084/jem.104.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELBERG S. S., SCHNEIDER P., FONG J. Cross-immunity between Brucella melitensis and Mycobacterium tuberculosis; intracellular behavior of Brucella melitensis in monocytes from vaccinated animals. J Exp Med. 1957 Oct 1;106(4):545–554. doi: 10.1084/jem.106.4.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FONG J., CHIN D., ELBERG S. S. Studies on tubercle bacillusmonocyte relationship. IV. Effects of passage in normal and immune systems upon virulent bacilli. J Exp Med. 1961 Jul 1;114:75–87. doi: 10.1084/jem.114.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREEMAN B. A., VANA L. R. Host-parasite relationships in brucellosis. I. Infection of normal guinea pig macrophages in tissue culture. J Infect Dis. 1958 May-Jun;102(3):258–267. doi: 10.1093/infdis/102.3.258. [DOI] [PubMed] [Google Scholar]
- Fauve R. M., Bouanchaud D., Delaunay A. Résistance cellulaire à l'infection bactérienne. IV. Immunisation active et résistance des macrophages de souris NCS à la multiplication intracellulaire de Listeria monocytogenes, Corynebacterium kutscheri et Brucella melitensis. Ann Inst Pasteur (Paris) 1966 Mar;110(3 Suppl):106–117. [PubMed] [Google Scholar]
- Fitzgeorge R. B., Solotorovsky M., Smith H. The behaviour of Brucella abortus within macrophages separated from the blood of normal and immune cattle by adherence to glass. Br J Exp Pathol. 1967 Oct;48(5):522–528. [PMC free article] [PubMed] [Google Scholar]
- HANKS J. H., WALLACE J. H. Determination of cell viability. Proc Soc Exp Biol Med. 1958 May;98(1):188–192. doi: 10.3181/00379727-98-23985. [DOI] [PubMed] [Google Scholar]
- HOLLAND J. J., PICKETT M. J. A cellular basis of immunity in experimental Brucella infection. J Exp Med. 1958 Sep 1;108(3):343–360. doi: 10.1084/jem.108.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOPPS H. E., SMADEL J. E., BERNHEIM B. C., DANAUSKAS J. X., JACKSON E. B. Effect of antibiotics on intracellular Salmonella typhosa. II. Elimination of infection by prolonged treatment. J Immunol. 1961 Aug;87:162–174. [PubMed] [Google Scholar]
- Hatten B. A., Sulkin S. E. Intracellular Production of Brucella L Forms I. Recovery of L Forms from Tissue Culture Cells Infected with Brucella abortus. J Bacteriol. 1966 Jan;91(1):285–296. doi: 10.1128/jb.91.1.285-296.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKIN C. R., ROWLEY D., AUZINS I. THE BASIS FOR IMMUNITY TO MOUSE TYPHOID. I. THE CARRIER STATE. Aust J Exp Biol Med Sci. 1964 Apr;42:215–228. doi: 10.1038/icb.1964.23. [DOI] [PubMed] [Google Scholar]
- JENKIN C., BENACERRAF B. In vitro studies on the interaction between mouse peritoneal macrophages and strains of Salmonella and Escherichia coli. J Exp Med. 1960 Aug 1;112:403–417. doi: 10.1084/jem.112.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger R. G. Properties of antibodies synthesized by cells in vitro in the presence and absence of streptomycin. Proc Natl Acad Sci U S A. 1966 May;55(5):1206–1213. doi: 10.1073/pnas.55.5.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. THE IMMUNOLOGICAL BASIS OF ACQUIRED CELLULAR RESISTANCE. J Exp Med. 1964 Jul 1;120:105–120. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORELLO J. A., BAKER E. E. INTERACTION OF SALMONELLA WITH PHAGOCYTES IN VITRO. J Infect Dis. 1965 Apr;115:131–141. doi: 10.1093/infdis/115.2.131. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B., Blanden R. V., Collins F. M. Host-parasite relations in mouse typhoid. J Exp Med. 1966 Oct 1;124(4):573–583. doi: 10.1084/jem.124.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POMALES-LEBRON A., STINEBRING W. R. Intracellular multiplication of Brucella abortus in normal and immune mononuclear phagocytes. Proc Soc Exp Biol Med. 1957 Jan;94(1):78–83. doi: 10.3181/00379727-94-22860. [DOI] [PubMed] [Google Scholar]
- RALSTON D. J., ELBERG S. S. Intramonocytic destruction of Brucella: potentiating effect of glycine on intracellular lysozyme activity. J Infect Dis. 1961 Jul-Aug;109:71–80. doi: 10.1093/infdis/109.1.71. [DOI] [PubMed] [Google Scholar]
- SUTER E. Multiplication of tubercle bacilli within mononuclear phagocytes in tissue cultures derived from normal animals and animals vaccinated with BCG. J Exp Med. 1953 Feb 1;97(2):235–245. doi: 10.1084/jem.97.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern J. L., Barner H. D., Cohen S. S. The lethality of streptomycin and the stimulation of RNA synthesis in the absence of protein synthesis. J Mol Biol. 1966 May;17(1):188–217. doi: 10.1016/s0022-2836(66)80103-1. [DOI] [PubMed] [Google Scholar]
- TURNER K. J., JENKIN C. R., ROWLEY D. THE BASIS FOR IMMUNITY TO MOUSE TYPHOID. 2. ANTIBODY FORMATION DURING THE CARRIER STATE. Aust J Exp Biol Med Sci. 1964 Apr;42:229–236. doi: 10.1038/icb.1964.24. [DOI] [PubMed] [Google Scholar]
- Umezawa H., Okanishi M., Kondo S., Hamana K., Utahara R., Maeda K., Mitsuhashi S. Phosphorylative inactivation of aminoglycosidic antibiotics by Escherichia coli carrying R factor. Science. 1967 Sep 29;157(3796):1559–1561. [PubMed] [Google Scholar]