Abstract
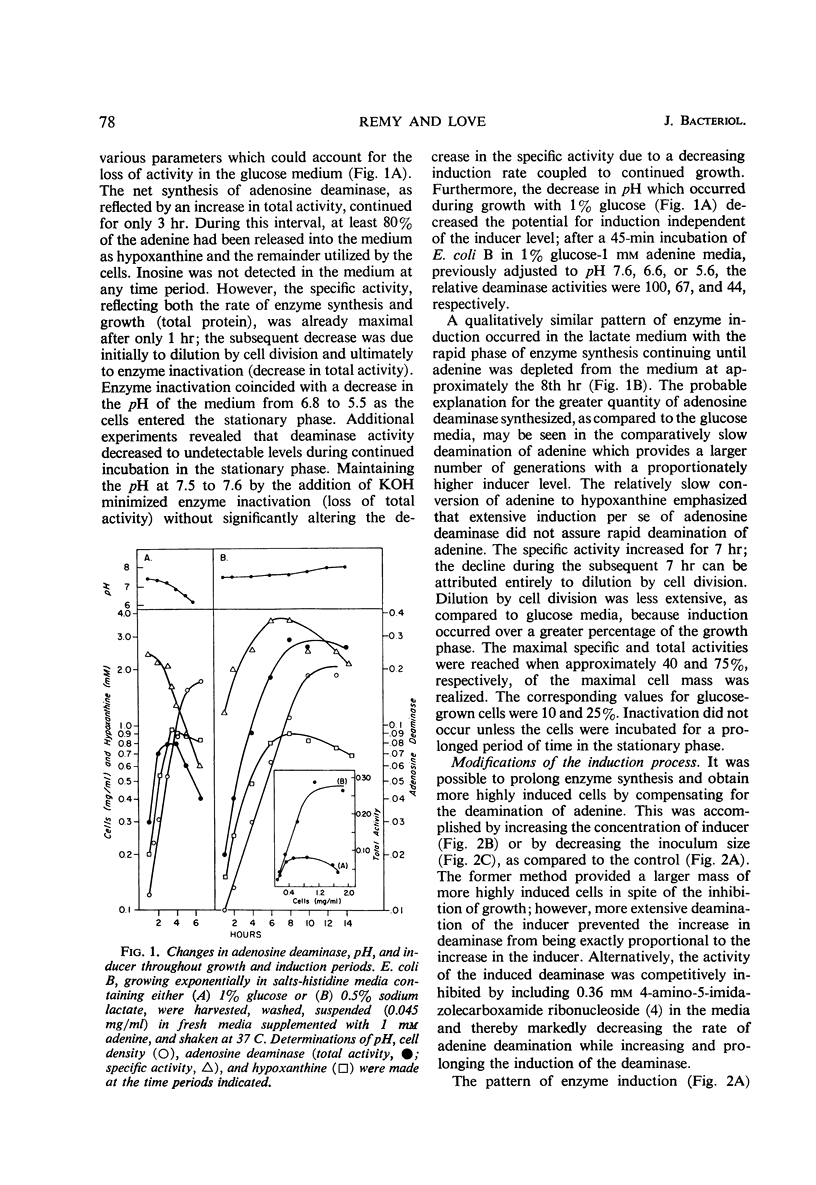
Supplementing the salts-glucose medium of Escherichia coli with adenine initiates induction of adenosine deaminase (adenosine aminohydrolase, EC 3.5.4.4), growth inhibition, and an increased potential for the net deamination of adenine. The extent and duration of these events are proportional to the initial adenine concentration and are dependent upon adenylate pyrophosphorylase and repression of histidine biosynthesis for maximal expression. The conversion of adenine to hypoxanthine, though limited in rate, occurs concurrently with induction and accounts for the progressively decreasing rate of deaminase induction, since hypoxanthine is a relatively ineffective inducer. The subsequent decrease in deaminase activity is due to dilution by continued cell division and by enzyme inactivation which occurs during the late-log and early-stationary phases. The partially purified deaminase is labile to a number of environmental conditions, particularly to phosphate buffers of pH 6.8 or less. A disproportionately slow rate of adenine deamination by cells utilizing lactate permits a more prolonged period of induction and, consequently, a greater quantity of enzyme to be synthesized; cell division, but not enzyme inactivation, reduces enzyme concentration. The adenosine deaminases of Aerobacter aerogenes and Salmonella typhimurium are not inducible.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buttin G., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. XXI. Utilization of deoxyribonucleoside triphosphates by Escherichia coli cells. J Biol Chem. 1966 Nov 25;241(22):5419–5427. [PubMed] [Google Scholar]
- Heppel L. A. Selective release of enzymes from bacteria. Science. 1967 Jun 16;156(3781):1451–1455. doi: 10.1126/science.156.3781.1451. [DOI] [PubMed] [Google Scholar]
- KOCH A. L., VALLEE G. The properties of adenosine deaminase and adenosine nucleoside phosphorylase in extracts of Escherichia coli. J Biol Chem. 1959 May;234(5):1213–1218. [PubMed] [Google Scholar]
- KURAMITSU H. K., UDAKA S., MOYED H. S. INDUCTION OF INOSINE 5'-PHOSPHATE DEHYDROGENASE AND XANTHOSINE 5'-PHOSPHATE AMINASE BY RIBOSYL-4-AMINO-5-IMIDAZOLECARBOXAMIDE IN PURINE-REQUIRING MUTANTS OF ESCHERICHIA COLI B. J Biol Chem. 1964 Oct;239:3425–3430. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Love S. H., Remy C. N. Metabolism of methylated purines in Escherichia coli: derepression of purine biosynthesis. J Bacteriol. 1966 Mar;91(3):1037–1049. doi: 10.1128/jb.91.3.1037-1049.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGASANIK B., KARIBIAN D. Purine nucleotide cycles and their metabolic role. J Biol Chem. 1960 Sep;235:2672–2681. [PubMed] [Google Scholar]
- MANS R. J., KOCH A. L. Metabolism of adenosine and deoxyadenosine by growing cultures of Escherichia coli. J Biol Chem. 1960 Feb;235:450–456. [PubMed] [Google Scholar]
- MOYED H. S. INHIBITION OF THE BIOSYNTHESIS OF THE PYRIMIDINE PORTION OF THIAMINE BY ADENOSINE. J Bacteriol. 1964 Oct;88:1024–1029. doi: 10.1128/jb.88.4.1024-1029.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama H., Mizuno D. Reutilization of degradation products of ribosomal ribonucleic acid in Escherichia coli strain B during the phosphorus-deficient stage. Biochim Biophys Acta. 1966 Sep;123(3):510–522. doi: 10.1016/0005-2787(66)90219-x. [DOI] [PubMed] [Google Scholar]
- Maruyama H., Mizuno D. The participation of ribonuclease in the degradation of Escherichia coli ribosomal ribonucleic acid as revealed by oligonucleotides accumulation in the phorphorus-deficient stage. Biochim Biophys Acta. 1965 Dec 9;108(4):593–604. doi: 10.1016/0005-2787(65)90056-0. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of ribonuclease into the medium when E. coli cells are converted to spheroplasts. Biochem Biophys Res Commun. 1964;14:109–112. doi: 10.1016/0006-291x(64)90238-4. [DOI] [PubMed] [Google Scholar]
- Neu H. C. The 5'-nucleotidase of Escherichia coli. I. Purification and properties. J Biol Chem. 1967 Sep 10;242(17):3896–3904. [PubMed] [Google Scholar]
- Nossal N. G., Heppel L. A. The release of enzymes by osmotic shock from Escherichia coli in exponential phase. J Biol Chem. 1966 Jul 10;241(13):3055–3062. [PubMed] [Google Scholar]
- Peterson R. N., Koch A. L. The relationship of adenosine and inosine transport in Escherichia coli. Biochim Biophys Acta. 1966 Sep 5;126(1):129–145. doi: 10.1016/0926-6585(66)90043-4. [DOI] [PubMed] [Google Scholar]
- REMY C. N. Metabolism of 6-methylaminopurine: synthesis and demethylation by Escherichia coli. J Biol Chem. 1961 Nov;236:2999–3005. [PubMed] [Google Scholar]
- WYNGAARDEN J. B., GREENLAND R. A. The inhibition of succinoadenylate kinosynthetase of Escherichia coli by adenosine and guanosine 5'-monophosphates. J Biol Chem. 1963 Mar;238:1054–1057. [PubMed] [Google Scholar]
- ZIMMERMAN E. F., MAGASANIK B. UTILIZATION AND INTERCONVERSION OF PURINE BASES AND RIBONUCLEOSIDES BY SALMONELLA TYPHIMURIUM. J Biol Chem. 1964 Jan;239:293–300. [PubMed] [Google Scholar]



