Abstract
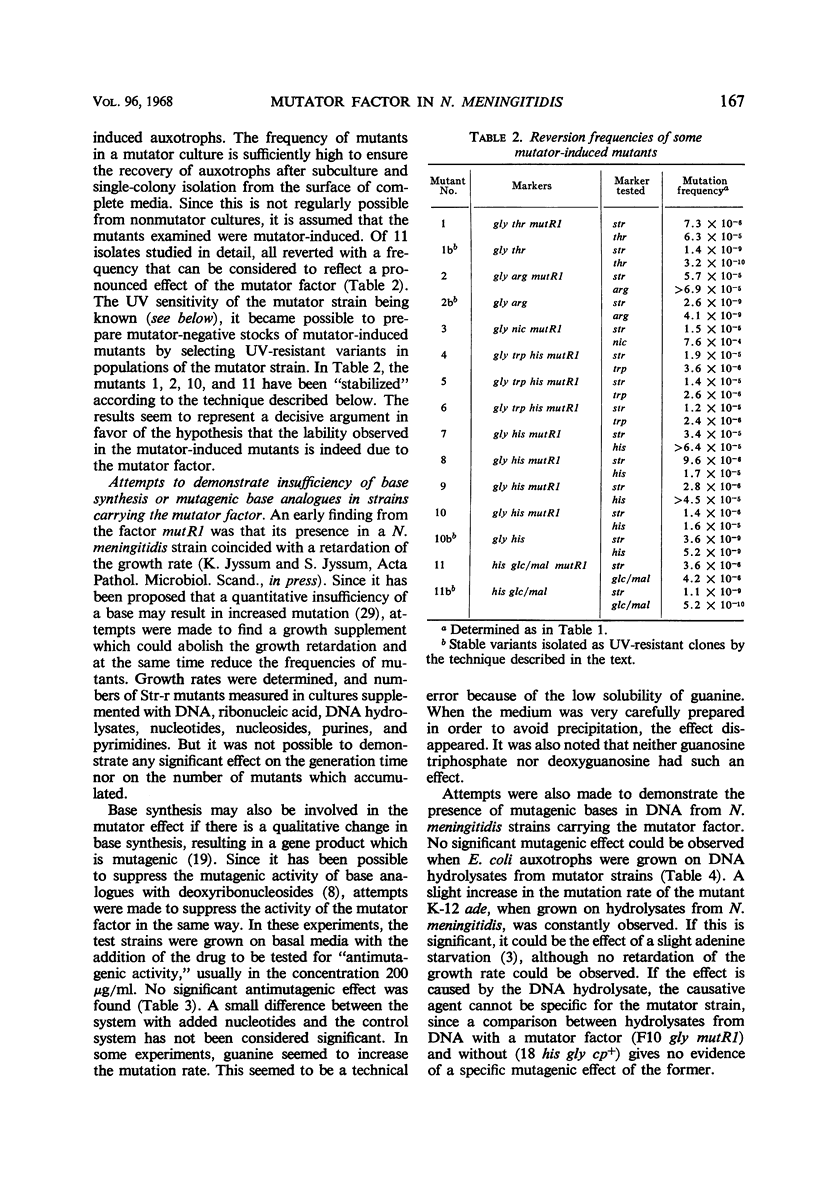
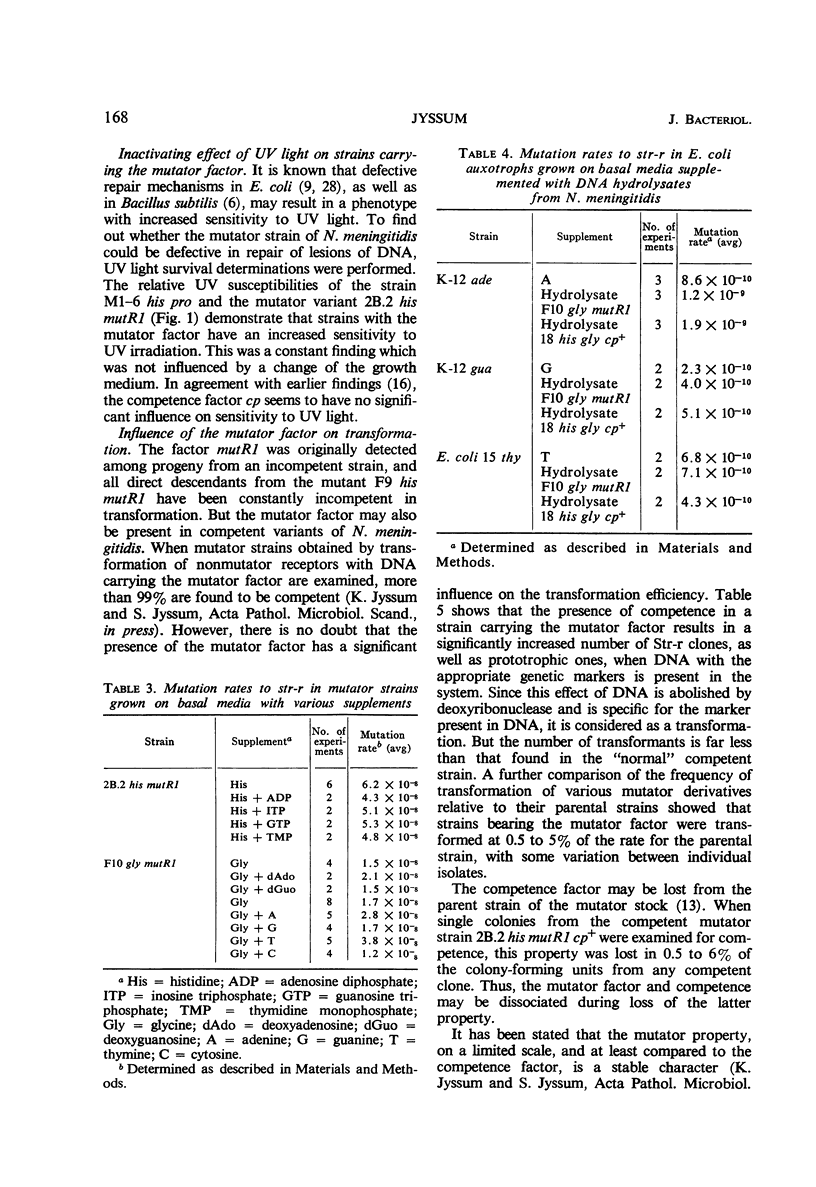
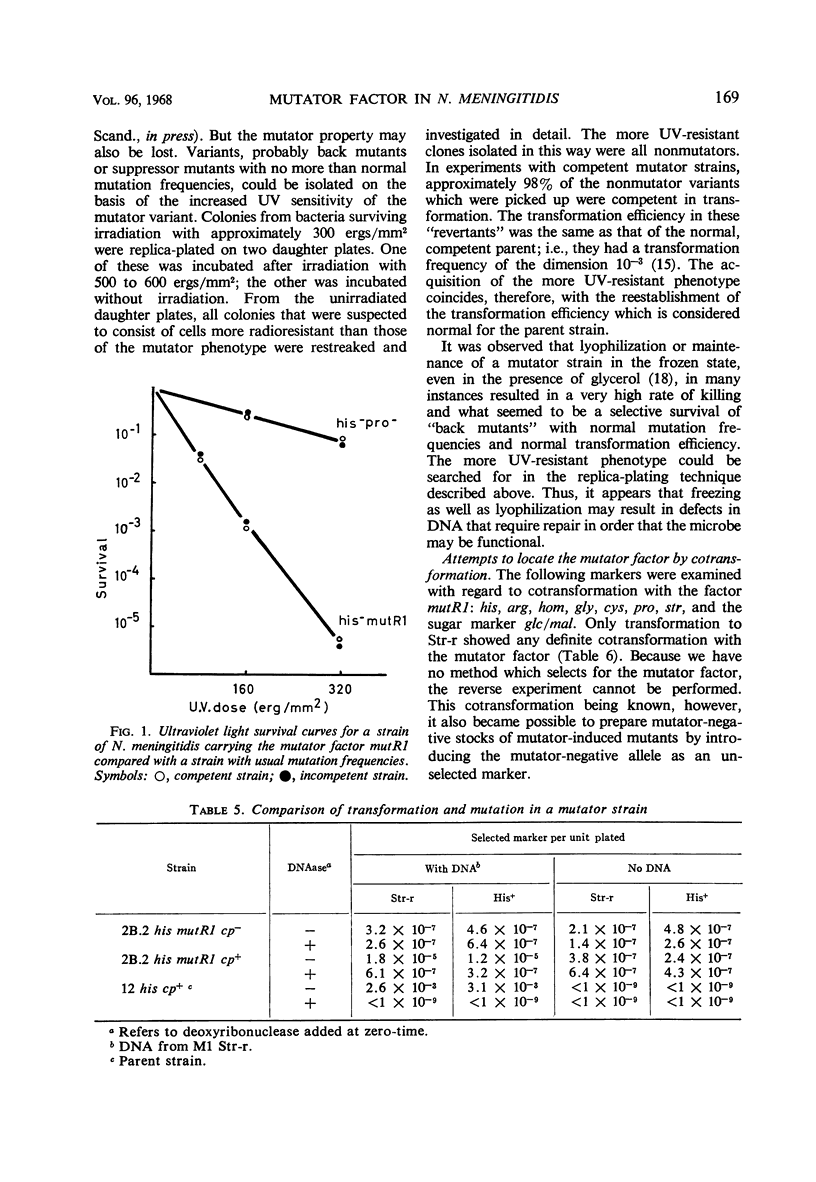
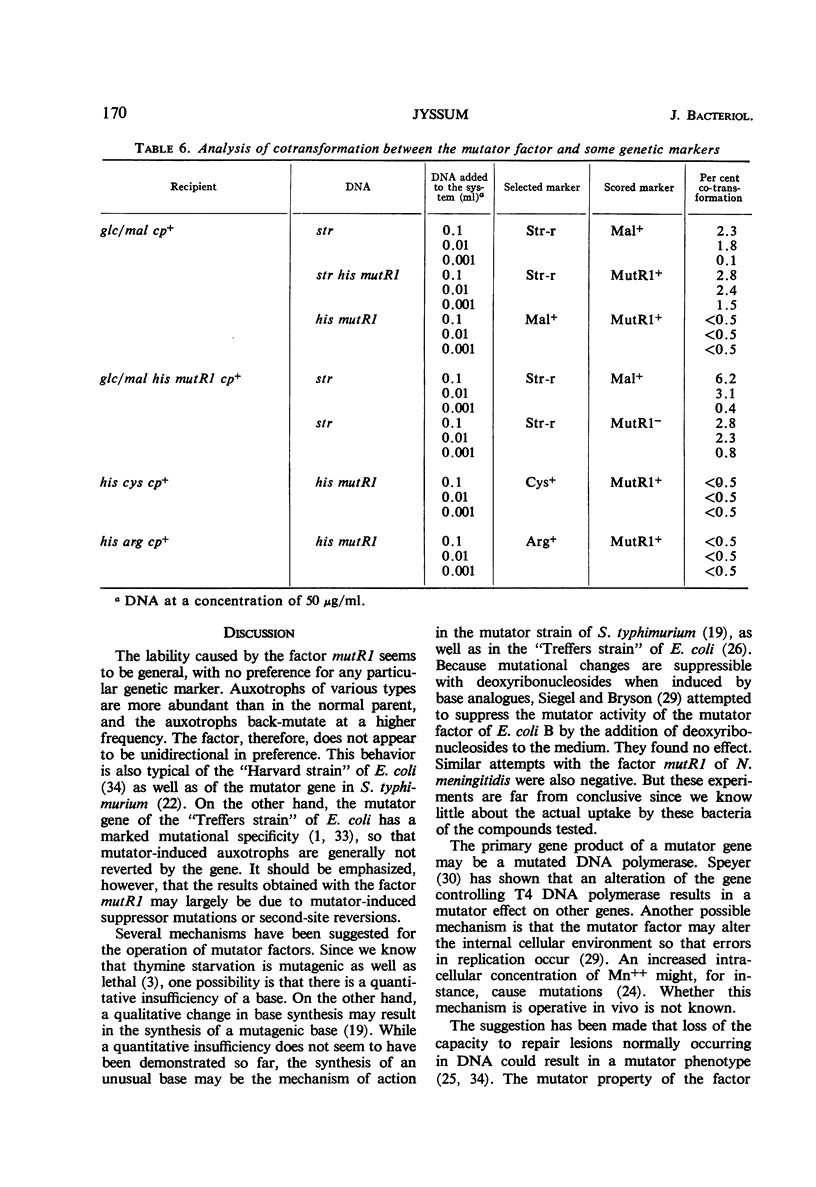
A variant of Neisseria meningitidis was found to carry a mutator factor which endowed the bacteria with generalized genetic instability. The reversion frequencies of several biochemical mutants were increased up to 1,000-fold when the factor was introduced. The factor is not unidirectional in preference, since the mutator induced mutants generally reverted with increased frequency in its presence. There could be found no indication of insufficient synthesis of nucleic acid precursors. Attempts to demonstrate an unusual, mutagenic base incorporated in deoxyribonucleic acid (DNA) were negative. Strains carrying the mutator factor had significantly increased sensitivity to ultraviolet light. A mutation to a more ultraviolet-resistant type coincided with a disappearance of the mutator property. The presence of the mutator factor in a competent strain resulted in a reduction of the transformation frequency to between 0.5 and 5% of that in the parental strain. A mutation to the more ultraviolet-resistant type resulted in simultaneous loss of the mutator property and reestablishment of a normal transformation efficiency. It has been suggested that this mutator factor may represent a defect in the DNA repair mechanism, which is also of importance for genetic recombination. The mutator factor showed cotransformation with the locus for streptomycin resistance, but a true linkage could not be proved.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADELBERG E. A., COUGHLIN C. A. Bacterial mutation induced by thymine starvation. Nature. 1956 Sep 8;178(4532):531–532. doi: 10.1038/178531a0. [DOI] [PubMed] [Google Scholar]
- BACON D. F., TREFFERS H. P. Spontaneous and mutator-induced reversions of an Escherichia coli auxotroph. I. Prototrophic types and their growth characteristics. J Bacteriol. 1961 May;81:786–793. doi: 10.1128/jb.81.5.786-793.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhme H. Genetic instability of an ultraviolet-sensitive mutant of Proteus mirabilis. Biochem Biophys Res Commun. 1967 Jul 21;28(2):191–196. doi: 10.1016/0006-291x(67)90428-7. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN A., SMOOT J. S. A strain of Escherichia coli with an unusually high rate of auxotrophic mutation. J Bacteriol. 1955 Nov;70(5):588–595. doi: 10.1128/jb.70.5.588-595.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUNDERSEN W. B., JYSSUM K., LIE S. Genetic instability with episome-mediated transfer in Escherichia coli. J Bacteriol. 1962 Mar;83:616–623. doi: 10.1128/jb.83.3.616-623.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD B. D., TESSMAN I. IDENTIFICATION OF THE ALTERED BASES IN MUTATED SINGLE-STRANDED DNA. II. IN VIVO MUTAGENESIS BY 5-BROMODEOXYURIDINE AND 2-AMINOPURINE. J Mol Biol. 1964 Aug;9:364–371. doi: 10.1016/s0022-2836(64)80213-8. [DOI] [PubMed] [Google Scholar]
- Hoch J. A., Barat M., Anagnostopoulos C. Transformation and transduction in recombination-defective mutants of Bacillus subtilis. J Bacteriol. 1967 Jun;93(6):1925–1937. doi: 10.1128/jb.93.6.1925-1937.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P., Theriot L. Three loci in Escherichia coli K-12 that control the excision of pyrimidine dimers and certain other mutagen products from DNA. Genetics. 1966 Jun;53(6):1119–1136. doi: 10.1093/genetics/53.6.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P., Theriot L. Mutants of Escherichia coli K-12 defective in DNA repair and in genetic recombination. Genetics. 1966 Jun;53(6):1137–1150. doi: 10.1093/genetics/53.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JYSSUM K., LIE S. TRANSFORMATION OF AUXOTROPHS OF NEISSERIA MENINGITIDIS. Acta Pathol Microbiol Scand. 1965;63:445–455. doi: 10.1111/apm.1965.63.3.445. [DOI] [PubMed] [Google Scholar]
- Jyssum K. Genetic factors determining competence in transformation of Neisseria meningitidis. II. A reversible loss of competence. Acta Pathol Microbiol Scand. 1966;67(4):493–502. doi: 10.1111/apm.1966.67.4.493. [DOI] [PubMed] [Google Scholar]
- Jyssum K. Polarity of chromosome replication in Neisseria meningitidis. J Bacteriol. 1965 Nov;90(5):1182–1187. doi: 10.1128/jb.90.5.1182-1187.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRCHNER C. E. The effects of the mutator gene on molecular changes and mutation of Salmonella typhimurium. J Mol Biol. 1960 Dec;2:331–338. doi: 10.1016/s0022-2836(60)80044-7. [DOI] [PubMed] [Google Scholar]
- Kirchner C. E., Rudden M. J. Location of a mutator gene in Salmonella typhimurium by cotransduction. J Bacteriol. 1966 Nov;92(5):1453–1456. doi: 10.1128/jb.92.5.1453-1456.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIE S. PRODUCTION OF AUXOTROPHIC MUTANTS OF NEISSERIA MENINGITIDIS BY NITROUS ACID. Acta Pathol Microbiol Scand. 1965;63:615–622. doi: 10.1111/apm.1965.63.4.615. [DOI] [PubMed] [Google Scholar]
- LIE S. STUDIES ON THE PHENOTYPIC EXPRESSION OF COMPETENCE IN NEISSERIA MENINGITIDIS. Acta Pathol Microbiol Scand. 1965;64:119–129. doi: 10.1111/apm.1965.64.1.119. [DOI] [PubMed] [Google Scholar]
- LIE S. THE INACTIVATING AND MUTAGENIC EFFECT OF ULTRAVIOLET IRRADIATION ON NEISSERIA MENINGITIDIS. Acta Pathol Microbiol Scand. 1965;63:456–468. doi: 10.1111/apm.1965.63.3.456. [DOI] [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake T. Mutator Factor in Salmonella Typhimurium. Genetics. 1960 Jan;45(1):11–14. doi: 10.1093/genetics/45.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcombe H. B. Delayed Phenotypic Expression of Spontaneous Mutations in Escherichia Coli. Genetics. 1948 Sep;33(5):447–476. doi: 10.1093/genetics/33.5.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orgel A., Orgel L. E. Induction of mutations in bacteriophage T4 with divalent manganese. J Mol Biol. 1965 Dec;14(2):453–457. doi: 10.1016/s0022-2836(65)80195-4. [DOI] [PubMed] [Google Scholar]
- Pauling C., Hanawalt P. Nonconservative DNA replication in bacteria after thymine starvation. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1728–1735. doi: 10.1073/pnas.54.6.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce B. L. The effect of a bacterial mutator gene upon mutation rates in bacteriophage T4. Genetics. 1966 Aug;54(2):657–662. doi: 10.1093/genetics/54.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel E. C., Bryson V. Mutator gene of Escherichia coli B. J Bacteriol. 1967 Jul;94(1):38–47. doi: 10.1128/jb.94.1.38-47.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speyer J. F. Mutagenic DNA polymerase. Biochem Biophys Res Commun. 1965 Oct 8;21(1):6–8. doi: 10.1016/0006-291x(65)90417-1. [DOI] [PubMed] [Google Scholar]
- Treffers H. P., Spinelli V., Belser N. O. A Factor (or Mutator Gene) Influencing Mutation Rates in Escherichia Coli. Proc Natl Acad Sci U S A. 1954 Nov;40(11):1064–1071. doi: 10.1073/pnas.40.11.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C., Cox E. C., Horn V. The unusual mutagenic specificity of an E. Coli mutator gene. Proc Natl Acad Sci U S A. 1966 Feb;55(2):274–281. doi: 10.1073/pnas.55.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamenhof P. J. A genetic locus responsible for generalized high mutability in Escherichia coli. Proc Natl Acad Sci U S A. 1966 Sep;56(3):845–852. doi: 10.1073/pnas.56.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Putte P., Zwenk H., Rörsch A. Properties of four mutants of Escherichia coli defective in genetic recombination. Mutat Res. 1966 Oct;3(5):381–392. doi: 10.1016/0027-5107(66)90048-0. [DOI] [PubMed] [Google Scholar]



