Abstract
Certain chemical compounds increase mutation frequency of Escherichia coli B/r significantly when used in conjunction with nonlethal ultraviolet (UV) dosages. Studies were done to elucidate the mechanism of this enhancing mutational effect. Dark survival curves showed that 500 μg of caffeine per ml in the postirradiation medium markedly decreased survival to 60 ergs/mm2 of UV in strain B/r. Caffeine did not markedly decrease survival to UV in strain B/r WP-2 hcr−. At least 90% of the mutations induced to streptomycin resistance by UV and 85% of those induced by UV with caffeine could be photoreversed. Experiments with thymine analogues suggested that thymine dimerization at the streptomycin locus was the primary premutational photoproduct induced by sublethal UV dosages. Caffeine did not interfere with the photoreversal of induced mutants, indicating that it probably does not bind to the photoreactivating enzyme or to a UV-induced lesion in the DNA. Addition of DNA or irradiated DNA with 500 μg of caffeine per ml resulted in no loss of the caffeine activity. The excision of UV-induced thymine-containing dimers from E. coli B/r T− was investigated in the presence and absence of caffeine. Our results indicated that caffeine prevents excision of thymine dimers, presumably by binding to the excising enzyme. This binding results in an impairment of repair, which produces the increase in mutant numbers.
Full text
PDF

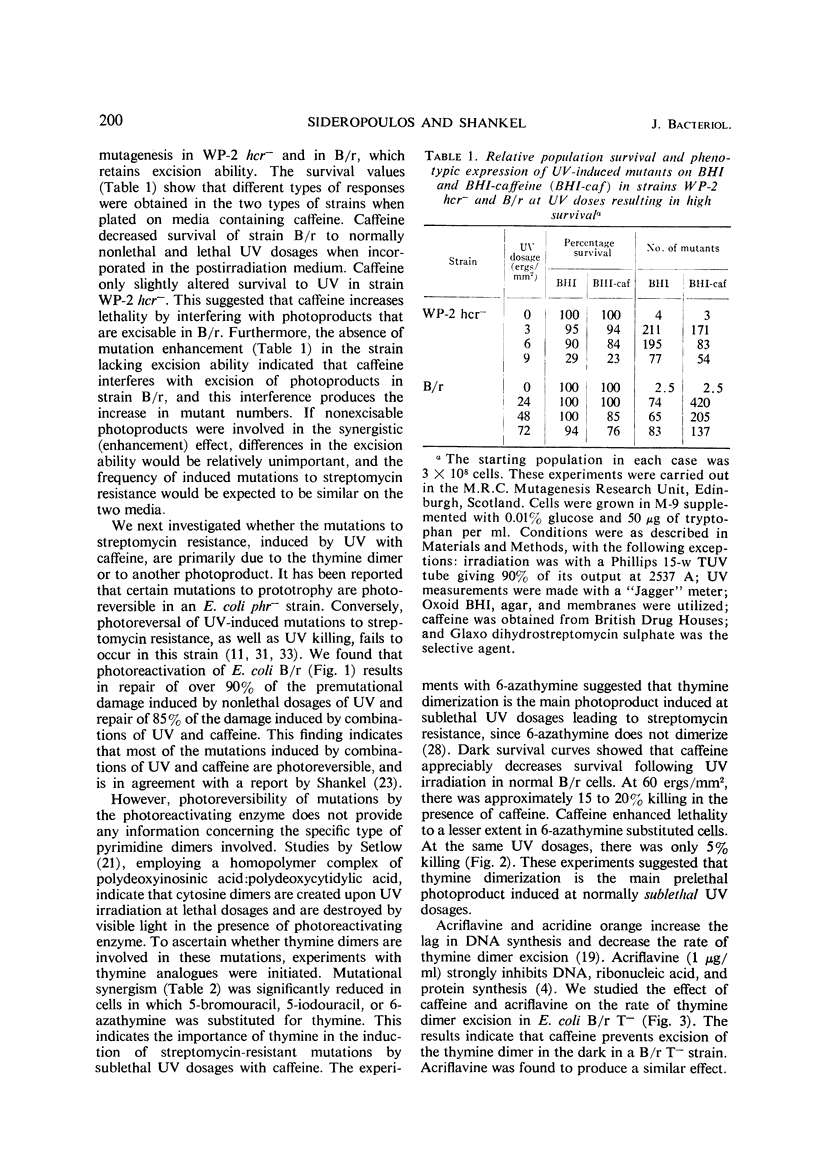


Selected References
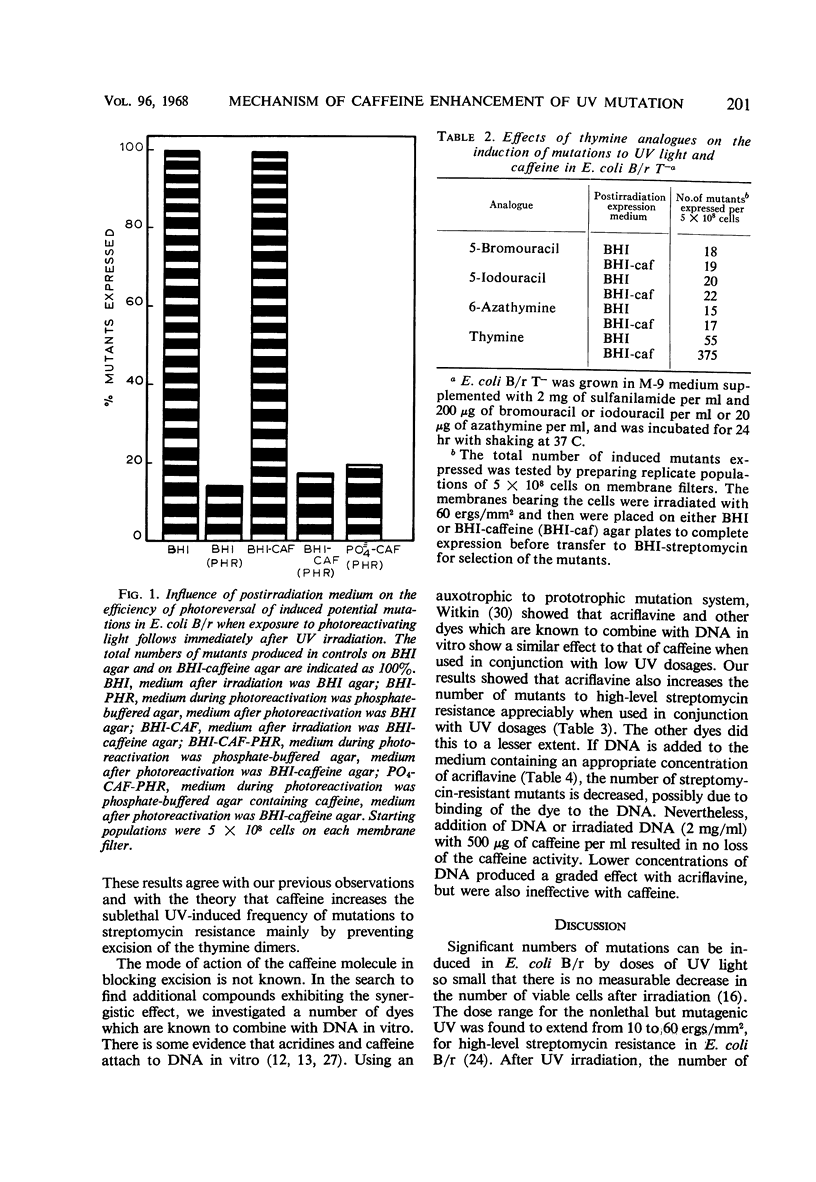
These references are in PubMed. This may not be the complete list of references from this article.
- ALPER T. EFFECTS ON IRRADIATED MICRO-ORGANISMS OF GROWTH IN THE PRESENCE OF ACRIFLAVINE. Nature. 1963 Nov 9;200:534–536. doi: 10.1038/200534a0. [DOI] [PubMed] [Google Scholar]
- BOYCE R. P., HOWARD-FLANDERS P. RELEASE OF ULTRAVIOLET LIGHT-INDUCED THYMINE DIMERS FROM DNA IN E. COLI K-12. Proc Natl Acad Sci U S A. 1964 Feb;51:293–300. doi: 10.1073/pnas.51.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DONESON I. N., SHANKEL D. M. MUTATIONAL SYNERGISM BETWEEN RADIATIONS AND METHYLATED PURINES IN ESCHERICHIA COLI. J Bacteriol. 1964 Jan;87:61–67. doi: 10.1128/jb.87.1.61-67.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
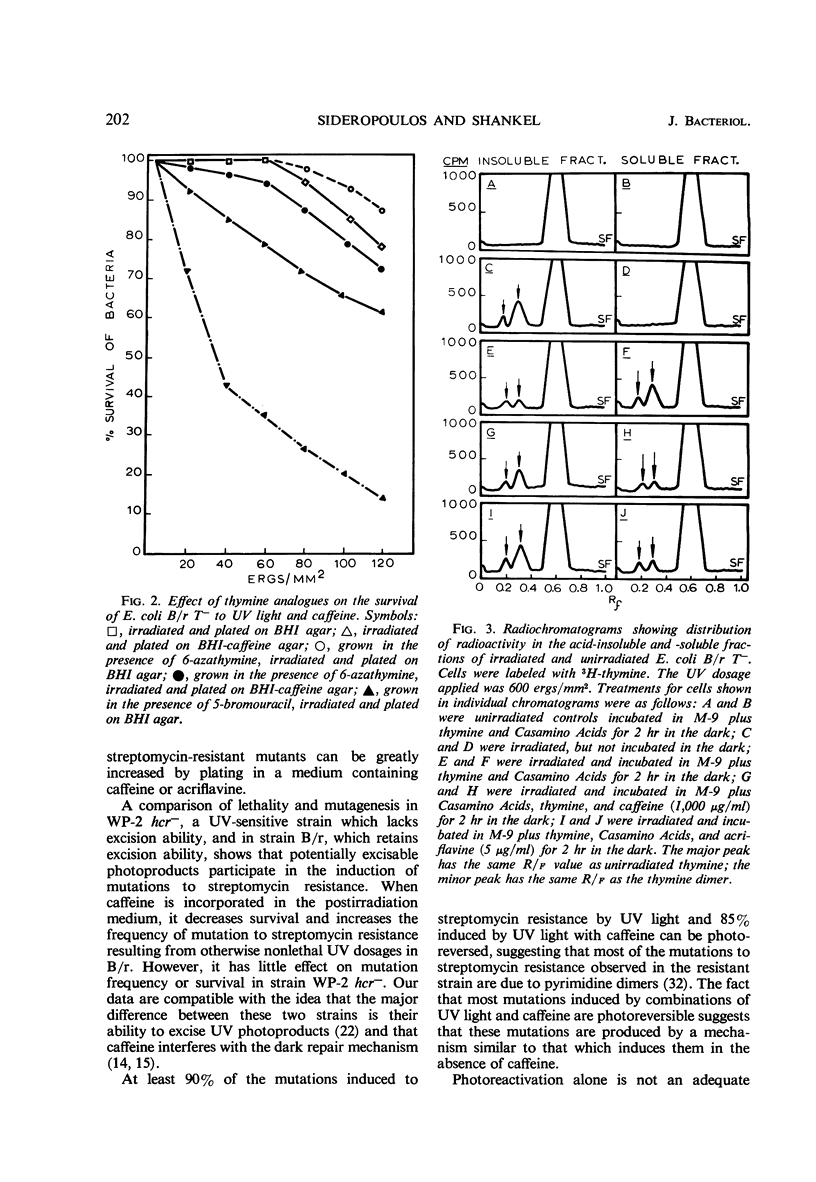
- Doudney C. O., White B. F., Bruce B. J. Acriflavine modification of nucleic acid formation, mutation induction and survival in ultraviolet light exposed bacteria. Biochem Biophys Res Commun. 1964 Feb 18;15(1):70–75. doi: 10.1016/0006-291x(64)90105-6. [DOI] [PubMed] [Google Scholar]
- FEINER R. R., HILL R. F. EFFECT OF BASIC DYES ON HOST-CELL REACTIVATION OF ULTRA-VIOLETIRRADIATED PHAGE. Nature. 1963 Oct 19;200:291–293. doi: 10.1038/200291a0. [DOI] [PubMed] [Google Scholar]
- GREENBERG J. A LOCUS FOR RADIATION RESISTANCE IN ESCHERICHIA COLI. Genetics. 1964 May;49:771–778. doi: 10.1093/genetics/49.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD-FLANDERS P., BOYCE R. P., SIMSON E., THERIOT L. A genetic locus in E. coli K12 that controls the reactivation of UV-photoproducts associated with thymine in DNA. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2109–2115. doi: 10.1073/pnas.48.12.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harm W. Differential effects of acriflavine and caffeine on various ultraviolet-irradiated Escherichia coli strains and T1 phage. Mutat Res. 1967 Mar-Apr;4(2):93–110. doi: 10.1016/0027-5107(67)90061-9. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P., Theriot L. Three loci in Escherichia coli K-12 that control the excision of pyrimidine dimers and certain other mutagen products from DNA. Genetics. 1966 Jun;53(6):1119–1136. doi: 10.1093/genetics/53.6.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAGGER J., STAFFORD R. S. EVIDENCE FOR TWO MECHANISMS OF PHOTOREACTIVATION IN ESCHERICHIA COLI B. Biophys J. 1965 Jan;5:75–88. doi: 10.1016/s0006-3495(65)86703-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LERMAN L. S. Structural considerations in the interaction of DNA and acridines. J Mol Biol. 1961 Feb;3:18–30. doi: 10.1016/s0022-2836(61)80004-1. [DOI] [PubMed] [Google Scholar]
- LERMAN L. S. The structure of the DNA-acridine complex. Proc Natl Acad Sci U S A. 1963 Jan 15;49:94–102. doi: 10.1073/pnas.49.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIEB M. DARK REPAIR OF UV INDUCTION IN K12 (LAMBDA). Virology. 1964 Jul;23:381–388. doi: 10.1016/0042-6822(64)90260-0. [DOI] [PubMed] [Google Scholar]
- MATNEY T. S., SHANKEL D. M., WYSS O. Mutations induced by ultraviolet light without attendant lethality. J Bacteriol. 1958 Feb;75(2):180–183. doi: 10.1128/jb.75.2.180-183.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SETLOW R. B., CARRIER W. L. THE DISAPPEARANCE OF THYMINE DIMERS FROM DNA: AN ERROR-CORRECTING MECHANISM. Proc Natl Acad Sci U S A. 1964 Feb;51:226–231. doi: 10.1073/pnas.51.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SETLOW R. B. PHYSICAL CHANGES AND MUTAGENESIS. J Cell Physiol. 1964 Oct;64:SUPPL 1–1:68. [PubMed] [Google Scholar]
- SETLOW R. B., SWENSON P. A., CARRIER W. L. THYMINE DIMERS AND INHIBITION OF DNA SYNTHESIS BY ULTRAVIOLET IRRADIATION OF CELLS. Science. 1963 Dec 13;142(3598):1464–1466. doi: 10.1126/science.142.3598.1464. [DOI] [PubMed] [Google Scholar]
- SHANKEL D. M. "Mutational synergism" of ultraviolet light and caffeine in Escherichia coli. J Bacteriol. 1962 Sep;84:410–415. doi: 10.1128/jb.84.3.410-415.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH K. C. A chemical basis for the sensitization of bacteria to ultraviolet light by incorporated bromouracil. Biochem Biophys Res Commun. 1962 Jan 24;6:458–463. doi: 10.1016/0006-291x(62)90375-3. [DOI] [PubMed] [Google Scholar]
- Setlow R. B., Carrier W. L., Bollum F. J. Pyrimidine dimers in UV-irradiated poly dI:dC. Proc Natl Acad Sci U S A. 1965 May;53(5):1111–1118. doi: 10.1073/pnas.53.5.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuster R. C., Boyce R. P. The excision of thymine dimer from the DNA of UV-irradiated E. coli 15 T-A-U during thymine deprivation. Biochem Biophys Res Commun. 1964 Jul 27;16(5):489–496. doi: 10.1016/0006-291x(64)90381-x. [DOI] [PubMed] [Google Scholar]
- TS'O P. O., HELMKAMP G. K., SANDER C. Interaction of nucleosides and related compounds with nucleic acids as indicated by the change of helix-coil transition temperature. Proc Natl Acad Sci U S A. 1962 Apr 15;48:686–698. doi: 10.1073/pnas.48.4.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WACKER A., JACHERTS D. [Ultraviolet ray resistance of azathymine-treated bacterial cells]. J Mol Biol. 1962 May;4:413–414. doi: 10.1016/s0022-2836(62)80023-0. [DOI] [PubMed] [Google Scholar]
- WITKIN E. M. Modification of mutagenesis initiated by ultraviolet light through postteatment of bacteria with basic dyes. J Cell Comp Physiol. 1961 Dec;58(3):135–144. doi: 10.1002/jcp.1030580413. [DOI] [PubMed] [Google Scholar]
- WITKIN E. M. PHOTOREVERSAL AND "DARK REPAIR" OF MUTATIONS TO PROTOTROPHY INDUCED BY ULTRAVIOLET LIGHT IN PHOTOREACTIVABLE AND NON-PHOTOREACTIVABLE STRAINS OF ESCHERICHIA COLI. Mutat Res. 1964 May;106:22–36. doi: 10.1016/0027-5107(64)90049-1. [DOI] [PubMed] [Google Scholar]
- WITKIN E. M., SICURELLA N. A., BENNETT G. M. PHOTOREVERSIBILITY OF INDUCED MUTATIONS IN A NONPHOTOREACTIVABLE STRAIN OF ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1963 Dec;50:1055–1059. doi: 10.1073/pnas.50.6.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WULFF D. L., RUPERT C. S. Disappearance of thymine photodimer in ultraviolet irradiated DNA upon treatment with a photoreactivating enzyme from baker's yeast. Biochem Biophys Res Commun. 1962 Apr 20;7:237–240. doi: 10.1016/0006-291x(62)90181-x. [DOI] [PubMed] [Google Scholar]
- WULFF D. L. THE ROLE OF THYMINE DIMER IN THE PHOTO-INACTIVATION OF THE BACTERIOPHAGE T4V1. J Mol Biol. 1963 Oct;7:431–441. doi: 10.1016/s0022-2836(63)80035-2. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Radiation-induced mutations and their repair. Science. 1966 Jun 3;152(3727):1345–1353. doi: 10.1126/science.152.3727.1345. [DOI] [PubMed] [Google Scholar]


