Abstract
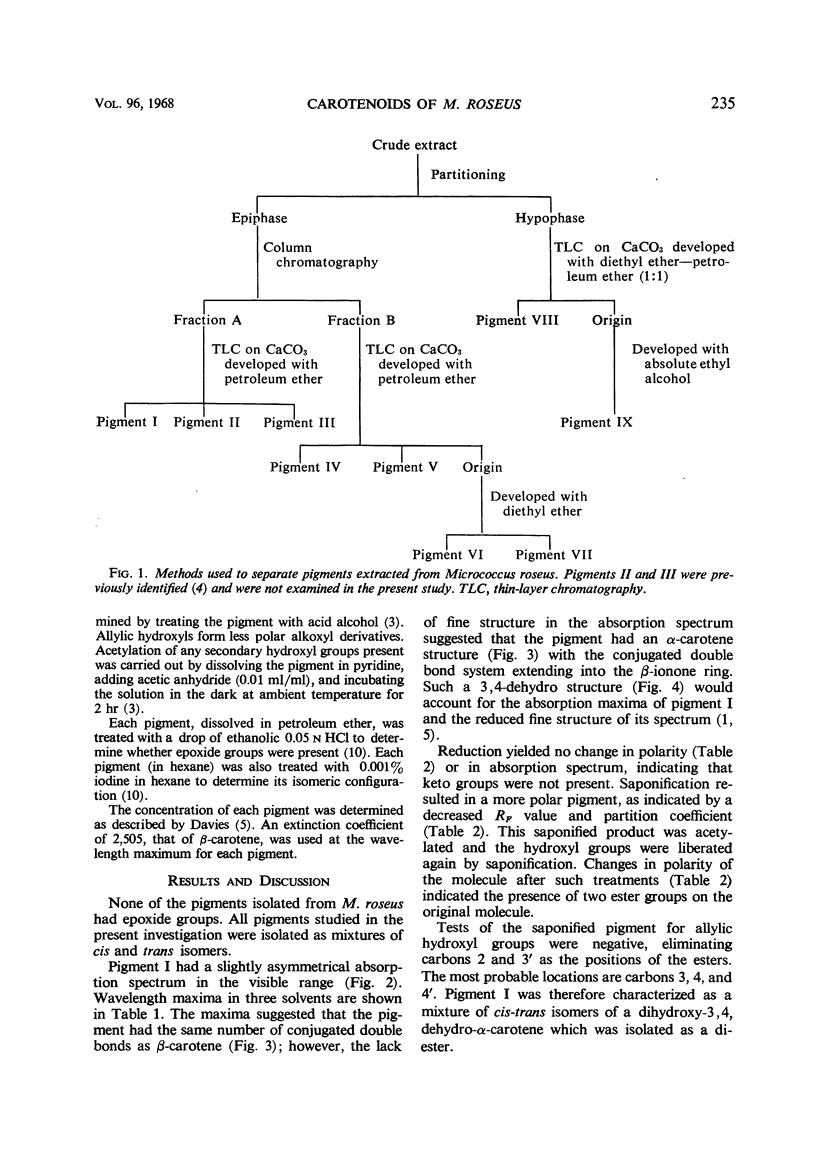
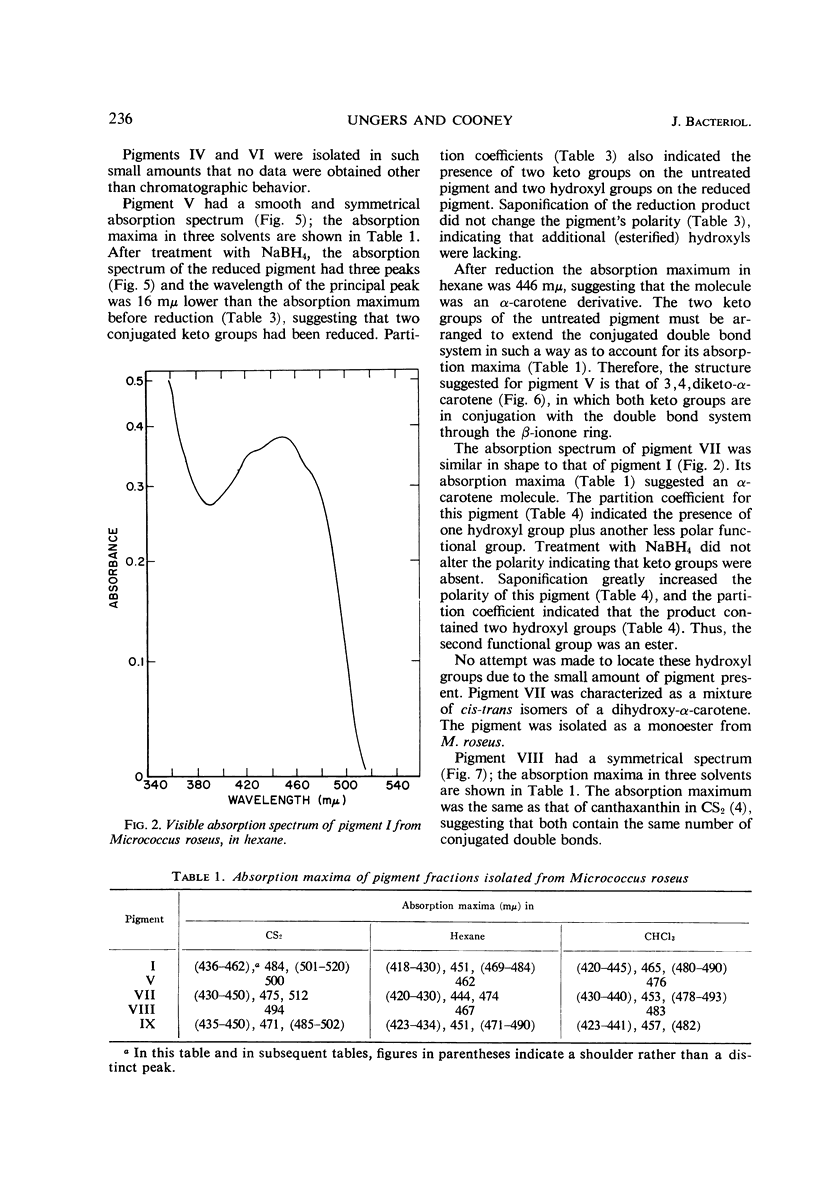
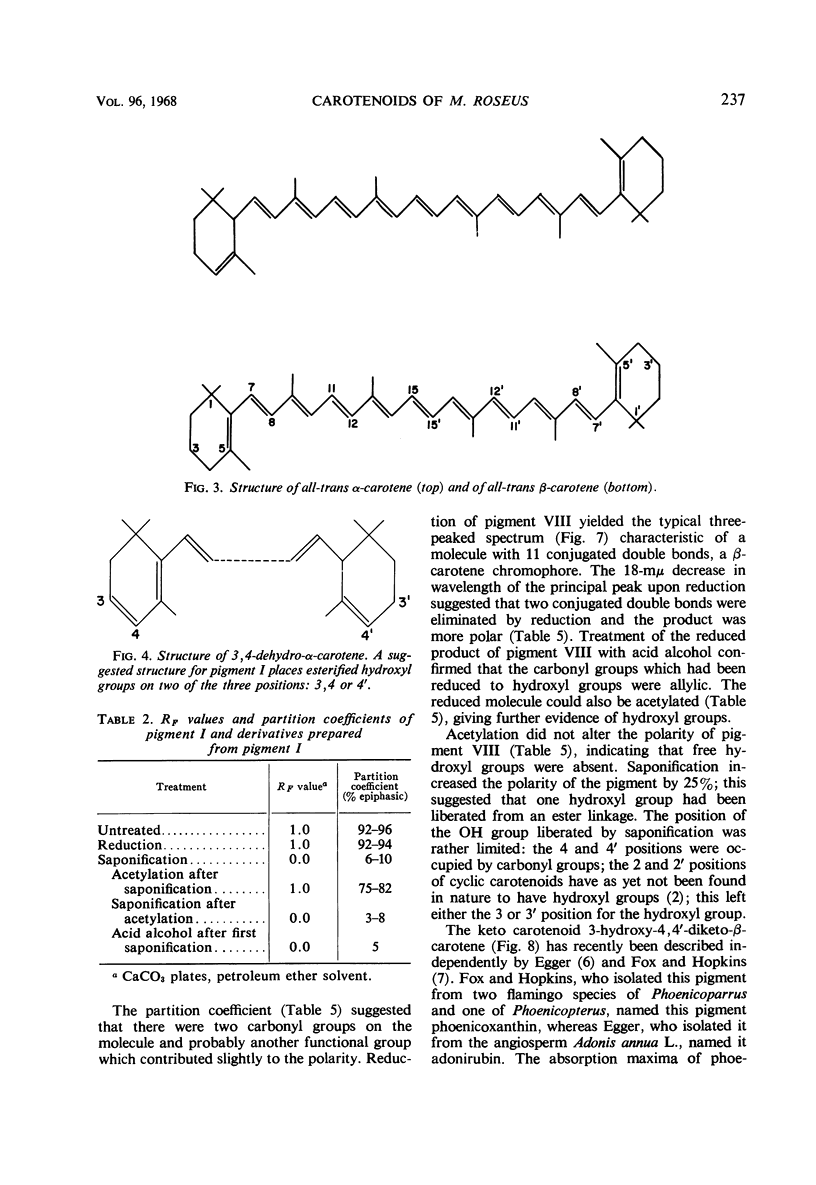
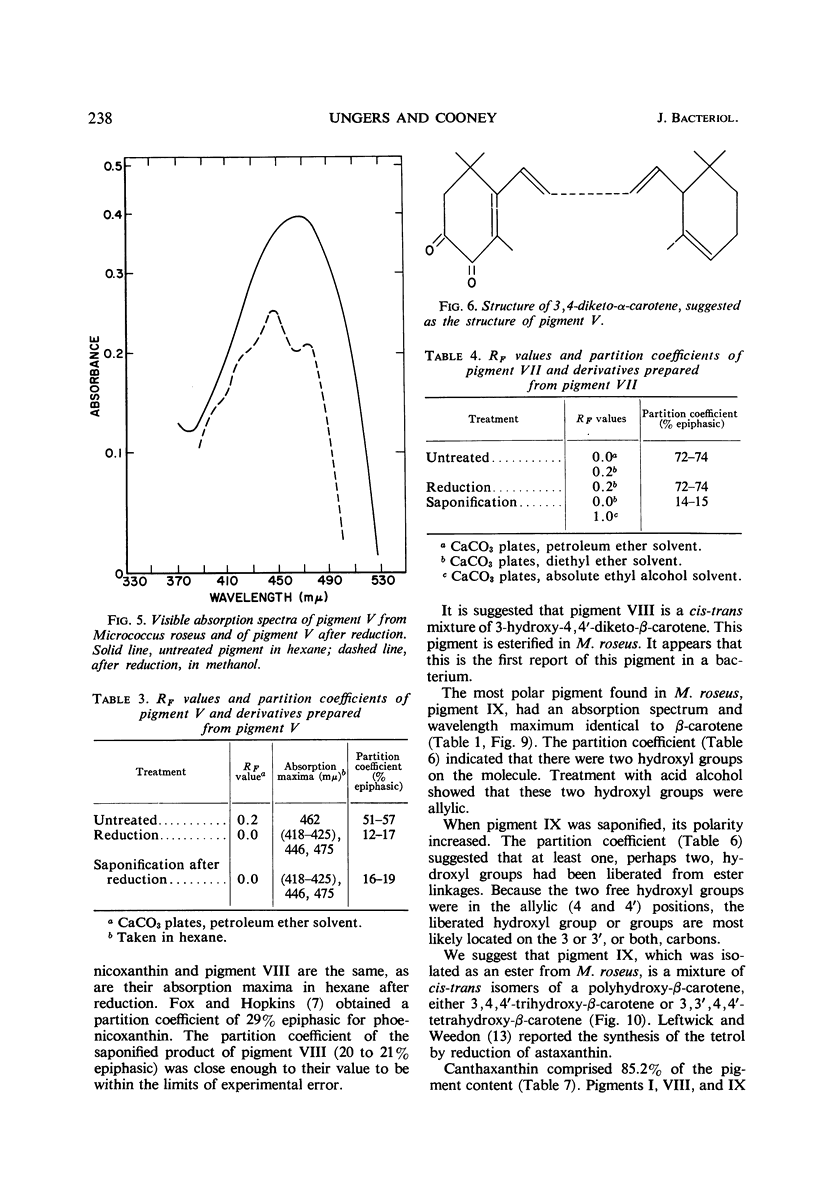
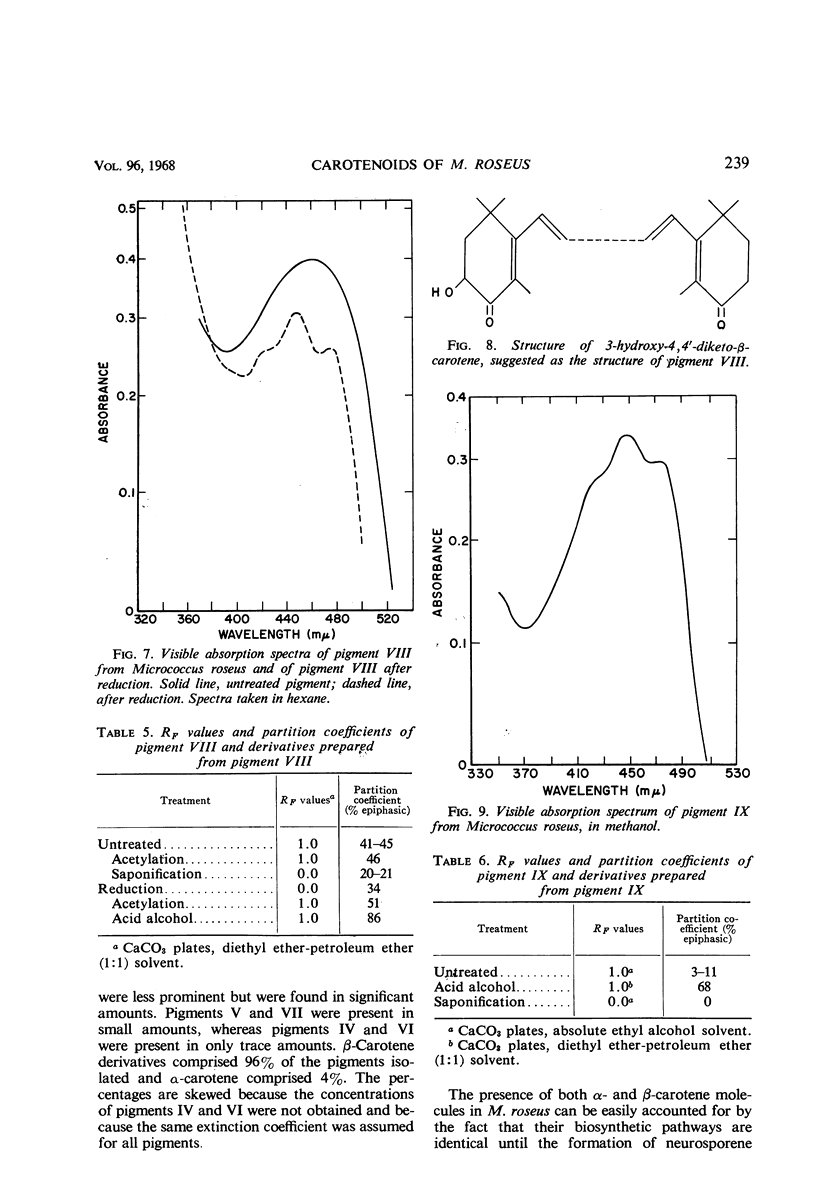
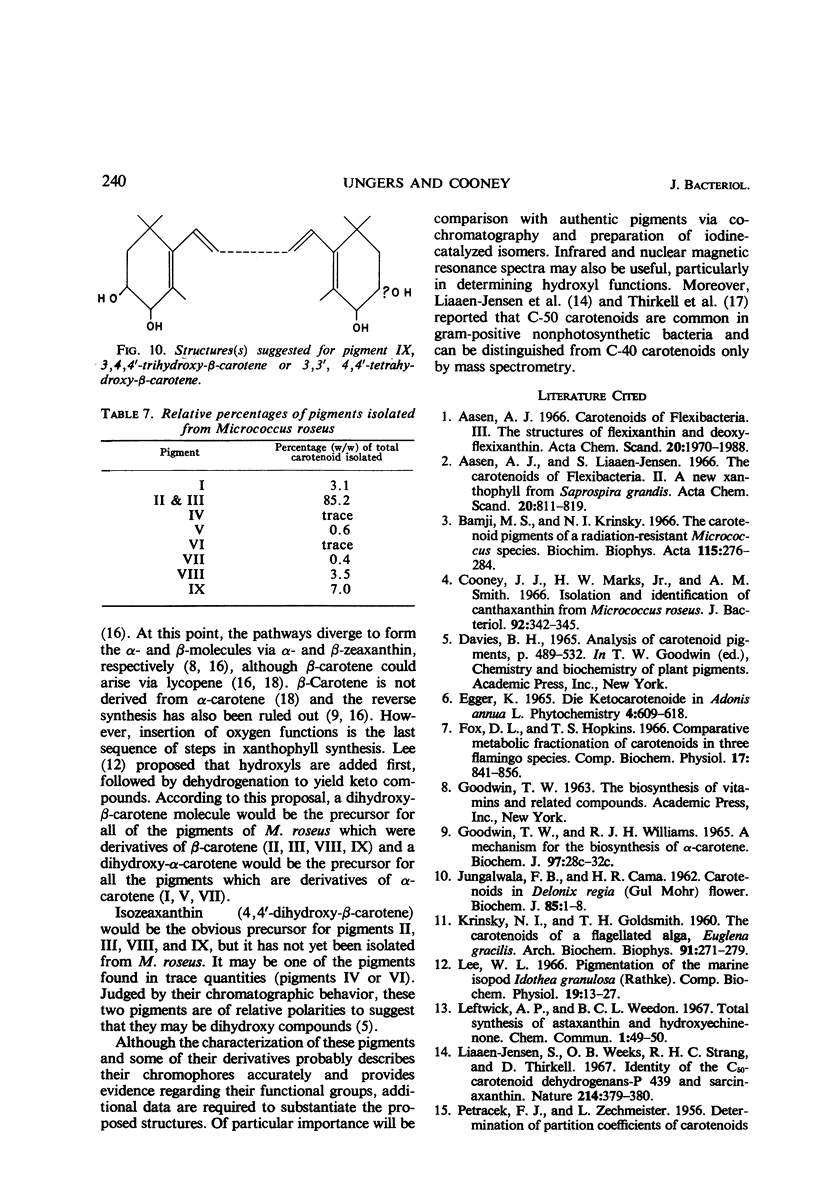
In addition to canthaxanthin, seven pigment fractions were isolated from Micrococcus roseus. They were purified by solvent partitioning and by column and thin-layer chromatography. Visible absorption spectra, chromatographic behavior, and partition coefficients of the pigments and derivatives prepared from the pigments were used in characterizing them. Both α- and β-carotene derivatives were present. The structure of one pigment was suggested as phoenicoxanthin (3-hydroxy-4,4′-diketo-β-carotene). Four other pigments were tentatively characterized as a dihydroxy-3,4-dehydro-α-carotene, a dihydroxy-α-carotene, a diketo-α-carotene, and a polyhydroxy-β-carotene. Two pigments were isolated in trace amounts and could not be characterized. All the pigments studied were isolated as mixtures of cis-trans isomers and all except the diketo-α-carotene were isolated as esters from M. roseus. Quantitation of the pigments showed that canthaxanthin (4,4′-diketo-β-carotene) represented 85% of the pigment recovered from extracts. Three of the other pigments contributed a significant proportion of the remaining pigments, whereas the other four were present in only small amounts. β-Carotene derivatives comprised 96% and α-carotene derivatives 4% of the pigments recovered from extracts.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aasen A. J., Jensen S. L. Carotenoids of flexibacteria. 3. The structures of flexixanthin and deoxy-flexixanthin. Acta Chem Scand. 1966;20(7):1970–1988. doi: 10.3891/acta.chem.scand.20-1970. [DOI] [PubMed] [Google Scholar]
- Aasen A. J., Jensen S. L. The carotenoids of flexibacteria. 2. A new xanthophyll from Saprospira grandis. Acta Chem Scand. 1966;20(3):811–819. doi: 10.3891/acta.chem.scand.20-0811. [DOI] [PubMed] [Google Scholar]
- Bamji M. S., Krinsky N. I. The carotenoid pigments of a radiation-resistant Micrococcus species. Biochim Biophys Acta. 1966 Feb 28;115(2):276–284. doi: 10.1016/0304-4165(66)90426-0. [DOI] [PubMed] [Google Scholar]
- Cooney J. J., Marks H. W., Smith A. M. Isolation and Identification of Canthaxanthin from Micrococcus roseus. J Bacteriol. 1966 Aug;92(2):342–345. doi: 10.1128/jb.92.2.342-345.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox D. L., Hopkins T. S. Comparative metabolic fractionation of carotenoids in three flamingo species. Comp Biochem Physiol. 1966 Mar;17(3):841–856. doi: 10.1016/0010-406x(66)90125-3. [DOI] [PubMed] [Google Scholar]
- Goodwin T. W., Williams R. J. A mechanism for the biosynthesis of alpha-carotene. Biochem J. 1965 Dec;97(3):28C–32C. doi: 10.1042/bj0970028c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUNGALWALA F. B., CAMA H. R. Carotenoids in Delonix regia (Gul Mohr) flower. Biochem J. 1962 Oct;85:1–8. doi: 10.1042/bj0850001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRINSKY N. I., GOLDSMITH T. H. The carotenoids of the flagellated alga. Euglena gracilis. Arch Biochem Biophys. 1960 Dec;91:271–279. doi: 10.1016/0003-9861(60)90501-4. [DOI] [PubMed] [Google Scholar]
- Liaaen-Jensen S., Weeks O. B., Strang R. H., Thirkell D. Identity of the C-50-carotenoid dehydrogenans-P439 and sarcinaxanthin. Nature. 1967 Apr 22;214(5086):379–380. doi: 10.1038/214379c0. [DOI] [PubMed] [Google Scholar]
- Thirkell D., Strang R. H., Chapman J. R. The pigments of Sarcina flava: a new series of C50 carotenoids. J Gen Microbiol. 1967 Oct;49(1):157–164. doi: 10.1099/00221287-49-1-157. [DOI] [PubMed] [Google Scholar]
- Williams R. J., Britton G., Goodwin T. W. The biosynthesis of cyclic carotenes. Biochem J. 1967 Oct;105(1):99–105. doi: 10.1042/bj1050099. [DOI] [PMC free article] [PubMed] [Google Scholar]


