Abstract
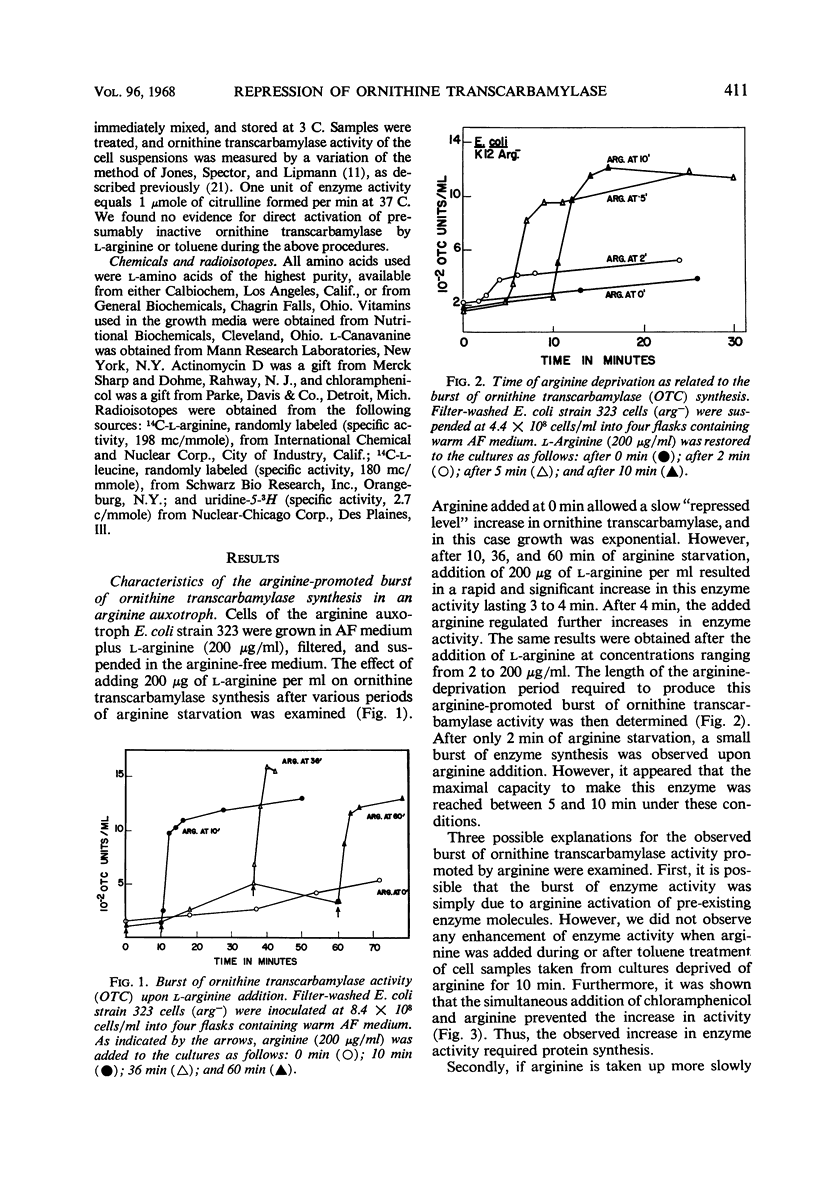
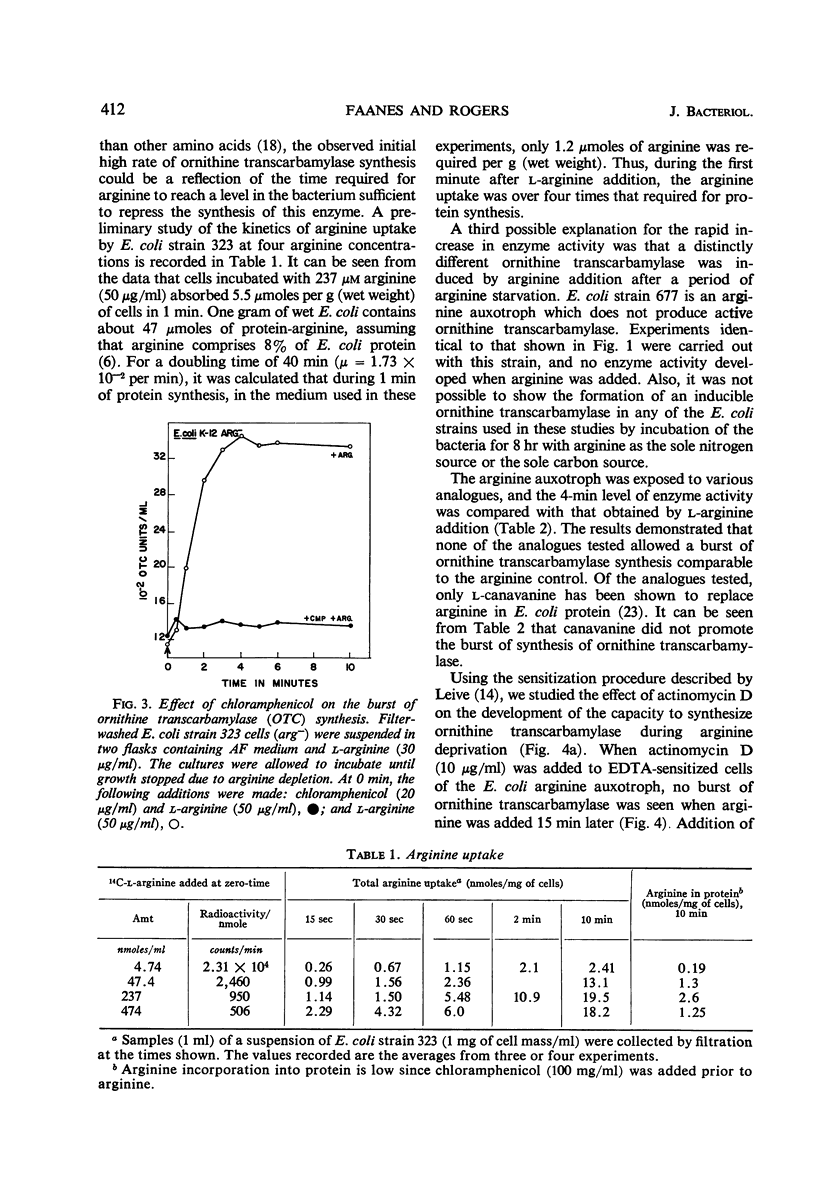
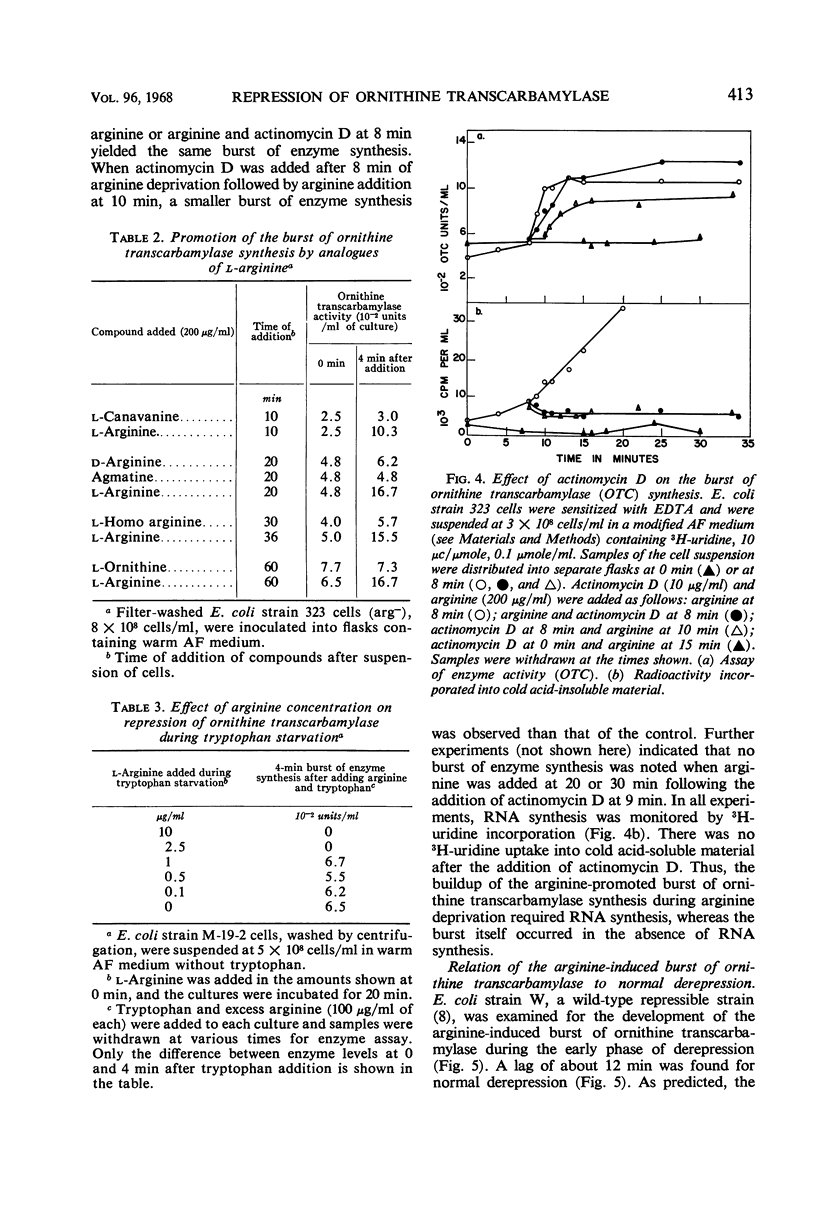
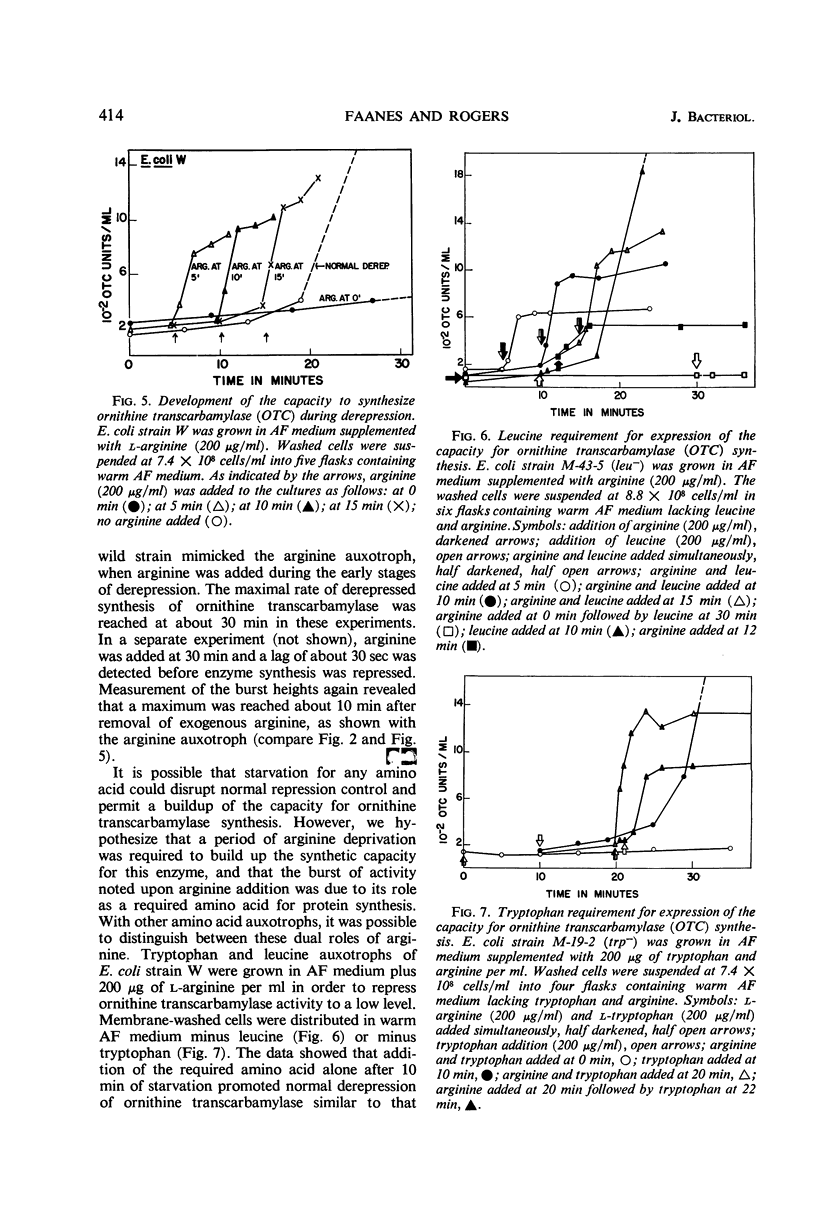
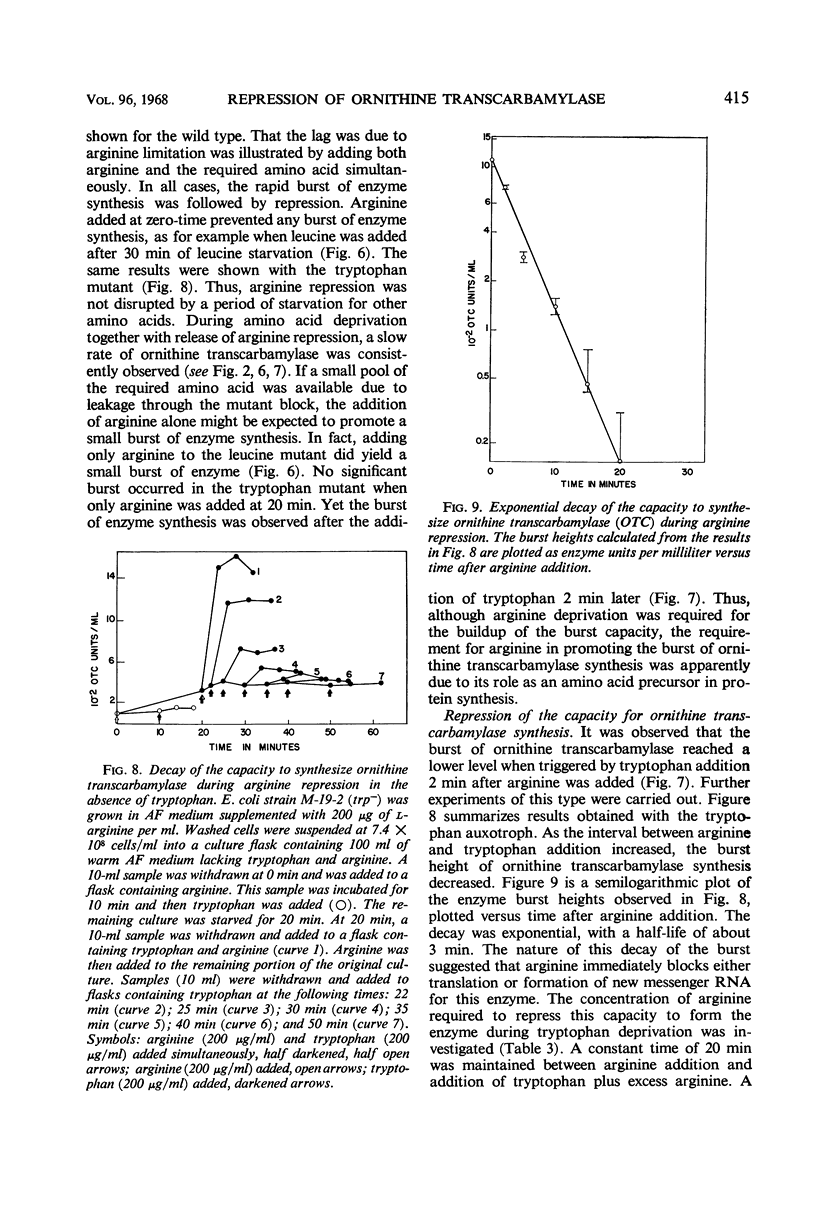
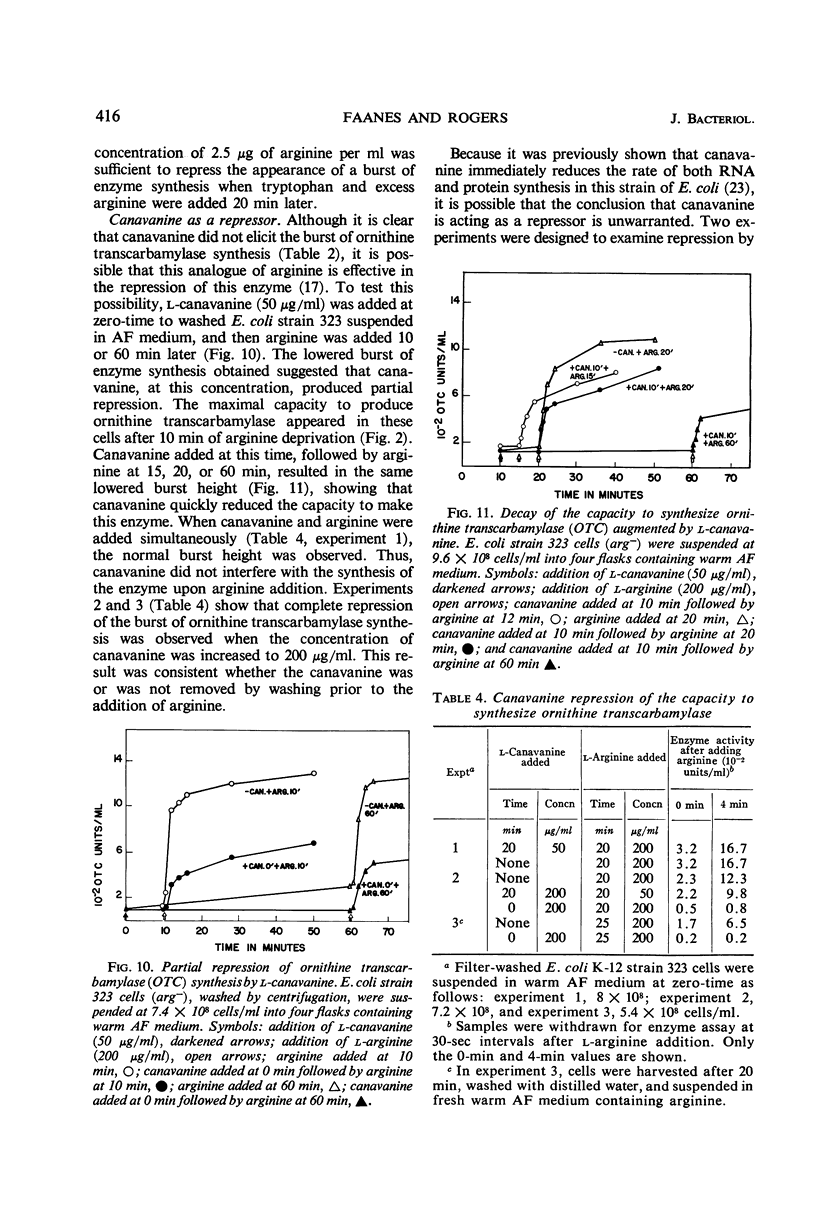
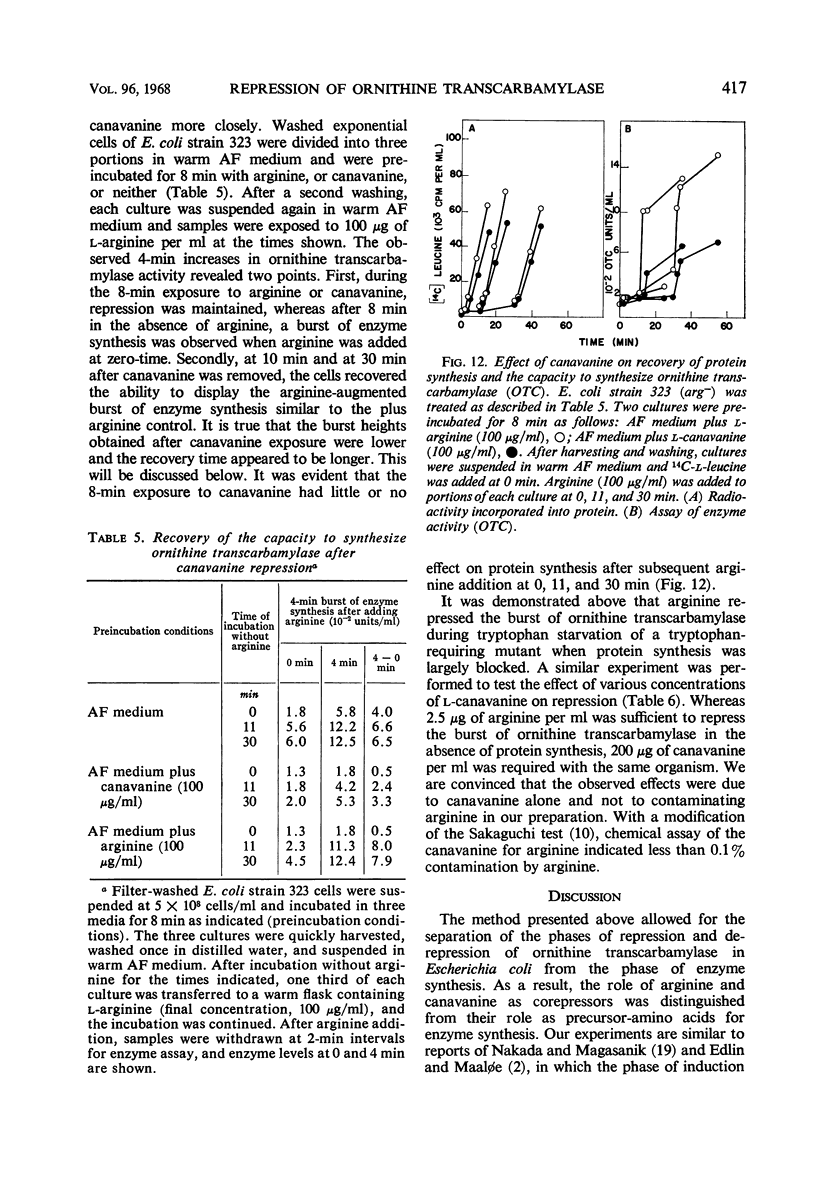
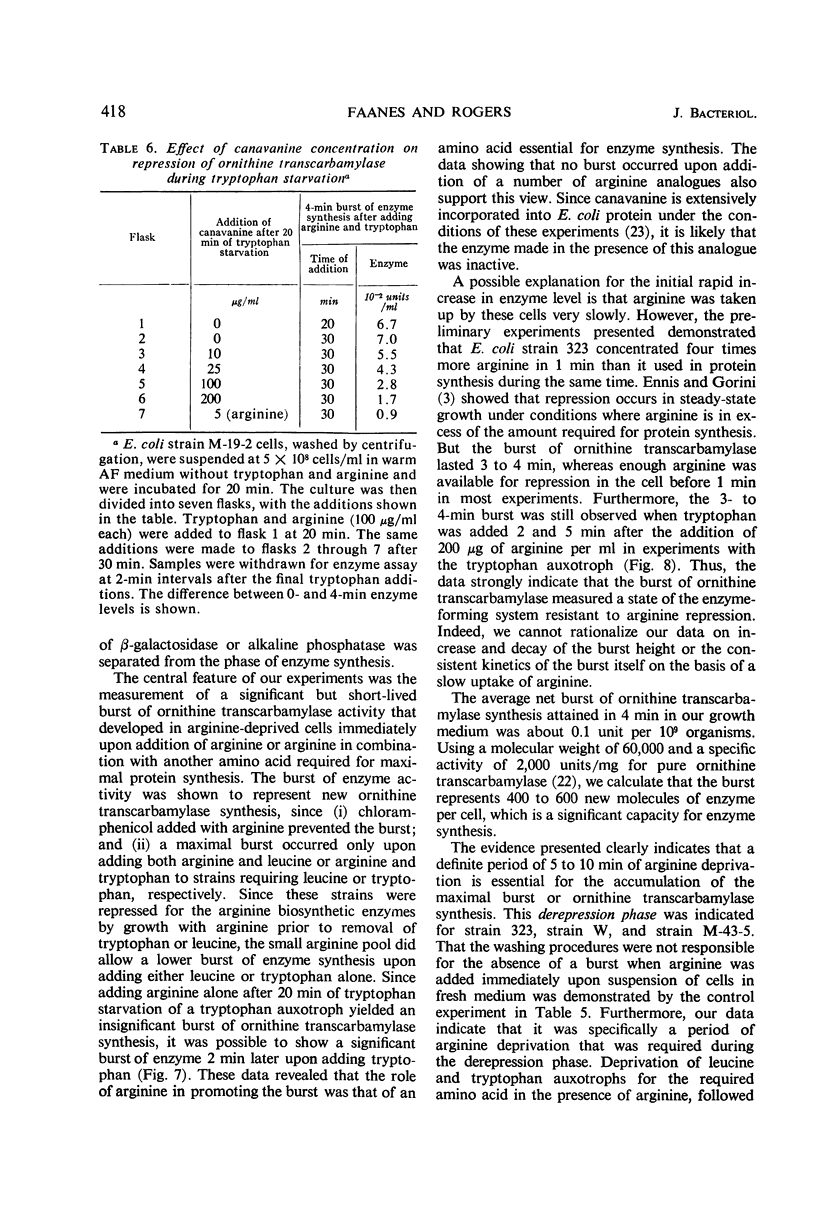
Conditions were found under which the processes of repression and derepression of ornithine transcarbamylase were separated from the process of enzyme synthesis. After 10 min of arginine deprivation followed by the addition of 2 to 200 μg of l-arginine per ml, a number of strains of Escherichia coli exhibited a significant burst of ornithine transcarbamylase synthesis which lasted 3 to 4 min before the onset of repression. The rapid increase of enzyme activity was shown to require protein synthesis, and was not due to a slow uptake of arginine or induction of an arginine-inducible ornithine transcarbamylase. The capacity of E. coli to synthesize the burst of ornithine transcarbamylase reached a maximum after 10 min of arginine deprivation and then remained constant. The observed increase in enzyme synthesis may reflect the level of unstable messenger ribonucleic acid (RNA) for ornithine transcarbamylase present in the cell at the time protein synthesis was reinitiated. After the addition of arginine in the absence of protein synthesis, the burst of ornithine transcarbamylase decayed with a half-life of about 3 min. The data implied that arginine prevents synthesis of new messenger RNA that can translate this enzyme. Repression of ornithine transcarbamylase by l-canavanine (100 to 200 μg/ml) was observed, and no active enzyme was formed in the presence of this analogue. The action of canavanine as a repressor was distinguished from the inhibitory effect of this compound on protein synthesis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENNIS H. L., GORINI L. Control of arginine biosynthesis in strains of Escherichia coli not repressible by arginine. J Mol Biol. 1961 Aug;3:439–446. doi: 10.1016/s0022-2836(61)80056-9. [DOI] [PubMed] [Google Scholar]
- Edlin G., Maaloe O. Synthesis and breakdown of messenger RNA without protein synthesis. J Mol Biol. 1966 Feb;15(2):428–434. doi: 10.1016/s0022-2836(66)80118-3. [DOI] [PubMed] [Google Scholar]
- Fan D. P. Decay of intact messengers in bacteria. J Mol Biol. 1966 Mar;16(1):164–179. doi: 10.1016/s0022-2836(66)80270-x. [DOI] [PubMed] [Google Scholar]
- Friesen J. D. Control of messenger RNA synthesis and decay in Escherichia coli. J Mol Biol. 1966 Oct;20(3):559–573. doi: 10.1016/0022-2836(66)90011-8. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN A., GOLDSTEIN D. B., LOWNEY L. I. PROTEIN SYNTHESIS OF 0 DEGREES C IN ESCHERICHIA COLI. J Mol Biol. 1964 Jul;9:213–235. doi: 10.1016/s0022-2836(64)80102-9. [DOI] [PubMed] [Google Scholar]
- GORINI L., GUNDERSEN W., BURGER M. Genetics of regulation of enzyme synthesis in the arginine biosynthetic pathway of Escherichia coli. Cold Spring Harb Symp Quant Biol. 1961;26:173–182. doi: 10.1101/sqb.1961.026.01.022. [DOI] [PubMed] [Google Scholar]
- GORINI L., MAAS W. K. The potential for the formation of a biosynthetic enzyme in Escherichia coli. Biochim Biophys Acta. 1957 Jul;25(1):208–209. doi: 10.1016/0006-3002(57)90450-x. [DOI] [PubMed] [Google Scholar]
- HIATT H. H., GROS F., JACOB F. The effect of induction and repression on the rate of synthesis of messenger ribonucleic acid. Biochim Biophys Acta. 1963 May 28;72:15–23. [PubMed] [Google Scholar]
- Izumi Y. New Sakaguchi reaction. II. Anal Biochem. 1965 Jul;12(1):1–7. doi: 10.1016/0003-2697(65)90136-3. [DOI] [PubMed] [Google Scholar]
- KEPES A. KINETICS OF INDUCED ENZYME SYNTHESIS. DETERMINATION OF THE MEAN LIFE OF GALACTOSIDASE-SPECIFIC MESSENGER RNA. Biochim Biophys Acta. 1963 Oct 15;76:293–309. [PubMed] [Google Scholar]
- Kepes A., Beguin S. Peptide chain initiation and growth in the induced synthesis of beta-galactosidase. Biochim Biophys Acta. 1966 Sep;123(3):546–560. doi: 10.1016/0005-2787(66)90222-x. [DOI] [PubMed] [Google Scholar]
- LEVINTHAL C., SIGNER E. R., FETHEROLF K. Reactivation and hybridization of reduced alkaline phosphatase. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1230–1237. doi: 10.1073/pnas.48.7.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leive L., Kollin V. Synthesis, utilization and degradation of lactose operon mRNA in Escherichia coli. J Mol Biol. 1967 Mar 14;24(2):247–259. doi: 10.1016/0022-2836(67)90330-0. [DOI] [PubMed] [Google Scholar]
- MAAS W. K. Studies on repression of arginine biosynthesis in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1961;26:183–191. doi: 10.1101/sqb.1961.026.01.023. [DOI] [PubMed] [Google Scholar]
- Maas W. K. Genetic defects affecting an arginine permease and repression of arginine synthesis in Escherichia coli. Fed Proc. 1965 Sep-Oct;24(5):1239–1242. [PubMed] [Google Scholar]
- NAKADA D., MAGASANIK B. THE ROLES OF INDUCER AND CATABOLITE REPRESSOR IN THE SYNTHESIS OF BETA-GALACTOSIDASE BY ESCHERICHIA COLI. J Mol Biol. 1964 Jan;8:105–127. doi: 10.1016/s0022-2836(64)80153-4. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P., MAAS W. K. Control by endogenously synthesized arginine of the formation of ornithine transcarbamylase in Escherichia coli. J Bacteriol. 1961 Feb;81:236–240. doi: 10.1128/jb.81.2.236-240.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGERS P., NOVELLI G. D. Formation of ornithine transcarbamylase in cells and protoplasts of Escherichia coli. Biochim Biophys Acta. 1959 Jun;33(2):423–436. doi: 10.1016/0006-3002(59)90132-5. [DOI] [PubMed] [Google Scholar]
- ROGERS P., NOVELLI G. D. Purification of ornithine transcarbamylase from derepressed cells of Escherichia coli W. Arch Biochem Biophys. 1962 Feb;96:398–407. doi: 10.1016/0003-9861(62)90426-5. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ J. H., MAAS W. K. Analysis of the inhibition of growth produced by canavanine in Escherichia coli. J Bacteriol. 1960 Jun;79:794–799. doi: 10.1128/jb.79.6.794-799.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtele C. F., Rogers P. Canavanine death in Escherichia coli. J Mol Biol. 1965 Dec;14(2):474–489. doi: 10.1016/s0022-2836(65)80197-8. [DOI] [PubMed] [Google Scholar]


