Abstract
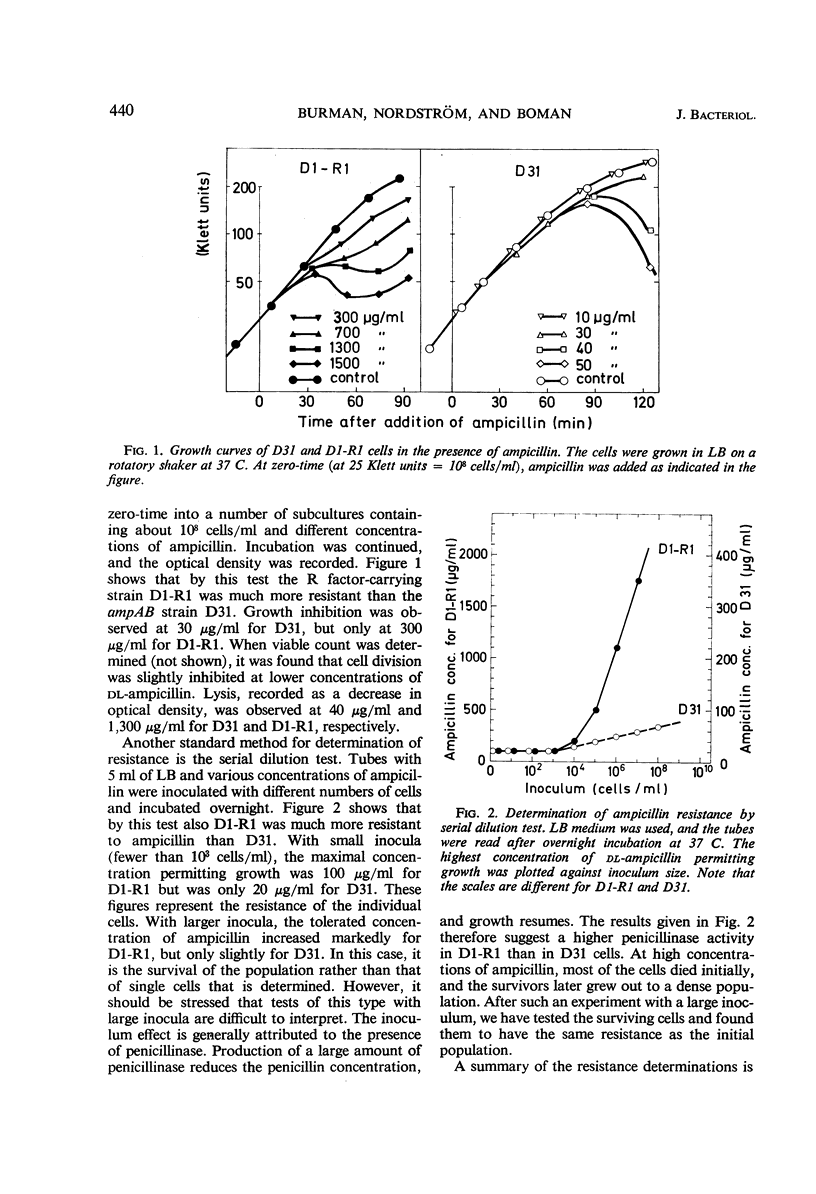
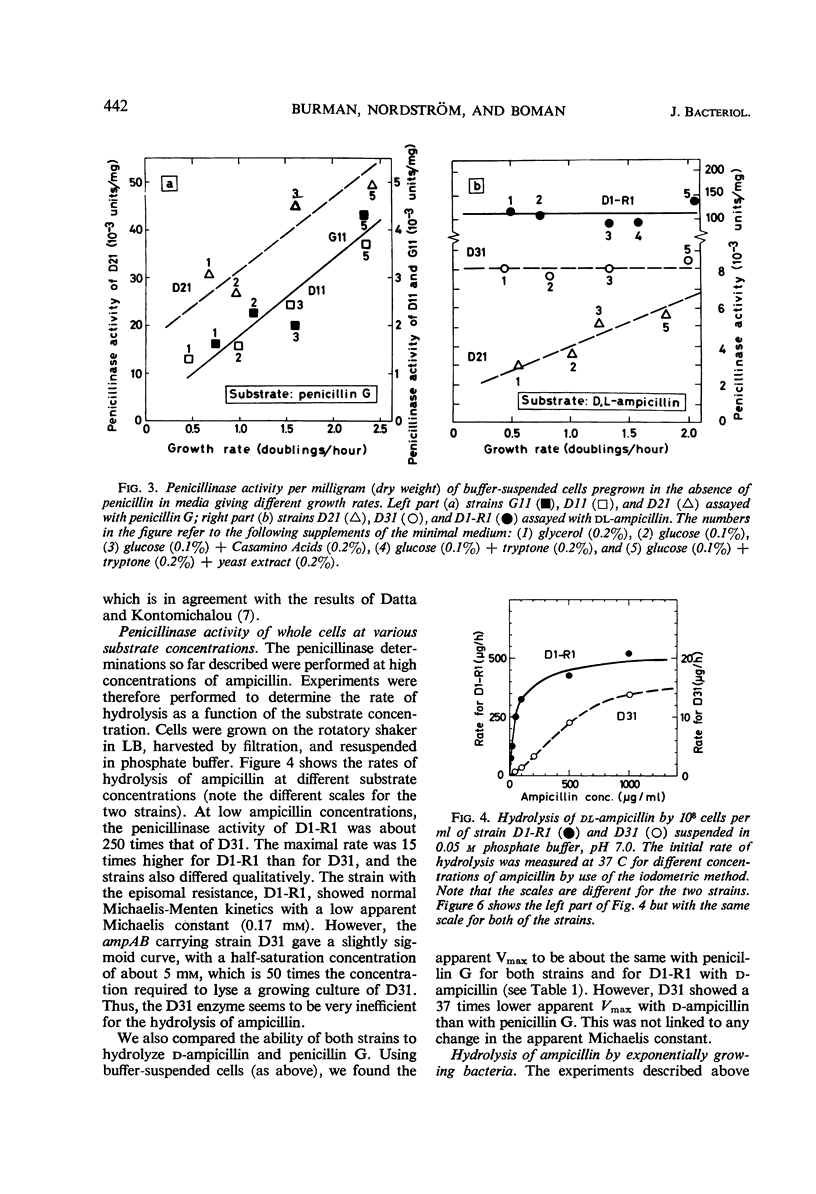
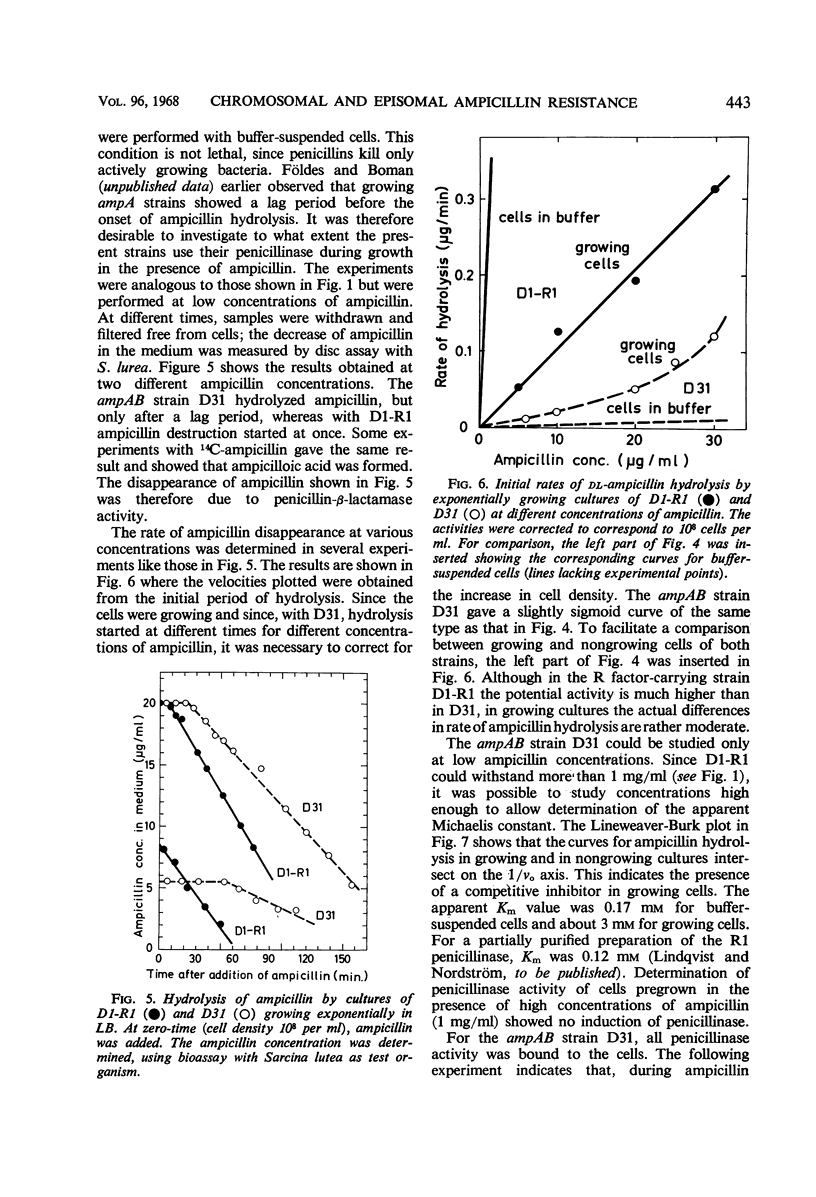
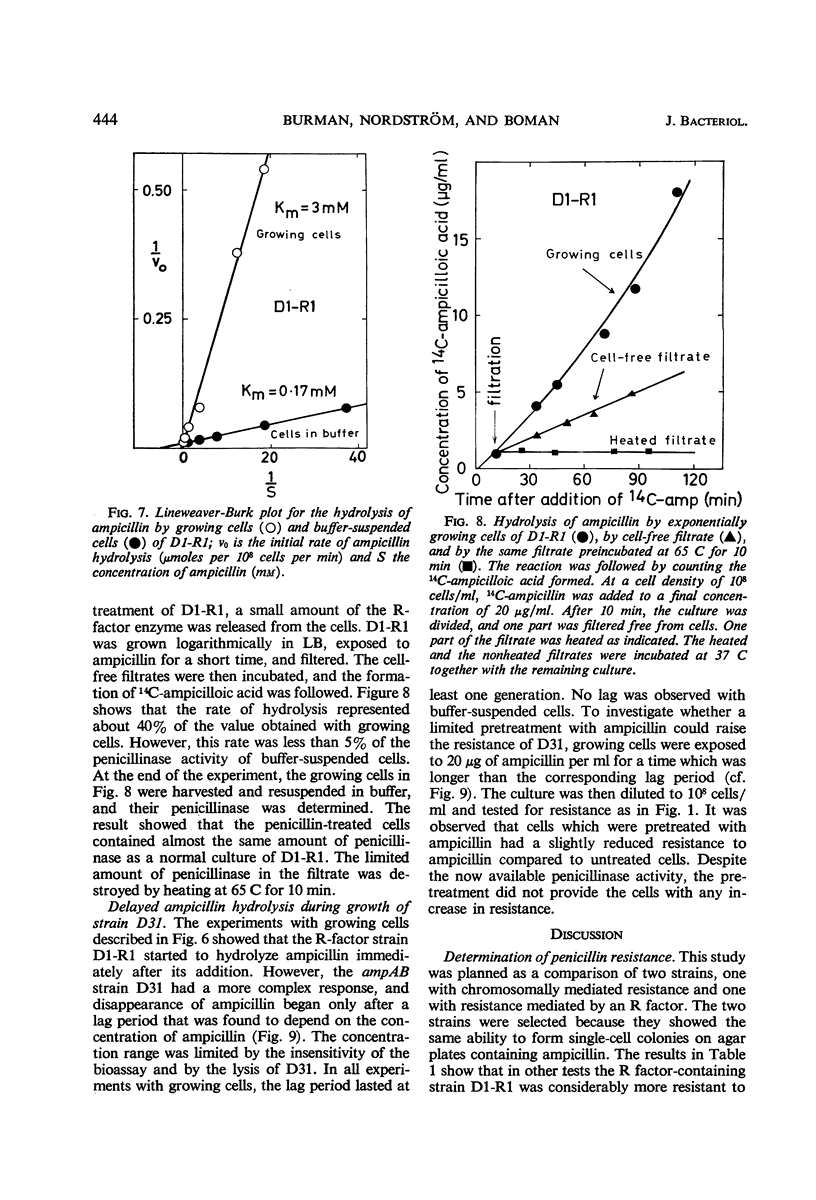
Two essentially isogenic strains of Escherichia coli K-12 were compared: D31 had chromosomally and D1-R1 episomally mediated resistance to ampicillin. The two strains had the same ability to form colonies on ampicillin plates, but in other tests they were quite different. In serial dilution tests as well as in exponentially growing cultures, D1-R1 was far more resistant to ampicillin than was D31. The inoculum effect with D1-R1 was large and with D31 was rather small. On plates, D31 was more resistant to penicillin G than was D1-R1. The penicillinase activity of buffer suspended cells against dl-ampicillin was 15 times higher for D1-R1 than for D31, but the two strains showed about the same rate of hydrolysis of penicillin G. With dl-ampicillin as substrate, for D1-R1 the apparent Km was 1.7 × 10−4m, whereas D31 gave a slightly sigmoid curve with a half-saturation concentration of about 5 × 10−3m. No induction of penicillinase activity was found. When the growth rate was varied by a factor of four, the amount of penicillinase per cell mass was constant in both D1-R1 and D31, whereas in two wild-type strains the amounts of penicillinase increased with increasing growth rates. With exponentially growing D1-R1, ampicillin disappearance started within 3 min, but at low ampicillin concentrations the rate was less than 10% of the rate of hydrolysis by buffer-suspended cells. Before D31 started hydrolysis, there was a lag period that lasted at least one generation and depended on the concentration of ampicillin. After this lag period, the rate of hydrolysis was 10 times higher than that observed with buffer-suspended cells. These differences between growing and nongrowing cells indicate that both the chromosomally and the episomally mediated penicillinases are controlled by some products present in growing cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERTANI G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951 Sep;62(3):293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOMAN H. G., ERIKSSON K. G. Penicillin-induced lysis in Escherichia coli. J Gen Microbiol. 1963 Jun;31:339–352. doi: 10.1099/00221287-31-3-339. [DOI] [PubMed] [Google Scholar]
- Citri N., Pollock M. R. The biochemistry and function of beta-lactamase (penicillinase). Adv Enzymol Relat Areas Mol Biol. 1966;28:237–323. doi: 10.1002/9780470122730.ch4. [DOI] [PubMed] [Google Scholar]
- Datta N. Infectious drug resistance. Br Med Bull. 1965 Sep;21(3):254–259. doi: 10.1093/oxfordjournals.bmb.a070405. [DOI] [PubMed] [Google Scholar]
- Datta N., Kontomichalou P. Penicillinase synthesis controlled by infectious R factors in Enterobacteriaceae. Nature. 1965 Oct 16;208(5007):239–241. doi: 10.1038/208239a0. [DOI] [PubMed] [Google Scholar]
- Datta N., Richmond M. H. The purification and properties of a penicillinase whose synthesis is mediated by an R-factor in Escherichia coli. Biochem J. 1966 Jan;98(1):204–209. doi: 10.1042/bj0980204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson-Grennberg K. G., Boman H. G., Jansson J. A., Thorén S. Resistance of Escherichia coli to Penicillins I. Genetic Study of Some Ampicillin-Resistant Mutants. J Bacteriol. 1965 Jul;90(1):54–62. doi: 10.1128/jb.90.1.54-62.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynell E., Datta N. The relation of resistance transfer factors to the F-factor (sex-factor) of Escherichia coli K12. Genet Res. 1966 Feb;7(1):134–140. doi: 10.1017/s0016672300009538. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P. Micro-iodometric assay for penicillinase. Biochem J. 1962 May;83:236–240. doi: 10.1042/bj0830236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POLLOCK M. R. PURIFICATION AND PROPERTIES OF PENICILLINASES FROM TWO STRAINS OF BACILLUS LICHENIFORMIS: A CHEMICAL, PHYSICOCHEMICAL AND PHYSIOLOGICAL COMPARISON. Biochem J. 1965 Mar;94:666–675. doi: 10.1042/bj0940666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock M. R. Origin and function of penicillinase: a problem in biochemical evolution. Br Med J. 1967 Oct 14;4(5571):71–77. doi: 10.1136/bmj.4.5571.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- WATANABE T. Infective heredity of multiple drug resistance in bacteria. Bacteriol Rev. 1963 Mar;27:87–115. doi: 10.1128/br.27.1.87-115.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T. Evolutionary relationships of R factors with other episomes and plasmids. Fed Proc. 1967 Jan-Feb;26(1):23–28. [PubMed] [Google Scholar]


