Abstract
Many eukaryotic cell surface proteins are anchored in the lipid bilayer through glycosylphosphatidylinositol (GPI). GPI anchors are covalently attached in the endoplasmic reticulum (ER). The modified proteins are then transported through the secretory pathway to the cell surface. We have identified two genes in Saccharomyces cerevisiae, LAG1 and a novel gene termed DGT1 (for “delayed GPI-anchored protein transport”), encoding structurally related proteins with multiple membrane-spanning domains. Both proteins are localized to the ER, as demonstrated by immunofluorescence microscopy. Deletion of either gene caused no detectable phenotype, whereas lag1Δ dgt1Δ cells displayed growth defects and a significant delay in ER-to-Golgi transport of GPI-anchored proteins, suggesting that LAG1 and DGT1 encode functionally redundant or overlapping proteins. The rate of GPI anchor attachment was not affected, nor was the transport rate of several non–GPI-anchored proteins. Consistent with a role of Lag1p and Dgt1p in GPI-anchored protein transport, lag1Δ dgt1Δ cells deposit abnormal, multilayered cell walls. Both proteins have significant sequence similarity to TRAM, a mammalian membrane protein thought to be involved in protein translocation across the ER membrane. In vivo translocation studies, however, did not detect any defects in protein translocation in lag1Δ dgt1Δ cells, suggesting that neither yeast gene plays a role in this process. Instead, we propose that Lag1p and Dgt1p facilitate efficient ER-to-Golgi transport of GPI-anchored proteins.
INTRODUCTION
A large number of proteins are transported across or integrated into the endoplasmic reticulum (ER)1 membrane (for review, see Walter and Johnson, 1994; Rapoport et al., 1996). These include most integral membrane and secreted proteins and most proteins destined to be stored in the lumen of intracellular organelles such as the ER, Golgi apparatus, lysosomes, and endosomes. In yeast, translocation of secretory precursors may follow one of two pathways (Ng et al., 1996). Membrane transit may occur either cotranslationally, in a reaction that requires targeting of ribosomes to the ER membrane, or posttranslationally, in an alternate pathway that allows polypeptides to traverse the ER membrane after their synthesis has been completed.
Cotranslational translocation across the ER membrane is initiated by the binding of the signal recognition particle (SRP) to ribosomes synthesizing polypeptides with hydrophobic signal sequences (for review, see Walter and Johnson, 1994). Interaction of SRP with the SRP receptor, a heterodimeric protein on the cytosolic surface of the ER membrane, targets the ribosome–nascent polypeptide complex to the ER membrane where a membrane complex termed the “translocon” catalyzes the transmembrane translocation of the nascent polypeptide. The core of the mammalian translocon consists of the heterotrimeric Sec61p complex, which is essential for translocation of all proteins and forms a hydrophilic, protein-conducting channel spanning the entire membrane (Simon and Blobel, 1991; Crowley et al., 1994).
Another component of the mammalian translocon is the multispanning membrane protein termed “trans-locating chain-associated membrane protein” (TRAM) which was found to be a principal cross-linking partner of different secretory proteins that are in transit across the membrane (Görlich et al., 1992). TRAM becomes cross-linked mostly to the charged, N-terminal region of the signal sequence (Görlich et al., 1992) and is no longer cross-linked to the nascent chain once the signal sequence is cleaved off by the signal peptidase (Mothes et al., 1994). Furthermore, nascent chains that carry a nonfunctional signal sequence are not cross-linked to TRAM, suggesting that nascent chains contact TRAM during an early phase of the translocation process (Jungnickel and Rapoport, 1995).
Protein translocation across the ER membrane can be reproduced using proteoliposomes reconstituted from detergent-solubilized ER membrane proteins (for review, see Rapoport et al., 1996). Interestingly, the heterotrimeric Sec61p complex, together with the SRP receptor, is sufficient for the translocation of some polypeptides into reconstituted proteoliposomes (Görlich and Rapoport, 1993). However, the translocation of most polypeptides depends on the presence of TRAM (Voigt et al., 1996). Whether a protein requires TRAM for translocation is determined by structural features of its signal sequence, supporting the notion that TRAM functions at an early phase of the translocation process (Voigt et al., 1996). Furthermore, TRAM was recently shown to be involved in regulating “translocational pausing,” a mechanism by which certain nascent secretory proteins are transiently exposed to the cytosol (Hegde et al., 1998). That study suggested that one role of TRAM may be to prevent cytosolic exposure of already translocated domains of the nascent chain. In contrast, studies on the integration of an integral membrane protein revealed cross-links between TRAM and the transmembrane segment rather late in the integration process, when the transmembrane domain can no longer be cross-linked to Sec61p and has laterally left the core of the translocon (Do et al., 1996). However, a functional role for TRAM in the release of transmembrane sequences into a lipid environment has not yet been demonstrated. Thus, the precise role of the mammalian TRAM protein remains obscure.
After translocation into the ER lumen, many proteins are further modified by posttranslational modifications such as signal sequence cleavage, side chain glycosylation, and disulfide bond formation. One additional modification is the addition of a glycosylphosphatidylinositol (GPI) anchor to the C terminus of the protein (for review, see Takeda and Kinoshita, 1995). GPI-anchored proteins represent a subclass of cell surface proteins serving diverse cellular functions such as transmembrane signaling, cell wall synthesis, and cell adhesion (Englund, 1993; Klis, 1994). In yeast, GPI-anchoring is an essential process for viability (Leidich et al., 1994; Hamburger et al., 1995; Schönbächler et al., 1995; Vossen et al., 1995).
GPI proteins are synthesized as precursors with two signal sequences: a classical cleavable signal sequence at the N terminus required for translocation of the protein into the ER lumen, and an additional hydrophobic region at the C terminus of the protein, which directs GPI anchoring in the lumen of the ER. During or after translocation of the protein into the ER, the C-terminal hydrophobic region is removed and replaced en bloc with a complete, preformed GPI anchor, which has been synthesized by the stepwise addition of sugars and phosphoethanolamine to phosphatidylinositol (Englund, 1993). Several of the genes involved in the synthesis of the yeast GPI anchor have been cloned and characterized (Hamburger et al., 1995; Leidich et al., 1995; Schönbächler et al., 1995; Benghezal et al., 1996).
GPI anchors have been proposed to play a role in protein sorting (Takeda and Kinoshita, 1995). In yeast, GPI anchor attachment is necessary for exit of GPI proteins from the ER (Nuoffer et al., 1993; Doering and Schekman, 1996). Only after anchor attachment are GPI proteins packaged into transport vesicles that leave the ER and are transported to the Golgi compartment (Schekman and Orci, 1996). How specific cargo proteins are recruited and concentrated into ER-derived transport vesicles is still poorly understood and the subject of intense investigation; GPI-anchored proteins may have unique requirements for this process (Brown, 1992; Brown and Rose, 1992; Futerman, 1995).
In this paper, we present evidence for two proteins that facilitate the vesicular transport of GPI proteins. In an attempt to identify the molecular function of TRAM, we identified two yeast proteins with significant sequence similarity to TRAM that are localized to the ER membrane. Unexpectedly, we found no evidence that these membrane proteins are involved in protein translocation across the ER membrane; instead, they appear to be specifically involved in the ER-to-Golgi transport of GPI-anchored proteins.
MATERIALS AND METHODS
Yeast Strains, Media, Reagents, and General Methods
Saccharomyces cerevisiae strains used in this study are listed in Table 1. Complete medium (YPD) and synthetic minimal media with glucose or galactose as the carbon source were used as described (Sherman, 1991). Synthetic complete minimal medium lacking inositol (SC − inositol) was prepared as published before (Culbertson and Henry, 1975). Growth rates were determined quantitatively at 15, 22, 30, 37, 39, and 40°C by measuring the optical density at 600 nm (OD600) of aliquots taken from exponentially growing YPD liquid cultures.
Table 1.
Haploid yeast strains used in this study
| Strain | Genotype | Reference/Source |
|---|---|---|
| W303-1A | MATa ade2-1 his3-11 leu2-3,-112 trp1-1 ura3-1 can1-100 | Deshaies and Schekman, 1990 |
| W303-1B | MATα ade2-1 his3-11 leu2-3,-112 trp1-1 ura3-1 can1-100 | Deshaies and Schekman, 1990 |
| DNY116 | MATα ade2-101ochre leu2Δ1 trp1Δ99 ura3Δ99 sec61-101 | Ng et al., 1996 |
| NY414 | MATaura3-52 sec13-1 | Novick et al., 1980 |
| RH2856 | MATα gaa1-1 leu2 ura3 | H. Riezman |
| WBY283 | MATα ade2-1 his3-11 leu2-3-112 trp1-1 ura3-1 can1-100 lag1Δ∷HIS3 | This study |
| WBY286 | MATa ade2-1 his3-11 leu2-3-112 trp1-1 ura3-1 can1-100 dgt1Δ∷ADE2 | This study |
| WBY616 | MATα ade2-1 his3-11 leu2-3-112 trp1-1 ura3-1 can1-100 lag1Δ∷HIS3 dgt1Δ∷ADE2 | This study |
| WBY739 | MATaade2-1 his3-11 leu2-3-112 trp1-1 ura3-1 can1-100 lag1Δ∷HIS3 dgt1Δ∷ADE2 [pWB94] | This study |
| WBY741 | MATaade2-1 his3-11 leu2-3-112 trp1-1 ura3-1 can1-100 lag1Δ∷HIS3 dgt1Δ∷ADE2 [pWB95] | This study |
| WBY743 | MATaade2-1 his3-11 leu2-3-112 trp1-1 ura3-1 can1-100 lag1Δ∷HIS3 dgt1Δ∷ADE2 [pWB96] | This study |
| WBY777 | MATα ade2-1 his3-11 leu2-3-112 trp1-1 ura3-1 can1-100 lag1Δ∷HIS3 dgt1Δ∷ADE2 [pWB98] | This study |
Recombinant DNA techniques were performed using standard techniques (Sambrook et al., 1989). Yeast strains were transformed with plasmids using electroporation (Becker and Guarente, 1991) or lithium acetate procedures (Ito et al., 1983). Western blots were visualized using enhanced chemiluminescence (Amersham, Arlington Heights, IL) as described by the manufacturer. SDS-PAGE was performed on 10–15% gradient gels. Chemicals were from Sigma Chemicals (St. Louis, MO) unless otherwise noted. DNA sequencing was performed using an Applied Biosystems A220 fluorescent DNA sequencer (Perkin Elmer-Cetus, Foster City, CA) and cycle sequencing.
Disruption of LAG1 and DGT1
The chromosomal deletion alleles lag1Δ::HIS3 and dgt1Δ::ADE2 were created by replacing the entire coding sequences of LAG1 and DGT1 with yeast-integrative plasmids (Sikorski and Hieter, 1989). To disrupt LAG1, the noncoding flanking regions of this gene (400–600 base pairs [bp]) were amplified by genomic PCR using oligonucleotides that incorporated EcoRI restriction sites at the ends of the fragments distant from LAG1 and EcoRV and XbaI sites at the inside ends. These fragments were then digested with the relevant restriction enzymes and cloned in a three-way ligation into EcoRV- and XbaI-cut pRS303 (HIS3; Sikorski and Hieter, 1989). The resulting plasmid, pWB281, was linearized with EcoRI and used to transform haploid W303-1B cells to His+ prototrophy. One of the His+ transformants was purified and designated WBY283. Correct chromosomal deletion of LAG1 was confirmed by extensive genomic PCR analysis using several primer pairs specific for either the wild-type allele LAG1 or the disruption allele lag1Δ::HIS3.
A similar strategy was used to replace the entire coding region of DGT1 with a yeast-integrative vector, pSO403 (ADE2; kindly provided by S.C. Ogg, Department of Biochemistry, University of Dundee, Dundee, United Kingdom). pSO403 had been constructed by subcloning a 2.3-kilobase (kb) BglII–BglII fragment containing the ADE2 gene from S. cerevisiae (Stotz and Linder, 1990) into the BamHI site of pBluescript II SK(+) (Stratagene, La Jolla, CA). The 5′- and 3′-flanking sequences of DGT1 (400–600 bp) were amplified by genomic PCR and cloned in a three-piece ligation into PstI- and XhoI-digested pSO403. The resulting plasmid, pWB280, was linearized with EcoRI and used to transform haploid W303-1A cells to Ade+ prototrophy. Correct replacement of DGT1 was confirmed in one Ade+ transformant (termed WBY286) by genomic PCR analysis.
Diploid W303 cells were also transformed with EcoRI-linearized pWB280, giving rise to a heterozygous chromosomal disruption of DGT1. Diploid Ade+ transformants were induced to sporulate in liquid sporulation media, and asci were dissected into tetrads. Both at 25 and 37°C, all tetrads analyzed gave rise to four equally well-growing colonies, showing that DGT1 is not important for growth.
Generation of a lag1Δ dgt1Δ Double Disruption Mutant
WBY283 and WBY286 were mated to construct a diploid that is heterozygous for both LAG1 and DGT1. The resulting His+Ade+ diploid (designated WBY600) was sporulated in liquid sporulation medium, and asci were dissected into tetrads. All tetrads gave rise to four viable spores, most of which grew with the wild-type rate, whereas some grew with a drastically reduced rate. Markers analysis revealed that the growth defect always cosegregated with the His+Ade+ phenotype. Genomic PCR analysis of all four colonies produced from spores of a single tetrad of nonparental ditype confirmed that the slowly growing His+Ade+ colonies contain the lag1Δ::HIS3 and dgt1Δ::ADE2 disruptions but not the LAG1 and DGT1 wild-type alleles. One haploid lag1Δ::HIS3 dgt1Δ::ADE2 clone was designated WBY616.
Cloning and Epitope Tagging of LAG1 and DGT1
LAG1 and DGT1 were amplified with flanking sequences (350–550 bp) by genomic PCR using Vent polymerase (New England Biolabs, Beverly, MA) and oligonucleotides that incorporated restriction sites at their 5′ ends (HindIII and XbaI for the amplification of LAG1; SacII and XhoI for amplifying DGT1). The resulting 2.0- to 2.2-kb DNA fragments were digested with the relevant restriction enzymes and cloned into the centromeric vector pRS316 (URA3; Sikorski and Hieter, 1989) digested with the same enzymes. Sequence analysis revealed that the inserts of the resulting plasmids, pWB98 (carrying LAG1) and pWB95 (carrying DGT1), agreed with the published genomic sequence. pWB96 was constructed by inserting the 2.0-kb SacII–XhoI fragment of pWB95 (bearing DGT1) into the centromeric vector pRS314 (TRP1; Sikorski and Hieter, 1989) to replace the SacII–XhoI fragment in the polylinker.
To construct an epitope-tagged HA-Dgt1p protein, a double-stranded DNA cassette was assembled by annealing the complementary oligonucleotides 5′-TCGACATACCCATATGATGTTCCAGATTACGCT-3′ and 5′-TCGAAGCGTAATCTGGAACATCAT-ATGGGTATG-3′. The protruding termini of this cassette are identical to those generated by the restriction endonuclease SalI. One copy of this construct was ligated into SalI-digested pWB96. The resulting plasmid, pWB94, carries an N-terminal hemagglutinin (HA) epitope inserted between the first and second amino acids of Dgt1p. Both strands of the modified region of pWB94 were sequenced to show that only the desired mutation had been introduced.
Haploid yeast strains carrying pWB94, pWB95, pWB96, or pWB98 were constructed by individually transforming these plasmids into the heterozygous diploid WBY600 (see above). Trp+ or Ura+ transformants were sporulated in liquid sporulation medium. Asci were dissected into tetrads and allowed to germinate on minimal plates lacking tryptophan or uracil. All Ade+ His+ spores (containing the disruption alleles lag1Δ::HIS3 and dgt1Δ::ADE2) that were also Trp+ (containing pWB94 or pWB96) or Ura+ (containing pWB95 or pWB98) gave rise to colonies of identical size as the Ade− His− colonies (containing the wild-type alleles). The resulting haploids were purified and termed WBY739, WBY741, WBY743, and WBY777 (see Table 1).
Antibodies
Monoclonal anti-HA ascites fluid was purchased from Babco (Berkeley Antibody, Richmond, CA). TRITC-conjugated donkey anti-mouse immunoglobulin G (IgG) and donkey anti-rabbit IgG were purchased from Jackson ImmunoResearch (West Grove, PA). Polyclonal anti-Kar2p antibodies were prepared in our laboratory using Kar2p overexpressed in Escherichia coli from a clone kindly provided by M. Rose (Princeton University, Princeton, NJ). The following polyclonal antisera were kindly provided by the indicated investigators: anti-CPY, R. Schekman (University of California, Berkeley, CA); anti-DPAP B, T. Stevens (University of Oregon, Eugene, OR); anti-Gas1p, A. Conzelmann (Université de Fribourg, Fribourg, Switzerland); and anti-Yap3p, Y. Bourbonnais (Université Laval, Québec, Canada).
Immunofluorescence
The intracellular location of HA-Dgt1p was examined by indirect immunofluorescence performed essentially as described (Pringle et al., 1991). Strains expressing HA-Dgt1p (WBY739) or untagged Dgt1p (WBY743) were grown at 30°C to early exponential growth phase (106–107 cells/ml) in SC medium lacking tryptophan. Cells were fixed for 2 h with 3.7% formaldehyde in the medium. Antibody incubations were performed in a humid chamber at 25°C for 1 h. HA-Dgt1p was detected using a 1:5000 dilution of monoclonal anti-HA serum. Polyclonal antibodies against Kar2p were used at a dilution of 1:10,000. Bound primary antibodies were decorated with TRITC-conjugated donkey anti-mouse IgG or donkey anti-rabbit IgG diluted 1:200. Slides were mounted in 90% glycerol containing 1 mg/ml p-phenylenediamine, pH 9.0, and 1 μg/ml DAPI. Cell remnants were examined at 1000-fold magnification on a Zeiss Axioskop microscope (Carl Zeiss, Thornwood, NY). Images were recorded on Eastman Kodak (Rochester, NY) TMAX 400 film with a Zeiss MC 80 camera and controller.
Electron Microscopy
Yeast cells were grown to early logarithmic phase at 30°C in YPD medium and prepared for electron microscopy by fixation with glutaraldehyde and KMnO4 (Kaiser and Schekman, 1990). Fixed cells were dehydrated through a graded ethanol series and embedded in Spurr’s resin (PolyScience, Niles, IL) overnight at 60°C. Thin sections (thickness, 50–60 nm) were stained with uranyl acetate and lead citrate and examined with a Zeiss 10 CA electron microscope.
In Vivo Labeling with 35S and Immunoprecipitations
Yeast cells were grown to early logarithmic phase in YPD medium at 30°C except for sec13-1 cells, which were grown at 22°C and shifted to 37°C for 90 min before labeling. Cells were harvested and washed three times with SC minimal medium lacking methionine. For in vivo translocation studies, pulse labeling with [35S]methionine and [35S]cysteine (ProMix 35S cell labeling mix; Amersham) and non-native immunoprecipitations were performed as described before (Hann and Walter, 1991). Pulse–chase experiments were performed by pulse labeling cells for 2 min at 30°C with 50 μCi/OD600 ProMix followed by the addition of a 1/50 vol of 200 mM methionine/200 mM cysteine (10,000-fold molar excess). During the 30-min chase period, aliquots containing 5 OD600 units were collected at the indicated times. Cell lysis and immunoprecipitations were performed as described (Hann and Walter, 1991). The immunoprecipitates were analyzed by SDS-PAGE on 10–15% gradient gels, followed by exposure and quantitation of the gel on a PhosphorImager (Molecular Dynamics, Sunnyvale, CA).
GPI Anchor Attachment Assay
Yeast cells were grown at 22°C to early logarithmic phase in SC minimal medium. Twenty OD600 units were collected, washed three times with SC − inositol, resuspended in 1.0 ml of SC − inositol, and preincubated for 5 min at 30°C (W303-1A and WBY616) or 37°C (RH2856). After the addition of 200 μCi of [3H]myo-inositol (Amersham), cells were incubated at 30°C (W303-1A and WBY616) or 37°C (RH2856). At the indicated times, 400-μl aliquots were removed and frozen in liquid nitrogen. NaN3 and NaF were added to a 10 mM final concentration. The cells were thawed on ice, washed twice in 10 mM NaN3 and 10 mM NaF, resuspended in 1 ml of a mixture of CHCl3:CH3OH:H2O (10:10:3), and lysed by vortexing with glass beads. The beads were removed, and proteins were precipitated at 10,000 × g for 5 min and delipidated by reextracting the pellet twice with CHCl3:CH3OH:H2O (10:10:3). The pellet was dried, resuspended in protein sample buffer, and boiled for 5 min. After centrifugation at 10,000 × g for 5 min, the proteins were separated by SDS-PAGE and prepared for fluorography by soaking the gel for 10 min in Amplify (Amersham).
RESULTS
Characterization of Two Proteins in S. cerevisiae That Are Structurally Related to TRAM
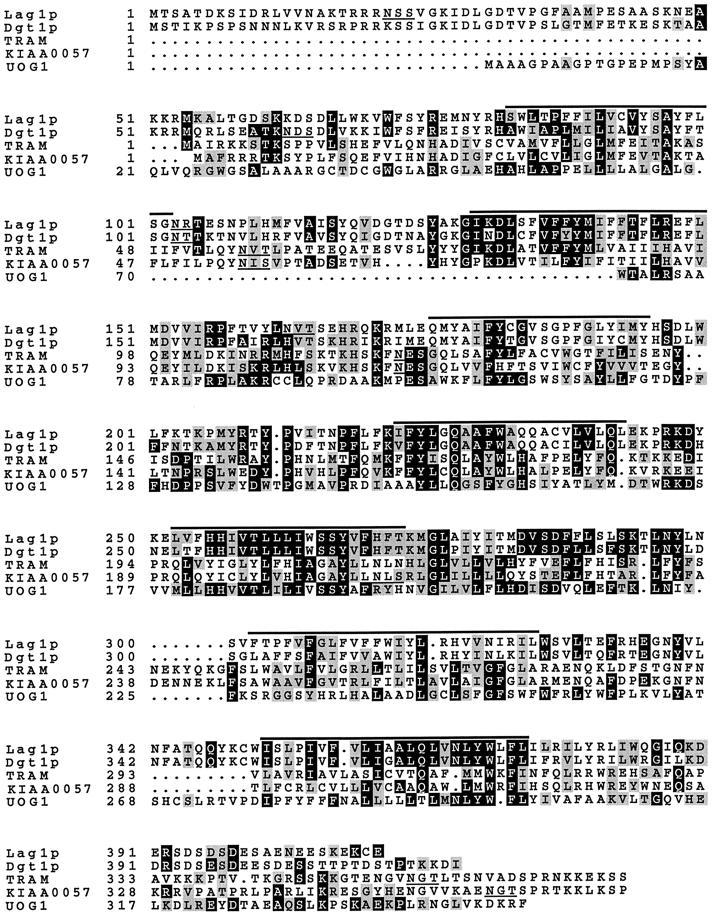
To identify yeast homologues of TRAM, the genomic DNA sequence of S. cerevisiae was analyzed. A database search revealed two yeast genes encoding proteins with significant sequence similarity to the mammalian TRAM protein (Figure 1): the previously described gene LAG1, identified as a regulator of longevity and aging (D’mello et al., 1994; see DISCUSSION), and a novel ORF on chromosome XI (YKL008c). For reasons discussed below, we refer to this second gene as DGT1, for “delayed GPI-anchored protein transport.” By convention, the corresponding gene products are termed Lag1p and Dgt1p, respectively. Both proteins have a predicted molecular mass of 48–49 kDa and show 70% sequence identity, suggesting that they may have similar or functionally redundant activities.
Figure 1.
Multiple alignment of the predicted amino acid sequence of TRAM homologues. The alignment includes the deduced protein sequence of S. cerevisiae LAG1 (D’mello et al. 1994), S. cerevisiae YKL008c (termed DGT1 in this work), human TRAM (Görlich et al. 1992), a human TRAM homologue (KIAA0057; Nomura et al. 1994), and human UOG-1. The alignment was generated using the PileUp (Genetics Computer Group, Madison, WI) and BOXSHADE (Institute for Animal Health, Surrey, United Kingdom) programs. Gaps that were inserted during the alignment are denoted by dots. Potential membrane-spanning domains were predicted using four different algorithms: PepPlot (Kyte and Doolittle, 1982), TMAP (Persson and Argos, 1994), TMpred (Hofmann and Stoffel, 1993), and TopPred2 (von Heijne, 1992). The positions of these putative transmembrane domains are indicated by black bars above the alignment. N-linked glycosylation consensus sequences are underlined. Black boxes indicate identical residues, whereas gray boxes show amino acid similarity.
In addition to their homology to TRAM, the yeast proteins Lag1p and Dgt1p have a sequence similarity to two additional mammalian proteins (Figure 1). KIAA0057 is very closely related to TRAM (53% sequence identity; Nomura et al., 1994), whereas UOG-1 is a human ORF with less sequence similarity to TRAM (Lee, 1991). Over their entire lengths, both predicted proteins are 19–21% identical to Lag1p and Dgt1p. The functions of KIAA0057 and UOG-1 are unknown. From sequence analysis, all family members are integral membrane proteins, Lag1p, Dgt1p, TRAM, and KIAA0057 having seven clusters of hydrophobic residues that are predicted to form membrane-spanning domains (Figure 1, overlined), whereas UOG-1 is shorter and has only six predicted transmembrane regions.
Like many proteins with multiple membrane-spanning domains, all TRAM family members shown in Figure 1 do not contain N-terminal cleaved signal sequences. Lag1p and Dgt1p as well as TRAM and KIAA0057 have several potential N-linked glycosylation sites (Figure 1, underlined). One remarkable feature of the two yeast proteins is the presence of a stretch of acidic and hydroxylated amino acids at the very C terminus (>75% Asp, Glu, Ser, or Thr over a range of 20–25 residues). The significance of this structural feature is unknown.
The C termini of all TRAM family members contain dilysine motifs (KKXX or KXKXX) known to be required for membrane protein retrieval from the Golgi compartment back to the ER (Jackson et al., 1990). Therefore, these proteins are likely to be localized to the ER or to shuttle between the ER and the Golgi apparatus. Because the ER retrieval signal has been shown to interact with cytosolic coatomer (COP) subunits (Cosson and Letourneur, 1994), the C termini of Lag1p and Dgt1p are likely to face the cytosol.
Cellular Localization of Dgt1p in the ER
Based on their C-terminal dilysine signals, Lag1p and Dgt1p should be at least partially localized to the ER membrane. To test this prediction directly, we constructed an allele in which the DGT1 gene is appended with sequences encoding an N-terminal epitope derived from influenza virus HA (“HA epitope”; Field et al., 1988). This epitope-tagged protein, HA-Dgt1p, is functional, as judged by its ability to complement the growth defect of cells deleted for LAG1 and DGT1 (see below). Western blot controls revealed that HA-Dgt1p was specifically recognized by monoclonal anti-HA antibodies (our unpublished results).
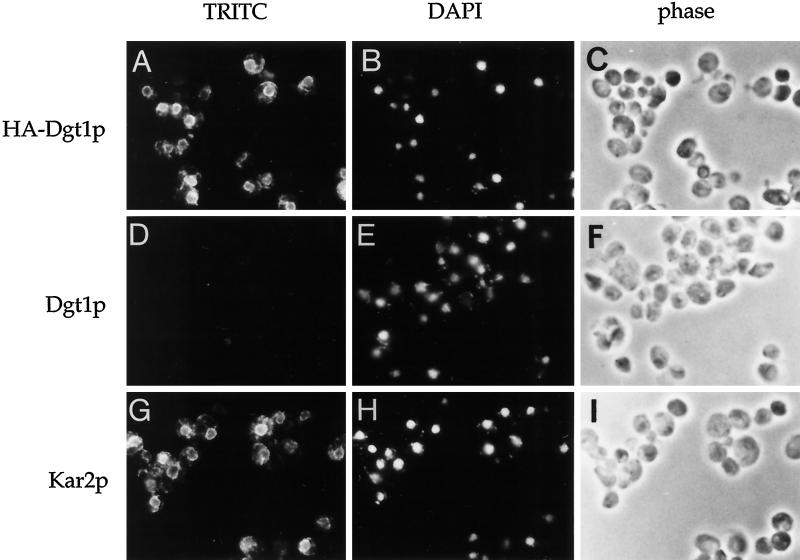
We visualized the intracellular distribution of HA-Dgt1p by indirect immunofluorescence microscopy. Cells expressing HA-Dgt1p or, as a control, an untagged version of DGT1 were fixed and processed for immunofluorescence (see MATERIALS AND METHODS). Nuclei and mitochondria were visualized using the DNA-binding dye DAPI (Figure 2, B, E, and H).
Figure 2.
Immunofluorescence localization of Dgt1p to the ER. lag1Δ dgt1Δ cells harboring an HA-tagged (WBY739) or an untagged (WBY743) version of DGT1 were fixed and processed for immunofluorescence using monoclonal anti-HA (A–F) or polyclonal anti-Kar2p (G–I) antibodies followed by secondary decoration with TRITC-conjugated anti-mouse or anti-rabbit IgG. Panels show the staining pattern of TRITC to visualize HA-Dgt1p and Kar2p localization. DNA was stained with DAPI to indicate nuclei, and cells were visualized by light microscopy (phase) to observe cell morphology.
In the presence of HA-Dgt1p, anti-HA antibodies detected the characteristic yeast ER staining pattern (Figure 2A), consisting of a distinct perinuclear ring and fine strands of staining connecting the perinuclear ER to ER cisternae underlying the plasma membrane. This pattern closely resembled that obtained in control samples stained with polyclonal antibodies specific to the ER-lumenal protein Kar2p (Figure 2G). Cells expressing Dgt1p without an epitope tag showed only background staining (Figure 2D). Thus HA-Dgt1p—and by extension Dgt1p—is localized to the ER membrane.
Very similar results were obtained when HA-tagged alleles of LAG1 and DGT1 were overexpressed using a GAL1/10 promoter (our unpublished results), demonstrating that both proteins are localized to the ER membrane.
Deletion of LAG1 and DGT1 Causes Growth Defects
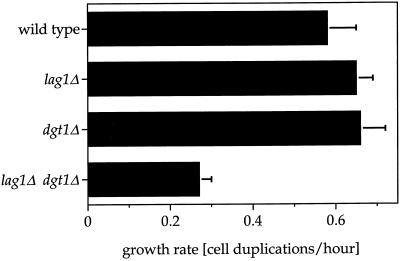
As a first step toward understanding the role of Lag1p and Dgt1p, we examined the phenotypes of haploid strains deleted for either of these genes. To this end, the entire coding regions of LAG1 and DGT1 were replaced by the selectable marker genes HIS3 and ADE2 (see MATERIALS AND METHODS). The resulting lag1Δ and dgt1Δ null mutants grew with wild-type rates both on solid media and in liquid culture (Figure 3), demonstrating that neither of these genes is essential for vegetative growth. Also, the lag1Δ and dgt1Δ mutations had no apparent effect on the shape, size, budding pattern, or viability of the cells.
Figure 3.
Lag1p and Dgt1p are important for growth. Growth rates in YPD medium of wild type and strains in which the chromosomal copies of LAG1 DGT1 or both LAG1 and DGT1 have been disrupted are shown. Growth rates were determined at 30°C as described in MATERIALS AND METHODS. Values are the average of four to six measurements; error bars indicate SD. The growth defect was observed in different rich and minimal media containing either glucose or galactose as the carbon source. When growth rates were studied on YPD at various temperatures between 15 and 40°C, lag1Δ dgt1Δ cells grew drastically slower at all temperatures tested. The growth defect was more pronounced above 35°C, indicating a slightly increased temperature sensitivity. Although lag1Δ dgt1Δ cells formed tiny colonies at 37°C, no growth was observed at 39–40°C, at which wild-type, lag1Δ, and dgt1Δ single mutant cells grew normally.
To address the possibility of overlapping functions for LAG1 and DGT1, we constructed a haploid mutant deleted for both of these genes. After mating of the haploid lag1Δ and dgt1Δ strains, the resulting diploid (heterozygous for both genes) was sporulated. Tetrad dissections resulted in four viable spores, most of which grew like wild type, whereas some grew with a significantly reduced rate. Analysis of auxotrophic markers revealed that the growth defect cosegregated with the His+Ade+ phenotype in all 22 tetrads analyzed. In addition, genomic PCR analysis of four colonies produced from spores of a single tetrad confirmed directly that the slowly growing His+Ade+ colonies contain the lag1Δ::HIS3 and dgt1Δ::ADE2 disruptions (our unpublished results). Similar to the results on agar plates, the lag1Δ dgt1Δ double mutant also showed a drastically reduced growth rate in liquid culture when compared with wild type and the single disruption mutants lag1Δ and dgt1Δ (Figure 3). Taken together, the slow-growth phenotype of the lag1Δ dgt1Δ mutant strongly suggests an important, but not essential, function for Lag1p and Dgt1p.
To confirm this interpretation, we tested whether plasmids carrying genomic copies of LAG1 or DGT1 would rescue the lag1Δ dgt1Δ growth phenotype. DNA fragments containing the entire genes and their flanking sequences were subcloned into pRS316 (Sikorski and Hieter, 1989), generating the plasmids pWB98 and pWB95. To show that the information contained in these DNA fragments was sufficient to complement the lag1Δ dgt1Δ null mutant, pWB98 or pWB95 was transformed into diploid cells heterozygous for both LAG1 and DGT1. After sporulation and tetrad dissection, haploid cells bearing both chromosomal lag1Δ and dgt1Δ mutations and the plasmid were selected. By using this approach rather than transforming the haploid lag1Δ dgt1Δ double mutant strain directly, we avoided complications arising from the extremely low transformation efficiency of lag1Δ dgt1Δ cells (see below). In all tetrads analyzed, each His+Ade+Ura+ colony (carrying both gene disruptions and the URA3-marked plasmid pWB98 or pWB95) grew at the wild-type rate. This confirms that these plasmids contain all the information necessary for the functional expression of LAG1 and DGT1.
Lag1p and Dgt1p Are Not Required for Protein Translocation across the ER Membrane
The mammalian TRAM protein is thought to be involved in protein translocation across or into the ER membrane (Do et al., 1996; Voigt et al., 1996). Because Lag1p and Dgt1p were identified through their sequence similarity to the mammalian TRAM protein, we asked directly whether these yeast proteins play a role in protein translocation across or membrane protein integration into the ER membrane. To this end, the translocation of several endogenous secretory and membrane proteins of yeast (including SRP-dependent and -independent proteins) was assessed in vivo.
Wild-type and lag1Δ dgt1Δ mutant cells were pulse labeled with [35S]methionine and [35S]cysteine. As a control, we used cells carrying sec61-101, a mutant allele of SEC61 that impairs translocation of proteins using either an SRP-dependent or -independent pathway (Ng et al., 1996). Lysates from radiolabeled cells were analyzed by immunoprecipitation using antibodies raised against proteins that are translocated across or integrated into the ER membrane. Defects were monitored as the accumulation of precursor proteins lacking glycosylation and/or signal sequence cleavage, both of which occur in the ER lumen.
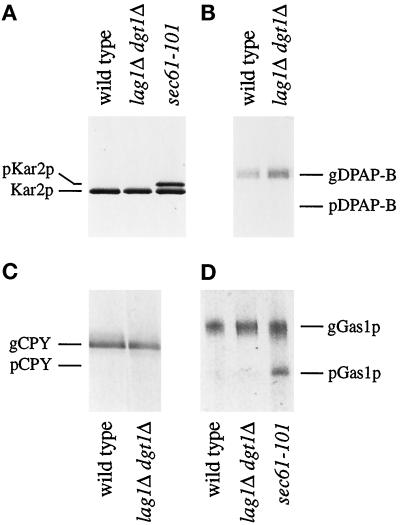
As shown in Figure 4A, no translocation defects were observed when lag1Δ dgt1Δ cells were analyzed using antibodies specific for Kar2p, an ER lumenal protein (Figure 4A, lane 2). As for wild-type cells (Figure 4A, lane 1) only the translocated form of Kar2p was observed in the mutant cells. In contrast, a significant fraction of the labeled Kar2p was recovered as the slower migrating precursor form in sec61-101 cells (Figure 4A, lane 3; the slower mobility of preKar2p in SDS-PAGE represents the presence of an uncleaved signal sequence), as was observed previously (Ng et al., 1996).
Figure 4.
Protein translocation across the ER membrane does not require LAG1 and DGT1. Cells of W303-1B (wild type), WBY616 (lag1Δ dgt1Δ), or DNY116 (sec61-101) were pulse labeled with [35S]methionine and [35S]cysteine for 5 min. Cell lysates were prepared, and immunoprecipitations were performed using polyclonal antibodies against the following proteins: (A) Kar2p, (B) dipeptidyl aminopeptidase B (DPAP B), (C) CPY, and (D) the GPI-anchored cell surface protein Gas1p. Eluates were subjected to SDS-PAGE followed by fluorography. Cytosolic precursor (p) and ER-glycosylated (g) forms of each protein are indicated.
Similar results were obtained for all other proteins tested. In particular, dipeptidyl aminopeptidase B (a vacuolar type II integral membrane protein; Figure 4B), carboxypeptidase Y (CPY, a soluble vacuolar protein; Figure 4C), Gas1p (a cell surface glycoprotein; Figure 4D), α-factor (a secreted yeast pheromone), protein disulfide isomerase (an ER-lumenal protein), and Pho8p (a type II integral membrane protein; our unpublished results) showed no evidence of translocation defects in lag1Δ dgt1Δ cells. Because translocation or membrane integration of this broad spectrum of proteins was not affected by the deletion of LAG1 and DGT1, these genes are unlikely to play a general role in protein translocation.
Deletion of LAG1 and DGT1 Causes Cell Wall Defects
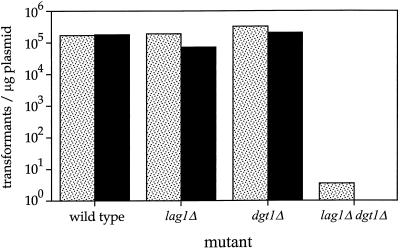
Attempts to transform the haploid lag1Δ dgt1Δ mutant with plasmids revealed that the deletion of these genes caused severe defects in transformation efficiency. Only very few transformants were obtained with either of two standard transformation procedures, using lithium acetate or electroporation. A quantitative analysis of this phenomenon is shown in Figure 5. When exponentially growing cells from wild-type or the single null mutants (lag1Δ or dgt1Δ) were transformed using lithium acetate, we obtained transformation rates of ∼105 transformants/μg of plasmid DNA. In contrast, the transformation efficiency of lag1Δ dgt1Δ cells was reduced by greater than four orders of magnitude. Although the addition of pWB96 (carrying DGT1) resulted in the transformation of a few lag1Δ dgt1Δ cells, not a single transformant was obtained with the control vector pRS314 (Figure 5). Additional experiments revealed that lag1Δ dgt1Δ cells gave rise to a drastically lower number of colonies than wild-type cells even when the transformation mixtures were plated on YPD plates instead of selective plates. Thus, the transformation procedure per se appeared to be lethal to lag1Δ dgt1Δ cells. Further characterization of the transformation procedure demonstrated that the death of lag1Δ dgt1Δ cells was primarily caused by the step involving treatment with DMSO and 42°C heat shock (our unpublished results), suggesting that the deletion of LAG1 and DGT1 generally destabilizes the cells possibly by impairing cell wall integrity.
Figure 5.
The transformation efficiency of lag1Δ dgt1Δ cells is drastically impaired. Yeast cells were grown at 30°C in YPD medium to logarithmic phase (OD600 = 0.4–0.6), and 0.5 OD600 cell equivalents were transformed with 3 μg of a plasmid with (pWB96; hatched bars) or without (pRS314; black bars) DGT1. Dilutions of the samples were plated onto selective minimal plates, and transformation rates were determined from the number of TRP+ colonies.
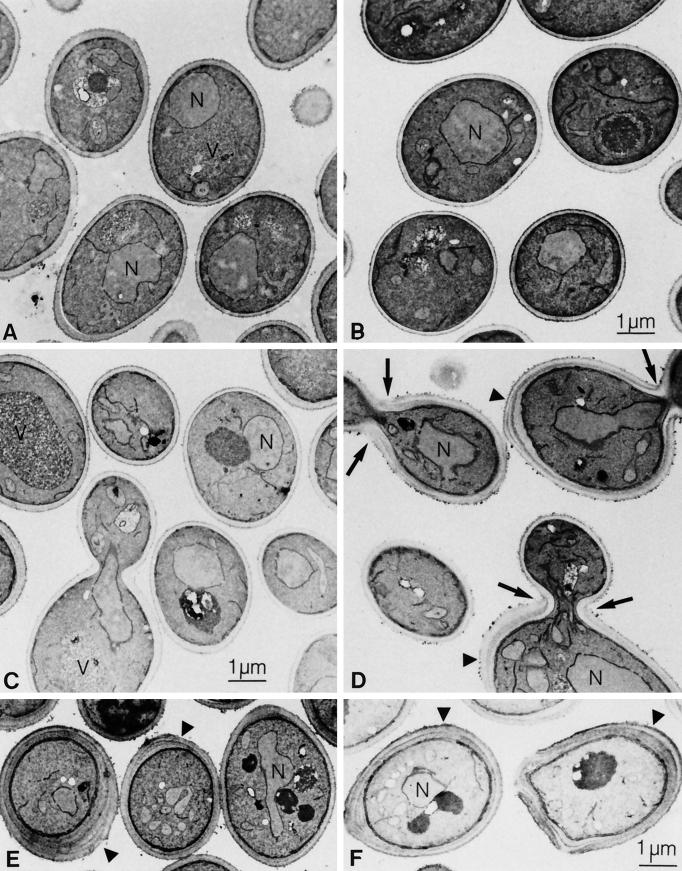
To determine directly whether the lag1Δ dgt1Δ mutant exhibited any ultrastructural changes indicative of cell wall defects, thin sections of the different mutants were studied by electron microscopy. Figure 6, B and C, shows that lag1Δ and dgt1Δ cells are morphologically indistinguishable from wild-type cells (Figure 6A). In contrast to the single disruption mutants, the lag1Δ dgt1Δ double mutants have severe cell wall defects (Figure 6, D–F). Normal cell walls were observed as a single electron translucent central layer, composed largely of β-glucan, which is surrounded by an electron-dense shell of mannoproteins (for review, see Klis, 1994). In contrast, lag1Δ dgt1Δ cell walls show several distinct layers (Figure 6, D–F, arrowheads), each with a thickness about that of a normal cell wall, suggesting that they are built through repeated deposition of cell wall material. Most of the lag1Δ dgt1Δ cells observed have cell walls that were drastically thicker (up to 500 nm) than the wild-type cell wall (150–200 nm). In some sections of lag1Δ dgt1Δ cells, budding daughter cells could be observed that were still attached to their mother cells (Figure 6D). Interestingly, in these images, the growing bud exhibited a relatively normal cell wall morphology. The inner cell wall layer of the mother cell appeared to be continuous with the cell wall of the bud, whereas the extra outer wall layers of the mother cell ended at the septum (Figure 6D, arrows).
Figure 6.
Altered cell wall morphology of the lag1Δ dgt1Δ mutant. Cells were grown in YPD medium at 30°C and fixed with permanganate, and thin sections were prepared for electron microscopy as described in MATERIALS AND METHODS. (A) Wild-type (W303-1A), (B) lag1Δ (WBY283), (C) dgt1Δ (WBY286), (D–F) lag1Δ dgt1Δ (WBY616). N, nucleus; V, vacuole. Note the thick, multilayered cell wall on lag1Δ dgt1Δ mother cells.
Several mutants with cell wall defects have been shown to grow at a reduced rate unless the medium is osmotically supported with 1 M sorbitol (Levin and Bartlett-Heubusch, 1992). However, the growth defect of lag1Δ dgt1Δ cells was not rescued by the addition of 1 M sorbitol to the growth medium (our unpublished results), excluding the possibility of an osmotic hypersensitivity of the lag1Δ dgt1Δ cells.
The aberrant cell wall structure of the lag1Δ dgt1Δ mutant may explain the drastically impaired transformation efficiency (Figure 5) and suggested that Lag1p and Dgt1p are involved, directly or indirectly, in some process related to cell wall biogenesis.
LAG1 and DGT1 Facilitate ER-to-Golgi Transport of GPI Proteins
The results presented above show that Lag1p and Dgt1p are localized to the ER membrane and are required for proper cell wall formation. Yet we could not obtain evidence that Lag1p or Dgt1p are involved in protein translocation, as originally suggested by their sequence similarity to mammalian TRAM. Because cell wall defects could, in principle, arise from an impaired delivery of some proteins to the cell surface, we addressed the possibility that Lag1p or Dgt1p might play a role in protein trafficking beyond the ER, such as from the ER to the Golgi compartment. To this end, we assessed the maturation kinetics of several marker proteins using pulse–chase analysis, followed by immunoprecipitation and SDS-PAGE analysis (Figure 7).
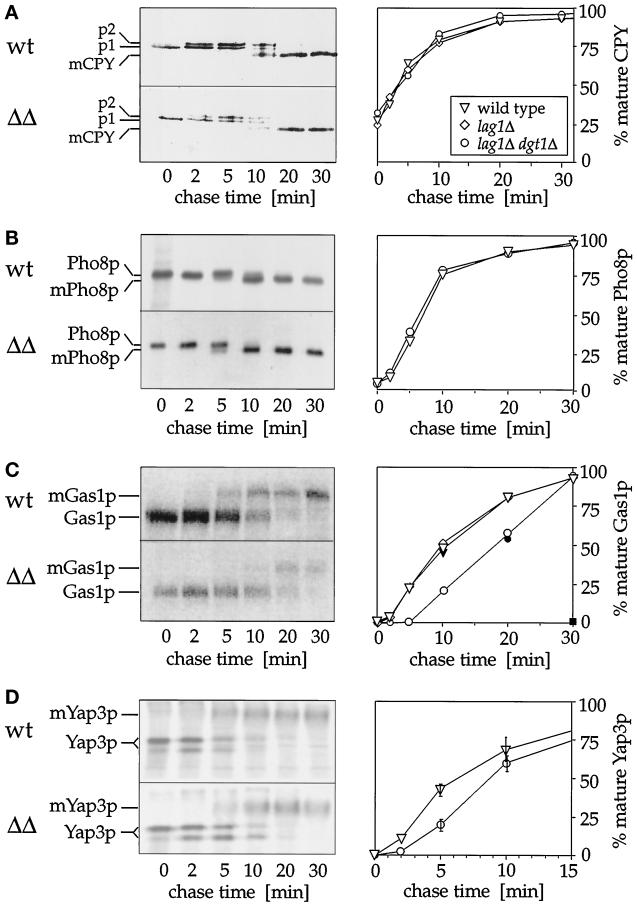
Figure 7.
Maturation of Gas1p and Yap3p, but not CPY and Pho8p, is defective in lag1Δ dgt1Δ cells. The rate of conversion of the precursor forms of CPY, Pho8p, Gas1p, or Yap3p to their mature products was determined by pulse labeling cells for 2 min with [35S]methionine and [35S]cysteine followed by a chase with an excess of unlabeled methionine and cysteine. At the indicated times, cell extracts of wild-type (wt) or lag1Δ dgt1Δ (ΔΔ) cells were made, and immunoprecipitations were performed using antibodies against CPY (A), Pho8p (B), Gas1p (C), or Yap3p (D). Eluates were subjected to SDS-PAGE followed by autoradiography. Precursor and mature (m) forms of each protein are indicated, ER-glycosylated and Golgi-modified forms of CPY are labeled p1 and p2, respectively. The graphs shown on the right indicate the percentages of mature proteins (determined by quantitating the radioactivity in the relevant bands using a PhosphorImager). Values are the average of three measurements; error bars indicate SD. Triangles, W303 (wild-type); diamonds, WBY283 (lag1Δ); circles, WBY616 (lag1Δ dgt1Δ); square, NY414 (sec13-1). Open symbols indicate experiments performed at 30°C, closed symbols represent results obtained at 37°C.
First, we studied the biogenesis of CPY, a vacuolar enzyme. The results in Figure 7A show that the maturation kinetics of CPY are indistinguishable in wild-type and lag1Δ dgt1Δ cells. The 67-kDa ER form (Figure 7A, p1) of CPY is rapidly converted to the 69-kDa Golgi form (Figure 7A, p2), which in turn is proteolytically cleaved in the vacuole to the 61-kDa mature form (Figure 7A, mCPY) (Stevens et al., 1982). These data demonstrate that the absence of Lag1p and Dgt1p does not delay ER-to-Golgi transport of CPY. Similar results were obtained using antibodies against Pho8p, a vacuolar type II integral membrane protein (Figure 7B). The 74-kDa ER precursor form of Pho8p is proteolytically cleaved in the vacuole to the 72-kDa mature form (Klionski and Emr, 1989). Because CPY and Pho8p maturation are indicative of vacuolar delivery, we conclude that Lag1p and Dgt1p do not play an important role in the general protein transport pathway.
Next, we addressed the possibility that these proteins might be involved in the vesicular transport of a specific subset of proteins (such as cell wall proteins). Specifically, we studied the maturation of GPI-anchored proteins, a group of cell surface proteins carrying a C-terminal lipid anchor that becomes attached onto specific proteins in the ER (Takeda and Kinoshita, 1995). The ER-to-Golgi transport of GPI proteins is dependent on the addition of the GPI anchor (Nuoffer et al., 1991; Doering and Schekman, 1996). Because Lag1p and Dgt1p are localized to the ER membrane, they might be required for GPI anchor attachments or the transport of GPI proteins. To address this possibility, we analyzed the ER-to-Golgi transport of Gas1p, a well-characterized GPI model protein (Nuoffer et al., 1991). Vesicular transport of Gas1p can be monitored by following the conversion of the 105-kDa core-glycosylated ER form to the 125-kDa mature form, which is formed upon arrival in the Golgi compartment (Fankhauser and Conzelmann, 1991; Nuoffer et al. 1991, 1993). When Gas1p does not receive a GPI anchor in the ER, it is not efficiently transported to the Golgi apparatus and remains in its immature 105-kDa form (Nuoffer et al., 1993; Doering and Schekman, 1996).
The maturation of Gas1p was examined in wild-type and the different disruption mutants using pulse–chase analysis, followed by immunoprecipitation and SDS-PAGE analysis (Figure 7C). As a control, we used a temperature-sensitive secretion mutant (sec13-1; Novick et al., 1980, 1981) in which the vesicle traffic is completely blocked between the ER and the Golgi apparatus (Figure 7C, filled square). As observed before, the maturation of Gas1p is rapid in wild-type cells, with a half-time of ∼10 min (Hamburger et al., 1995; Schimmöller et al., 1995; Sütterlin et al., 1997). Very similar results were obtained using the individual deletion mutants lag1Δ (Figure 7C) and dgt1Δ (our unpublished results). In lag1Δ dgt1Δ cells, however, maturation of Gas1p was significantly delayed with a half-time of ∼20 min (observed at both 30 and 37°C).
Quantitative analysis of the data (Figure 7C, right) indicates that even in the absence of LAG1 and DGT1 most of Gas1p was present in the mature Golgi form after a 30-min chase. At these later time points, however, we consistently observed a significant loss of the total Gas1p signal, suggesting that a significant portion of the unmature Gas1p molecules were being degraded. This effect may not reflect a phenotype resulting from the disruption of LAG1 and DGT1, however, because Gas1p matured equally inefficiently in wild-type cells (Figure 7C, wild-type control).
The delayed maturation of Gas1p observed in the lag1Δ dgt1Δ mutant suggests that Lag1p and Dgt1p may be required for efficient ER-to-Golgi transport of GPI-anchored proteins in general. To test this notion, we investigated the maturation kinetics of another GPI-protein, the aspartyl endoprotease Yap3p. Pulse–chase-labeled protein extracts were immunoprecipitated using anti-Yap3p antibodies (Ash et al., 1995). As shown in Figure 7D, two different proteins of 80 and 100 kDa were precipitated at early time points. These bands presumably correspond to differently core-glycosylated ER forms of Yap3p, because both can be converted to a single band of ∼70 kDa upon treatment with endoglycosidase H (our unpublished results). At later time points, hyperglycosylated Yap3p is formed, which migrates as a broad band on SDS polyacrylamide gels at ∼160 kDa and is indicative of the protein’s arrival in the Golgi apparatus (Ash et al., 1995).
We quantitated the amount of both ER forms as well as the 160-kDa Golgi form of Yap3p to measure the relative block in ER-to-Golgi transport. As shown in Figure 7D, right, the hyperglycosylated Golgi form of Yap3p was formed with a half-time of ∼5 min in wild-type cells. In contrast, Yap3p maturation was delayed in lag1Δ dgt1Δ cells (half-time, 8–9 min). Although this difference was less pronounced than for Gas1p, it was reproduced in three separate experiments (the average values and SDs are shown in Figure 7D). Thus, we conclude that Lag1p and Dgt1p facilitate efficient ER-to-Golgi transport of Yap3p.
GPI Anchor Attachment Is Not Defective in the lag1Δ dgt1Δ Mutant
Taken together, the results presented thus far show that the loss of Lag1p and Dgt1p causes a delay in the maturation of GPI-anchored proteins, whereas the vesicular transport of other proteins such as CPY and Pho8p occurs with wild-type kinetics. Thus, the phenotype of lag1Δ dgt1Δ is quite distinct from sec mutants, which completely block the secretory pathway. One possible explanation for the observed cargo specificity of the transport defect would be that GPI anchor attachment is defective in the lag1Δ dgt1Δ mutant. Indeed, a temperature-sensitive mutation of GAA1, encoding a protein involved in GPI anchor attachment, causes a similar delay in the ER-to-Golgi transport of Gas1p (Hamburger et al., 1995).
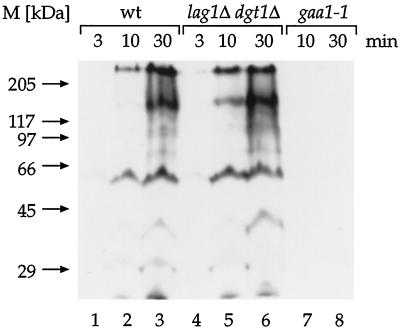
To test this possibility, we labeled cells with [3H]-myo-inositol, a biosynthetic precursor of the GPI anchor that becomes incorporated into the whole spectrum of GPI-anchored proteins (Conzelmann et al., 1990), and compared the rates of GPI anchor attachment in wild-type and lag1Δ dgt1Δ cells. As a control, we used a strain carrying a temperature-sensitive allele of GAA1, gaa1-1 (Hamburger et al., 1995).
The incorporation of [3H]myo-inositol into proteins was analyzed by labeling wild-type, lag1Δ dgt1Δ, and gaa1-1 cells for different lengths of time. Total protein extracts were prepared and delipidated, and the GPI proteins were visualized by SDS-PAGE and fluorography. As shown in Figure 8, lanes 1–3, wild-type cells incorporated [3H]inositol into a distinct pattern of bands reminiscent of that observed before (Hamburger et al., 1995; Schönbächler et al., 1995). Interestingly, the pattern of [3H]inositol-labeled proteins in wild-type cells closely resembled that of lag1Δ dgt1Δ mutant cells, indicating that all abundant GPI proteins were correctly modified in the absence of Lag1p and Dgt1p (Figure 8, lanes 1–6). Furthermore, the kinetics of [3H]-inositol incorporation were similar in both strains, demonstrating that Lag1p and Dgt1p are not required for the efficiency of GPI anchor biosynthesis and attachment. Because the banding pattern in the wild-type and lag1Δ dgt1Δ strains is indistinguishable, the kinetic delay in GPI-anchored protein maturation (see Figure 7) can only have a minor effect on the majority of the monitored proteins, because otherwise maturation intermediates with different mobilities should have been observed. As observed before (Hamburger et al., 1995), inositol incorporation into proteins was almost completely abolished in the gaa1-1 mutant (Figure 8, lanes 7–9).
Figure 8.
GPI anchor attachment does not require LAG1 and DGT1. Wild-type (wt), WBY616 (lag1Δ dgt1Δ), or RH2856 (gaa1-1) cells were preincubated for 5 min and pulse labeled with [3H]myo-inositol for the indicated times at 30°C, except for RH2856, which was preincubated and labeled at 37°C. Total protein extracts were prepared, delipidated, and separated by SDS-PAGE followed by fluorography at −70°C.
DISCUSSION
We have identified and characterized two ER transmembrane proteins that are members of an evolutionarily conserved family and, at least in yeast, appear to be involved in ER-to-Golgi transport of GPI-anchored proteins. This conclusion is suggested by the observation that the deletion of LAG1 and DGT1 delays the maturation of two GPI-anchored proteins, for which we have determined the ER-to-Golgi transport kinetics (Figure 7), but does not impair or delay the attachment of GPI anchors in general (Figure 8). The transport defect is only observed in the lag1Δ dgt1Δ double mutant but not in strains individually deleted for LAG1 or DGT1, suggesting redundant or overlapping functions for these gene products. Because the ER-to-Golgi transport of GPI-anchored proteins is only partially impaired in lag1Δ dgt1Δ cells, Lag1p and Dgt1p facilitate, but are not essential, for the transport of GPI-anchored proteins.
Possible Roles of Lag1p and Dgt1p in GPI-anchored Protein Transport
One possible function of Lag1p and Dgt1p is to serve as “escortins” that directly interact with and facilitate movement of GPI-anchored proteins out of the ER. In addition to mutations that impair GPI anchoring, deletion of EMP24 or ERV25, two genes encoding transmembrane proteins that are enriched in COPII transport vesicles, also delays the maturation of a subset of proteins, including Gas1p but also other, non–GPI-anchored proteins (Schimmöller et al., 1995; Belden and Barlowe, 1996). These proteins belong to the p24 class of type I membrane proteins that were proposed to serve as selective “cargo receptors” in the sorting and packaging of proteins into COPII vesicles (Fiedler et al., 1996; Sohn et al., 1996). Because cargo recruitment from the ER is thought to involve selective mechanisms, it is conceivable that Lag1p and Dgt1p serve as “GPI receptors,” possibly in collaboration with Emp24p and Erv25p, in selectively packaging of GPI-anchored proteins into transport vesicles.
The presence of ER retention signals in Lag1p and Dgt1p supports this interpretation. The KKXX signal is recognized by COPI, which is thought to be involved in recycling from the Golgi compartment (Letourneur et al., 1994). This suggests that Lag1p and Dgt1p cycle between the ER and Golgi, which would be an essential aspect of the function of cargo receptors that become copackaged into the transport vesicles. This view would also be consistent with the recent observation that the ER-to-Golgi transport of GPI-anchored proteins is selectively blocked in ret1-1, a mutant in the α-subunit of COPI (Sütterlin, et al., 1997). Because the primary role of COPI is thought to be the retrieval of proteins from the Golgi to the ER (Letourneur et al., 1994; Lewis and Pelham, 1996), this result suggests that cycling of component(s) involved in GPI-anchored protein transport is required. It remains to be shown whether Lag1p and Dgt1p cycle between the ER and Golgi. Alternatively, they could reside permanently in the ER, with the KKXX retrieval signal only providing a backup function to retrieve those molecules that have inappropriately escaped the ER.
Another possibility is that Lag1p and Dgt1p affect GPI-anchored protein maturation more indirectly. A reduced rate of GPI-anchored protein transport was also observed, for example, when the biosynthesis of sphingolipids was blocked either by treatment with myriocin, an inhibitor of ceramide synthesis (Horvath et al., 1994), or by a mutation in LCB1, encoding the first enzyme in sphingolipid biosynthesis (Sütterlin et al., 1997). Interestingly, this mutation also affects Gas1p and Yap3p transport to quantitatively different degrees, as we observed in Figure 7.
Thus, ER-to-Golgi transport of GPI proteins is dependent on sphingolipid synthesis, indicating that sphingolipids might interact with GPI proteins to initiate transport. Studies in polarized epithelial cells have shown that GPI-anchored proteins and sphingolipids are cotransported from the Golgi to the apical plasma membrane, suggesting that GPI proteins and sphingolipids associate to form microdomains, or “rafts,” in the trans-Golgi network, which are then recruited into vesicles bound for the apical surface (Simons and van Meer, 1988; Brown and Rose, 1992). The GPI anchor allows GPI-anchored proteins to enter such domains, from which other secretory proteins are excluded. A similar “clustering hypothesis” has been proposed in yeast, where ER-to-Golgi transport of GPI-anchored proteins seems to depend on their interaction with sphingolipids. Therefore, sphingolipids may stimulate the clustering of GPI proteins as a prelude to their packaging into specific transport vesicles (Horvath et al., 1994; Skrzypek et al., 1997) or facilitate the fusion of GPI-containing vesicles with the Golgi membrane (Sütterlin et al., 1997). Deletion of Lag1p and Dgt1p could therefore impair the biogenesis or availability of sphingolipids and hence delay maturation of GPI-anchored proteins more indirectly.
Clearly, other models could also explain the results obtained in this study. For example, Lag1p and Dgt1p could serve as molecular chaperones for the folding or assembly of GPI-anchored proteins immediately after GPI anchor attachment. Future studies will be necessary to distinguish between these conceptually distinct possibilities.
Relationship to the Longevity Phenotype of lag1Δ Cells
LAG1 (for “longevity-assurance gene 1”) has been described previously to play a role in regulating life span and aging in yeast (D’mello et al., 1994). In that study, LAG1 was found to be differentially expressed during the cell’s replicative life span, i.e., predominantly in younger cells. Furthermore, a mutant disrupted for LAG1 displayed a drastically increased life span, i.e., an increased average number of cell divisions performed by an individual cell (D’mello et al., 1994). The molecular cause for this increase in life span has not been described. However, the “longevity phenotype” was described for a lag1Δ single disruption, whereas the GPI transport phenotype was only observed after disruption of both LAG1 and DGT1. Therefore, we consider a direct link between these two phenotypes very unlikely.
Sequence Similarities to Mammalian Proteins
Although we initiated our studies of Lag1p and Dgt1p because of their sequence similarity to mammalian TRAM proteins, these proteins appear functionally distinct. Several studies have shown that TRAM is intimately associated with protein translocation across and integration into the ER membrane. In particular, TRAM was shown 1) to be required for the translocation of most but not all proteins (Görlich et al., 1992; Voigt et al., 1996), 2) to be in close proximity to the signal sequence during early phases of translocation (High et al., 1993; Mothes et al., 1994), 3) to be involved in the formation of a tight ribosome–membrane junction early in translocation (Voigt et al., 1996), 4) to interact with a transmembrane segment during its membrane integration (Do et al., 1996), and 5) to play a role in regulating “translocational pausing,” a mechanism by which certain nascent secretory proteins are transiently exposed to the cytosol (Hegde et al., 1998). All these results suggest that TRAM is stoichiometrically associated with the translocon and is involved in facilitating and/or regulating some aspect of the translocation–integration process per se.
In contrast, our results demonstrate that the yeast proteins Lag1p and Dgt1p play a different molecular role. Because all seven substrates tested (including soluble and membrane proteins as well as SRP-dependent and -independent proteins) are translocated normally in the lag1Δ dgt1Δ mutant, we conclude that Lag1p and Dgt1p are not involved in protein translocation and integration. Formally, however, we cannot rule out the possibility that some other, still unidentified protein(s) require(s) Lag1p and Dgt1p at the stage of membrane translocation or integration into the ER. Thus it appears that mammalian TRAM and yeast Lag1p and Dgt1p have a common evolutionary origin, but their functions have diverged. Because Lag1p and Dgt1p also show a similar degree of sequence similarity (∼20% identity over the entire length) to the products of two other mammalian genes of unknown function, KIAA0057 (Nomura et al., 1994) and UOG1 (Lee, 1991), it is possible that one or both of them perform a similar role in GPI transport as Lag1p and Dgt1p.
Cell Wall Defects
The pronounced cell wall defects in lag1Δ dgt1Δ cells are characterized by an extremely low efficiency of plasmid transformation and by thickened, multilayered cell walls (Figures 5 and 6). Cell wall thickening was only detected in mother cells, whereas most buds display normal cell wall thickness. These results suggest that the mutant walls are built through repeated deposition of cell wall material, possibly as the result of feedback regulation that continually tries to correct earlier defects.
Two lines of evidence underline the importance of GPI-anchored proteins for cell wall integrity. First, mutations that affect different steps of the GPI biosynthesis show increased sensitivities to calcofluor white and hygromycin B (Benghezal et al., 1995; Vossen et al., 1995, 1997), which are characteristic of defects in cell wall integrity. Consistent with this observation, lag1Δ dgt1Δ cells are hypersensitive to calcofluor white and hygromycin B (our unpublished results). Second, GPI-anchored proteins are known to play an important role in covalently cross-linking cell wall proteins to the cell wall glucan (Lu et al., 1995; Roemer and Bussey, 1995). Therefore, perturbation of the GPI pathway might change the rate of cell wall construction, possibly causing aberrant covalent binding or secretion of cell wall proteins. The deletion of the GPI-anchoring signal of agglutinin α, for example, prevents its incorporation into the cell wall and results in its secretion as an inactive molecule into the medium (Wojciechowicz et al., 1993). Also, a mutant in GPI3, a gene involved in the first step of GPI synthesis, produces only very limited amounts of GPI anchors, thus causing accumulation of ER precursors of GPI-dependent cell wall proteins (Vossen et al., 1997). The majority of cell wall proteins that eventually leave the ER of this mutant are not covalently incorporated into the cell wall but are secreted into the growth medium, indicating that improper delivery of GPI-anchored proteins can have profound effects on the proper incorporation of cell wall proteins. In gpi3 mutants, however, no thickening of the cell wall was apparent when they were analyzed at semipermissive temperature (Vossen et al., 1997). Although we do not understand the molecular differences that are responsible for the differences in cell wall morphology, it remains plausible that the observed kinetic retardation of GPI-anchored protein transport in lag1Δ dgt1Δ mutant cells accounts for the observed cell wall defects.
Similarly, the growth defect of lag1Δ dgt1Δ cells can also be explained by an impaired transport of GPI-anchored proteins. All GPI pathway mutants have lower growth rates compared with wild type (Benghezal et al., 1995; Hamburger et al., 1995; Vossen et al., 1997). Furthermore, growth is impaired if the synthesis or attachment of GPI anchors is selectively blocked by depletion of inositol (Doering and Schekman, 1996) or by alteration of the GPI-anchoring signal of Gas1p (Nuoffer et al., 1991). Our results are therefore consistent with the view that the availability of GPI proteins is a limiting factor in cell wall construction and growth.
ACKNOWLEDGMENTS
We are grateful to Sandra Huling for taking the electron micrographs and to Rita Wiemeyer for excellent technical assistance. We thank A. Conzelmann for antibodies against Gas1p, Y. Bourbonnais for antibodies against Yap3p, and H. Riezman for the gaa1-1 mutant strain RH2856. We also thank Corinna Barz, Ted Powers, and Stefan Schorling for helpful discussions and D. Oesterhelt for support. This work was supported by a research fellowship from the Deutsche Forschungsgemeinschaft to W.P.B. and by grants from the National Institutes of Health to P.W. P.W. is an Investigator of the Howard Hughes Medical Institute.
Abbreviations used:
- bp
base pair
- COP
coatomer protein
- CPY
carboxypeptidase Y
- ER
endoplasmic reticulum
- GPI
glycosylphosphatidylinositol
- HA
hemagglutinin
- Ig
immunoglobulin
- kb
kilobase
- SRP
signal recognition particle
- SC
synthetic complete
- TRAM
translocating chain-associated membrane protein
REFERENCES
- Ash J, Dominguez M, Bergeron JJ, Thomas DY, Bourbonnais Y. The yeast proprotein convertase encoded by YAP3 is a glycophosphatidylinositol-anchored protein that localizes to the plasma membrane. J Biol Chem. 1995;270:20847–20854. doi: 10.1074/jbc.270.35.20847. [DOI] [PubMed] [Google Scholar]
- Becker DM, Guarente L. High-efficiency transformation of yeast by electroporation. Methods Enzymol. 1991;194:182–187. doi: 10.1016/0076-6879(91)94015-5. [DOI] [PubMed] [Google Scholar]
- Belden WJ, Barlowe C. Erv25p, a component of COPII-coated vesicles, forms a complex with Emp24p that is required for efficient endoplasmic reticulum to Golgi transport. J Biol Chem. 1996;271:26939–26946. doi: 10.1074/jbc.271.43.26939. [DOI] [PubMed] [Google Scholar]
- Benghezal M, Benachour A, Rusconi S, Aebi M, Conzelmann A. Yeast Gpi8p is essential for GPI anchor attachment onto proteins. EMBO J. 1996;15:6575–6583. [PMC free article] [PubMed] [Google Scholar]
- Benghezal M, Lipke PN, Conzelmann A. Identification of six complementation classes involved in the biosynthesis of glycosylphosphatidyl inositol anchors in Saccharomyces cerevisiae. J Cell Biol. 1995;130:1333–1344. doi: 10.1083/jcb.130.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA. Interactions between GPI-anchored proteins and membrane lipids. Trends Cell Biol. 1992;2:338–343. [PubMed] [Google Scholar]
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Conzelmann A, Fankhauser C, Desponds C. Myo inositol gets incorporated into numerous membrane glycoproteins of Saccharomyces cerevisiae; incorporation is dependent on phosphomannomutase (sec53) EMBO J. 1990;9:653–661. doi: 10.1002/j.1460-2075.1990.tb08157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosson P, Letourneur F. Coatomer interaction with di-lysine endoplasmic reticulum retention motifs. Science. 1994;263:1629–1631. doi: 10.1126/science.8128252. [DOI] [PubMed] [Google Scholar]
- Crowley KS, Liao S, Worrell VE, Reinhart GD, Johnson AE. Secretory proteins move through the endoplasmic reticulum membrane via an aqueous, gated pore. Cell. 1994;78:461–471. doi: 10.1016/0092-8674(94)90424-3. [DOI] [PubMed] [Google Scholar]
- Culbertson MR, Henry SA. Inositol-requiring mutants of Saccharomyces cerevisiae. Genetics. 1975;80:23–40. doi: 10.1093/genetics/80.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’mello NP, Childress AM, Franklin DS, Kale SP, Pinswasdi C, Jazwinski SM. Cloning and characterization of LAG1, a longevity-assurance gene in yeast. J Biol Chem. 1994;269:15451–15459. (erratum 269, 28522) [PubMed] [Google Scholar]
- Do H, Falcone D, Lin J, Andrews DW, Johnson AE. The cotranslational integration of membrane proteins into the phospholipid bilayer is a multistep process. Cell. 1996;85:369–378. doi: 10.1016/s0092-8674(00)81115-0. [DOI] [PubMed] [Google Scholar]
- Doering TL, Schekman R. GPI anchor attachment is required for Gas1p transport from the endoplasmic reticulum in COP II vesicles. EMBO J. 1996;15:182–191. [PMC free article] [PubMed] [Google Scholar]
- Englund PT. The structure and biosynthesis of glycosyl phosphatidylinositol protein anchors. Annu Rev Biochem. 1993;62:121–138. doi: 10.1146/annurev.bi.62.070193.001005. [DOI] [PubMed] [Google Scholar]
- Fankhauser C, Conzelmann A. Purification, biosynthesis and cellular localization of a major 125-kDa glycophosphatidylinositol-anchored membrane glycoprotein of Saccharomyces cerevisiae. Eur J Biochem. 1991;195:439–448. doi: 10.1111/j.1432-1033.1991.tb15723.x. [DOI] [PubMed] [Google Scholar]
- Fiedler K, Veit M, Stamnes MA, Rothman JE. Bimodal interaction of coatomer with the p24 family of putative cargo receptors. Science. 1996;273:1396–1399. doi: 10.1126/science.273.5280.1396. [DOI] [PubMed] [Google Scholar]
- Field J, Nikawa J, Broek D, MacDonald B, Rodgers L, Wilson IA, Lerner RA, Wigler M. Purification of a RAS-responsive adenylyl cyclase complex from Saccharomyces cerevisiae by use of an epitope addition method. J Mol Biol. 1988;8:2159–2165. doi: 10.1128/mcb.8.5.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futerman AH. Inhibition of sphingolipid synthesis: effects on glycosphingolipid-GPI-anchored protein microdomains. Trends Cell Biol. 1995;5:377–378. doi: 10.1016/s0962-8924(00)89078-9. [DOI] [PubMed] [Google Scholar]
- Görlich D, Hartmann E, Prehn S, Rapoport TA. A protein of the endoplasmic reticulum involved early in polypeptide translocation. Nature. 1992;357:47–52. doi: 10.1038/357047a0. [DOI] [PubMed] [Google Scholar]
- Görlich D, Rapoport TA. Protein translocation into proteoliposomes reconstituted from purified components of the endoplasmic reticulum membrane. Cell. 1993;75:615–630. doi: 10.1016/0092-8674(93)90483-7. [DOI] [PubMed] [Google Scholar]
- Hamburger D, Egerton M, Riezman H. Yeast Gaa1p is required for attachment of a completed GPI anchor onto proteins. J Cell Biol. 1995;129:629–639. doi: 10.1083/jcb.129.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hann BC, Walter P. The signal recognition particle in S. cerevisiae. Cell. 1991;67:131–144. doi: 10.1016/0092-8674(91)90577-l. [DOI] [PubMed] [Google Scholar]
- Hegde RS, Lingappa VR. TRAM regulates the exposure of nascent secretory proteins to the cytosol during translocation into the endoplasmic reticulum. Cell. 1998;92:621–631. doi: 10.1016/s0092-8674(00)81130-7. [DOI] [PubMed] [Google Scholar]
- High S, et al. Site-specific photocross-linking reveals that Sec61p and TRAM contact different regions of a membrane-inserted signal sequence. J Biol Chem. 1993;268:26745–26751. [PubMed] [Google Scholar]
- Hofmann K, Stoffel W. TMbase—a database of membrane spanning proteins segments. Biol Chem. 1993;347:166. [Google Scholar]
- Horvath A, Sütterlin C, Manning-Krieg U, Movva NR, Riezman H. Ceramide synthesis enhances transport of GPI-anchored proteins to the Golgi apparatus in yeast. EMBO J. 1994;13:3687–3695. doi: 10.1002/j.1460-2075.1994.tb06678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Fukuda Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MR, Nilsson T, Peterson PA. Identification of a consensus motif for retention of transmembrane proteins in the endoplasmic reticulum. EMBO J. 1990;9:3153–3162. doi: 10.1002/j.1460-2075.1990.tb07513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungnickel B, Rapoport TA. A posttargeting signal sequence recognition event in the endoplasmic reticulum membrane. Cell. 1995;82:261–270. doi: 10.1016/0092-8674(95)90313-5. [DOI] [PubMed] [Google Scholar]
- Kaiser CA, Schekman R. Distinct sets of SEC genes govern transport vesicle formation and fusion early in the secretory pathway. Cell. 1990;61:723–733. doi: 10.1016/0092-8674(90)90483-u. [DOI] [PubMed] [Google Scholar]
- Klionski DJ, Emr SD. Membrane protein sorting: biosynthesis, transport and processing of yeast vacuolar alkaline phosphatase. EMBO J. 1989;8:2241–2250. doi: 10.1002/j.1460-2075.1989.tb08348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klis FM. Review: cell wall assembly in yeast. Yeast. 1994;10:851–869. doi: 10.1002/yea.320100702. [DOI] [PubMed] [Google Scholar]
- Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lee SJ. Expression of growth/differentiation factor 1 in the nervous system: conservation of a bicistronic structure. Proc Natl Acad Sci USA. 1991;88:4250–4254. doi: 10.1073/pnas.88.10.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidich SD, Drapp DA, Orlean P. A conditionally lethal yeast mutant blocked at the first step in glycosyl phosphatidylinositol anchor synthesis. J Biol Chem. 1994;269:10193–10196. [PubMed] [Google Scholar]
- Leidich SD, Kostova Z, Latek RR, Costello LC, Drapp DA, Gray W, Fassler JS, Orlean P. Temperature-sensitive yeast GPI anchoring mutants gpi2 and gpi3 are defective in the synthesis of N-acetylglucosaminyl phosphatidylinositol. Cloning of the GPI2 gene. J Biol Chem. 1995;270:13029–13035. doi: 10.1074/jbc.270.22.13029. [DOI] [PubMed] [Google Scholar]
- Letourneur F, Gaynor EC, Hennecke S, Demolliere C, Duden R, Emr SD, Riezman H, Cosson P. Coatomer is essential for retrieval of dilysine-tagged proteins to the endoplasmic reticulum. Cell. 1994;79:1199–1207. doi: 10.1016/0092-8674(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Levin DE, Bartlett-Heubusch E. Mutants in the S. cerevisiae PKC1 gene display a cell cycle-specific osmotic stability defect. J Cell Biol. 1992;116:1221–1229. doi: 10.1083/jcb.116.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MJ, Pelham HR. SNARE-mediated retrograde traffic from the Golgi complex to the endoplasmic reticulum. Cell. 1996;85:205–215. doi: 10.1016/s0092-8674(00)81097-1. [DOI] [PubMed] [Google Scholar]
- Lu CF, Montijn RC, Brown JL, Klis F, Kurjan J, Bussey H, Lipke PN. Glycosyl phosphatidylinositol-dependent cross-linking of α-agglutinin and β 1,6-glucan in the Saccharomyces cerevisiae cell wall. J Cell Biol. 1995;128:333–340. doi: 10.1083/jcb.128.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothes W, Prehn S, Rapoport TA. Systematic probing of the environment of a translocating secretory protein during translocation through the ER membrane. EMBO J. 1994;13:3973–3982. doi: 10.1002/j.1460-2075.1994.tb06713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng DTW, Brown JD, Walter P. Signal sequences specify the targeting route to the endoplasmic reticulum membrane. J Cell Biol. 1996;134:269–278. doi: 10.1083/jcb.134.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura N, Nagase T, Miyajima N, Sazuka T, Tanaka A, Sato S, Seki N, Kawarabayasi Y, Ishikawa K, Tabata S. Prediction of the coding sequences of unidentified human genes. II. The coding sequences of 40 new genes (KIAA0041-KIAA0080) deduced by analysis of cDNA clones from human cell line KG-1. DNA Res. 1994;1:223–229. doi: 10.1093/dnares/1.5.223. [DOI] [PubMed] [Google Scholar]
- Novick P, Ferro S, Schekman R. Order of events in the yeast secretory pathway. Cell. 1981;25:461–469. doi: 10.1016/0092-8674(81)90064-7. [DOI] [PubMed] [Google Scholar]
- Novick P, Field C, Schekman R. Identification of 23 complementation groups required for posttranslational events in the yeast secretory pathway. Cell. 1980;21:205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Nuoffer C, Horvath A, Riezman H. Analysis of the sequence requirements for glycosylphosphatidylinositol anchoring of Saccharomyces cerevisiae Gas1 protein. J Biol Chem. 1993;268:10558–10563. [PubMed] [Google Scholar]
- Nuoffer C, Jenö P, Conzelmann A, Riezman H. Determinants for glycophospholipid anchoring of the Saccharomyces cerevisiae GAS1 protein to the plasma membrane. Mol Cell Biol. 1991;11:27–37. doi: 10.1128/mcb.11.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson B, Argos P. Prediction of transmembrane segments in proteins utilizing multiple sequence alignments. J Mol Biol. 1994;237:182–192. doi: 10.1006/jmbi.1994.1220. [DOI] [PubMed] [Google Scholar]
- Pringle JR, Adams AEM, Drubin DG, Haarer BK. Immunofluorescence methods for yeast. Methods Enzymol. 1991;194:565–602. doi: 10.1016/0076-6879(91)94043-c. [DOI] [PubMed] [Google Scholar]
- Rapoport TA, Jungnickel B, Kutay U. Protein transport across the eukaryotic endoplasmic reticulum and bacterial inner membranes. Annu Rev Biochem. 1996;65:271–303. doi: 10.1146/annurev.bi.65.070196.001415. [DOI] [PubMed] [Google Scholar]
- Roemer T, Bussey H. Yeast Kre1p is a cell surface O-glycoprotein. Mol Gen Genet. 1995;249:209–216. doi: 10.1007/BF00290368. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EM, Maniatis T. Molecular Cloning: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Schekman R, Orci L. Coat proteins and vesicle budding. Science. 1996;271:1526–1533. doi: 10.1126/science.271.5255.1526. [DOI] [PubMed] [Google Scholar]
- Schimmöller F, Singer-Krüger B, Schröder S, Krüger U, Barlowe C, Riezman H. The absence of Emp24p, a component of ER-derived COPII-coated vesicles, causes a defect in transport of selected proteins to the Golgi. EMBO J. 1995;14:1329–1339. doi: 10.1002/j.1460-2075.1995.tb07119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönbächler M, Horvath A, Fassler J, Riezman H. The yeast SPT14 gene is homologous to the human PIG-A gene and is required for GPI anchor synthesis. EMBO J. 1995;14:1637–1645. doi: 10.1002/j.1460-2075.1995.tb07152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SM, Blobel G. A protein-conducting channel in the endoplasmic reticulum. Cell. 1991;65:371–380. doi: 10.1016/0092-8674(91)90455-8. [DOI] [PubMed] [Google Scholar]
- Simons K, van Meer G. Lipid sorting in epithelial cells. Biochemistry. 1988;27:6197–6202. doi: 10.1021/bi00417a001. [DOI] [PubMed] [Google Scholar]
- Skrzypek M, Lester RL, Dickson RC. Suppressor gene analysis reveals an essential role for sphingolipids in transport of glycosylphosphatidylinositol-anchored proteins in Saccharomyces cerevisiae. J Bacteriol. 1997;179:1513–1520. doi: 10.1128/jb.179.5.1513-1520.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn K, Orci L, Ravazzola M, Amherdt M, Bremser M, Lottspeich F, Fiedler K, Helms JB, Wieland FT. A major transmembrane protein of Golgi-derived COPI-coated vesicles involved in coatomer binding. J Cell Biol. 1996;135:1239–1248. doi: 10.1083/jcb.135.5.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens T, Esmon B, Schekman R. Early stages in the yeast secretory pathway are required for transport of carboxypeptidase Y to the vacuole. Cell. 1982;30:439–448. doi: 10.1016/0092-8674(82)90241-0. [DOI] [PubMed] [Google Scholar]
- Stotz A, Linder P. The ADE2 gene from Saccharomyces cerevisiae: sequence and new vectors. Gene. 1990;95:91–98. doi: 10.1016/0378-1119(90)90418-q. [DOI] [PubMed] [Google Scholar]
- Sütterlin C, Doering TL, Schimmöller F, Schröder S, Riezman H. Specific requirements for the ER to Golgi transport of GPI-anchored proteins in yeast. J Cell Sci. 1997;110:2703–2714. doi: 10.1242/jcs.110.21.2703. [DOI] [PubMed] [Google Scholar]
- Takeda J, Kinoshita T. GPI-anchor biosynthesis. Trends Biochem Sci. 1995;20:367–371. doi: 10.1016/s0968-0004(00)89078-7. [DOI] [PubMed] [Google Scholar]
- Voigt S, Jungnickel B, Hartmann E, Rapoport TA. Signal sequence-dependent function of the TRAM protein during early phases of protein transport across the endoplasmic reticulum membrane. J Cell Biol. 1996;134:25–35. doi: 10.1083/jcb.134.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Membrane protein structure prediction, hydrophobicity analysis and the positive-inside rule. J Mol Biol. 1992;225:487–494. doi: 10.1016/0022-2836(92)90934-c. [DOI] [PubMed] [Google Scholar]
- Vossen JH, Müller WH, Lipke PN, Klis FM. Restrictive glycosylphosphatidylinositol anchor synthesis in cwh6/gpi3 yeast cells causes aberrant biogenesis of cell wall proteins. J Bacteriol. 1997;179:2202–2209. doi: 10.1128/jb.179.7.2202-2209.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossen JH, Ram AF, Klis FM. Identification of SPT14/CWH6 as the yeast homologue of hPIG-A, a gene involved in the biosynthesis of GPI anchors. Biochim Biophys Acta. 1995;1243:549–551. doi: 10.1016/0304-4165(95)00002-s. [DOI] [PubMed] [Google Scholar]
- Walter P, Johnson AE. Signal sequence recognition and protein targeting to the endoplasmic reticulum membrane. Annu Rev Cell Biol. 1994;10:87–119. doi: 10.1146/annurev.cb.10.110194.000511. [DOI] [PubMed] [Google Scholar]
- Wojciechowicz D, Lu CF, Kurjan J, Lipke PN. Cell surface anchorage and ligand-binding domains of the Saccharomyces cerevisiae cell adhesion protein α-agglutinin, a member of the Ig superfamily. Mol Cell Biol. 1993;13:2554–2563. doi: 10.1128/mcb.13.4.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]