Abstract
Characterization of structural isomers has become increasingly important and extremely challenging in glycobiology. This communication demonstrates the capability of ion-trap mass spectrometry in conjunction with 157 nm photofragmentation to identify different structural isomers of permethylated N-glycans derived from ovalbumin without chromatographic separation. The results are compared with CID experiments. Photodissociation generates extensive cross-ring fragment ions as well as diagnostic glycosidic product ions that are not usually observed in CID MS/MS experiments. The detection of these product ions aids in characterizing indigenous glycan isomers. The ion-trap facilitates MSn experiments on the diagnostic glycosidic fragments and cross-ring product ions generated through photofragmentation, thus allowing unambiguous assignment of all of the isomeric structures associated with the model glycoprotein utilized in this study. Photofragmentation is demonstrated to be a powerful technique for the structural characterization of glycans.
Introduction
Glycans are biomolecules consisting of one or more monosaccharide units that are covalently N- or O-linked to a protein as one of the most common posttranslational modifications. They are either linear, consisting of repeating monosaccharide rings (commonly observed for O-linked glycans), or branched with multiple glycosidic linkages between monosaccharide units.[1,2] Their crucial role in a wide range of biological processes, including inter and intra cellular activities,[3] co-ordination of immune functions,[4] therapeutics[5] and protein regulations and interactions[6] is widely acknowledged[7]. To improve our understanding of these processes, it is important to explore glycan structure-function relationships. However, the detailed characterization of glycan structure and its attributes remains difficult task, due to the microheterogeneity and diversity of these molecules. Most importantly, the discrimination of numerous structural isomers differing in sequence, linkage, position, or branching features remains a challenging frontier of glycobiology.
For years, the characterization of glycan structures has almost exclusively been accomplished by tandem mass spectrometry (MS/MS).[1,8,9] This is due to its high sensitivity and minimum sample requirements relative to nuclear magnetic resonance (NMR). Different combinations of ionization techniques, ion activation and mass analyzers have been employed, including fast atom bombardment (FAB)-MS,[10-12] infrared-laser desorption MS,[13,14] matrix-assisted laser desorption/ionization (MALDI)/magnetic sector MS,[15-17] electrospray ionization (ESI)-MS,[18,19] MALDI/time-of-flight (TOF)-MS,[20-22] ESI ion-trap MS,[23-25] MALDI Fourier transform MS,[26] ESI- or MALDI-based quadrupole/TOF-MS,[27-30], MALDI/Postsource Decay (PSD) TOF-MS[31-33] and, more recently, MALDI-TOF/TOF-MS [34-38].
A glycan ion generally fragments in two ways: (a) glycosidic cleavages resulting from a bond rupture between two adjacent sugar residues; and (b) cross-ring cleavages in which any two bonds on the same sugar unit are broken. Cross-ring fragment ions are commonly observed in high-energy CID methods as demonstrated in the tandem TOF/TOF approach.[34–38] A limitation of this technique, however, is its inability to perform multi-stage tandem mass spectrometry experiments. On the other hand, glycosidic cleavage ions are predominantly observed in low-energy activation methods and are mainly used to derive sequence and limited branching information. Most recently, Reinhold and coworkers demonstrated the use of low-energy activation to identify the structural isomers of glycans in complex mixtures with the help of sequential tandem mass spectrometry (MS)n.[39] However, a disadvantage of CID is the decrease in both the degree and efficiency of dissociation with increasing mass and MSn events. Alternatively, other activation techniques have been utilized for the structural characterization of glycans including infrared multi-photon dissociation (IRMPD)[40-43] and electron capture dissociation (ECD)[44,45]. Although IRMPD and CID are both low-energy vibrational excitation techniques, Lebrilla and co-workers have shown that the fragmentation efficiency of IRMPD increases with increasing oligosaccharide size.[42] Recent efforts in the fragmentation of various metal-cationized oligosaccharides by ECD have provided structural information complementary to IRMPD.[45]
Recently, we have reported that 157 nm laser photodissociation of peptide ions generates high energy backbone and side-chain cleavages.[46–48] On the basis of previous spectroscopic studies, we proposed that the chromophore involved in this process is the backbone amide. The preferential cleavage between the α- and carbonyl-carbon atoms of a singly charged peptides having C- or N- terminal arginine yielded uniform and easily interpretable distributions of x- and a-type ions. We recently expanded the utility of photofragmentation to the characterization of native and derivatized linear oligosaccharides as well as permethylated acidic glycans.[49,50] Photodissociation has yielded intense cross-ring fragmentation of Girard’s T derivatized oligosaccharides and the product ions correspond to high-energy fragmentation pathways.[49] Significant progress has been reported with different permethylated glycan structures, including sialylated linear and branched, high-mannose type and fucosylated, complex types.[50] In the present study, we report further results with photofragmentation of selected permethylated N-glycans derived from ovalbumin. In particular, we explore the potential of utilizing an ion-trap mass spectrometer to perform MSn CID experiments on the characteristic photofragment ions.
EXPERIMENTAL
Materials
Ovalbumin (chicken egg white), endoglycosidase peptide-N-glycosidase (PNGase F; EC 3.4.1.52), di-hydroxy benzoic acid (DHB), sodium acetate, mercaptoethanol, and dimethyl sulfoxide (DMSO) were purchased from Sigma Chemical Company (St. Louis, MO, USA). Sodium hydroxide 20–40 mesh beads were obtained from Aldrich (Milwaukee, WI, USA). Micro spin-columns, suitable for volumes from 25 to 75 μL, chloroform, methanol and iodomethane were from EM Science (Gibbstown, NJ, USA), while glacial acetic acid was from Sigma Chemical Company. Acetonitrile was obtained from Fisher Scientific (Fair Lawn, NJ, USA). All water used in these experiments was purified by an E-pure water filtration system from Barnstead Thermolyne Co. (Dubuque, IA, USA).
Enzymatic Release of N-glycans from Ovalbumin
The enzymatic release of N-glycans from ovalbumin was carried out according to our previously published procedure [51]. Briefly, 50 μg glycoprotein was suspended in 25 μL of an incubation buffer consisting of 10 mM sodium phosphate, pH 7.0, and 1% mercaptoethanol. The sample was then thermally denatured by incubation at 95°C for 5 min. Next, it was allowed to cool to room temperature prior to the addition of 5 mU of PNGase F and incubation at 37°C in a water bath overnight. Finally, the sample was dried under a stream of nitrogen prior to permethylation.
Solid-phase spin-column Permethylation
All glycans were permethylated following our solid-phase permethylation method[52]. First, a capillary reactor consisting of PEEK (polyetherether ketone polymer) tubing with 1-mm inner diameter was connected to a PEEK union with a stainless-steel frit. Sodium hydroxide beads suspended in acetonitrile were then used to pack the capillary reactor. Samples prepared in DMSO were infused through the reactor at a flow rate of 2 μL/min. Typically, a 50 μg sample aliquot was suspended in 30 μL of DMSO, to which 0.3 μL of water and 5.6 μL of methyl iodide were added. The permethylated glycans eluted from the sodium hydroxide reactor were subsequently extracted from the mixture with chloroform and washed repeatedly with cold water prior to evaporation.
Sample Preparation
The dried permethylated samples were resuspended in 25 μL of 50/50 methanol/water solution containing 1 mM sodium acetate. Samples were infused directly into the mass spectrometer without any prior chromatographic separations at 300 nL/min, while the ESI capillary voltage was set to 1.8 kV.
Instrumentation
Electrospray experiments were performed on a Thermo Fisher LTQ mass spectrometer (San Jose, CA) equipped with an in-house fabricated nano-ESI source (75 μm i.d. fused silica capillary pulled into a tip needle). The system was upgraded with LTQ 1.0 developer’s Kit software and calibrated using PPG 2700 (for 2000–4000Da region). All samples were infused into the LTQ mass spectrometer at flow rates ranging between 300 to 500 nL/min using a syringe pump. The ion injection time was set to 500–1000ms with an isolation width of 2–3 daltons to select glycan precursor ions. Automatic gain control (AGC) was used to accumulate sufficient precursor ions (Full MS and MSn target value 1 × 105 ions). CID was accomplished using helium gas at normalized collision energy of 30–50% for 60 ms (single scan) with Q-value of 0.25. A total of 60 micro scans were averaged to produce a spectrum. The LTQ was also slightly modified to be compatible with 157nm photodissociation as recently reported [48–50,53]. Briefly, an F2 laser capable of 60 Hz repetition rate (EX100HF-60, GAM Laser, Orlando, FL), producing 2 mJ of light in a 10 ns pulse, was connected to the back of the LTQ instrument with a vacuum line. The unfocused light was introduced into the trap through a 1.7mm diameter aperture aligned with the preexisting 2mm hole in the back lens of the LTQ. Based on the size of the aperture and the laser beam profile, we estimate that about 40 μJ of light pass into the ion trap. After irradiating the trapped ions, most of the light then passes through the hole in the front lens of the trap. The precursor ion isolation conditions were the same as in CID experiments, except that the normalized collision energy was set to 0% at a Q-value of 0.10. Since collision gas is not introduced into the analyzer cell it is possible to use a lower ion trap Q value during photodissociation experiments.[54,55] The advantage of this, in principle, is that lower mass fragment ions should be detectable. The laser was triggered by the LTQ at the beginning of the activation stage. In order to pursue MS3 experiments, the signal intensities of MS2 fragment ions were enhanced by photodissociating with three laser shots. This significantly improves the signal intensities of MS2 photofragments. Once an ion of interest is selected for MS3 experiments, 20–30 % of normalized collision energy was used for further fragmentation. MS/MS interpretation was aided through the use of the Chem Draw Ultra 10 (Cambridge Soft, Cambridge, MA, USA).
An Applied Biosystems (Foster City, CA, USA) 4800 proteomics analyzer was utilized for high energy 1 keV CID experiments with argon collision gas at a pressure of 1 × 10− 7 Torr. MALDI spots were prepared by dissolving 10–20 picomoles of the dried samples in 10 μL of DHB matrix solution (20 g/L) in 1:1 v/v MeOH/water containing 1mM of sodium Acetate. The acquired spectra were the average of 1000 laser shots. MS and MS/MS data were further processed using Data Explorer 4.0 (Applied Biosystems).
RESULTS AND DISCUSSION
It is common in any fragmentation technique to evaluate how effectively precursor ions are converted into fragments; this can be done by measuring the extent of precursor ion depletion caused by laser irradiation. The precursor ion depletion rate in our study was calculated as the difference between the precursor ion intensity before and after photofragmentation divided by the precursor ion intensity prior to photofragmentation. It was possible to achieve consistent results with this method since the precursor ion signal intensities were stable to within about 3%. In our previous published work, precursor ion depletion rates of ~ 25% have been measured for peptides [47] and linear oligosaccharides [49]. Recently, we applied 15% of normalized collision energy during photodissociation experiments to increase the fragmentation efficiency. This significantly improves the signal intensities of MS2 stage photofragments.[53] It was of interest to study this efficiency using multiple laser shots that were employed to generate our photofragmentation spectra. Two ovalbumin glycans having m/z values of 2070 and 2153 were chosen as models to perform this study. We found that more laser shots consistently yield greater product ion signals. In one case, about 90 ± 3% precursor ion depletion was observed with three laser shots (Supplemental Figure 1). Typical experimental spectra obtained from the m/z 2070 ion with 1–3 laser shots are displayed in Figure 1a–c. It is evident that additional laser shots do not change the types of photofragments formed but simply improve the MSn signal. The intensities of the fragment ions generated using two laser shots (Figure 1b) were about 3-times higher than those observed using a single laser shot (Figure 1a). Similarly, the intensities of the fragment ions resulting from three laser shots (Figure 1c) were about 6-fold higher than those observed using a single shot. Therefore, 3 laser shots were used in subsequent experiments yielding cleaner spectra with better signal-to-noise ratios. It is noteworthy that the present depletion rates are considerably higher than what we previously reported for linear charge-tagged oligosaccharides.[49] This could arise for a few reasons. First, there are more sugar rings in these branched molecules leading to larger absorption coefficients. Second, the presence of N-acetyl groups in ovalbumin glycans and the permethylation of these structures could likewise provide additional absorption chromophores. Third, improved alignment of the 157nm light with the axis of the ion trap may now lead to irradiation of more of the trapped ions.
Figure 1.
Comparison of photodissociation spectra obtained by (a) 1 (b) 2 (c) 3 laser shots from [M+Na]+ permethylated glycan ion m/z 2071 of hen ovalbumin.
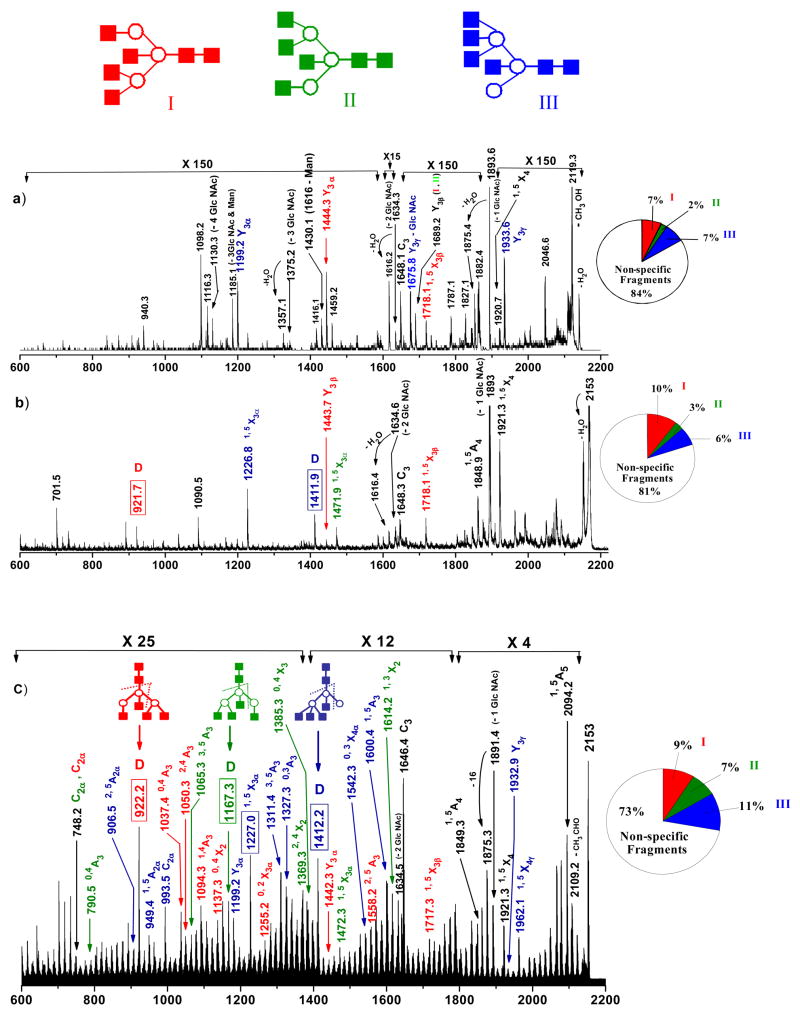
The nomenclature introduced by Dommon and Costello,[56] Spina et al[57] and by Stephens and coworkers [58] is utilized throughout this work to describe the fragmentation processes. Our recent work with permethylated acidic and neutral N-glycans demonstrated the ability to form abundant cross-ring fragments inside the ion-trap with 157 nm light activation.[50] We also learned that singly charged ions yielded more informative spectra than their multiply charged counterparts and concluded that photodissociation produced high-energy fragmentation pathways.[49,50] In this report, we explore the capabilities of the ion-trap photodissociation combination for the structural characterization of permethylated [M+Na]+ isomeric N-glycans derived from ovalbumin. Table 1 summarizes the masses and structures of different glycan isomers with some of their photofragment ions that are characterized in this manuscript. The structures of these glycans have been studied over the last twenty years and reported to be very heterogeneous [59–63]. The nano-ESI MS1 spectrum of the permethylated glycan mixture released from ovalbumin is in Supplemental Figure 2. To demonstrate the capability of photofragmentation, we specifically focused on glycans that contain at least three possible isomeric structures observed at masses of about 2000 daltons. The low energy CID spectrum displayed in Figure 2(a) was generated in the linear ion-trap instrument for the (GlcNAc)6 (Man)3 glycan structure (m/z 2153). It has been shown previously that this structure consists of three isomeric glycan structures (denoted in different colors) depicted as I, II and III in the inset of Figure 2(a) [62,63]. The most abundant product ion observed in the spectrum is m/z 1894 corresponding to the facile loss of GlcNAc (N-Acetyl Glucosamine) residue from the precursor. A peak at m/z 1648 (C3) belonging to the loss of two GlcNAc residues from the reducing end of the molecule. The formation of these non specific product ions (labeled in black letters) appears to be significantly limiting the yield of other product ions. Importantly, they can arise from any of the given isomeric structures and are not helpful either to identify or distinguish the isomers. Minor ions at m/z values of 1199 (Y3α), 1676 (Y3γ – GlcNAc), 1933 (Y3 γ) are indicative of isomer III (shown in blue). Other features at m/z 1444 (Y3α) and 1718 (1,5X3β) point to the presence of isomer I (shown in red). Also present in the Figure 2(a) is the ion at m/z 1689 (Y3β) that could be assigned to isomer I and II (shown in green). However, the signal intensities of all of these diagnostic fragments were low and the information was certainly not sufficient to distinguish all three isomeric structures. Thus the ion-trap MS2 analysis of these glycans would not be sufficient to reveal the presence of all of the isomeric structures of this m/z value. Similar observations were made by Reinhold and others [39]. The pie chart in Figure 2a displays the percentages of specific and non-specific fragment ions. It is evident that the majority of low energy CID fragments are non-specific.
Table 1.
Summary of characteristic photofragment ions subjected to collisional dissociation.
| m/z | Precursor ion Structures * | Fragments used for MS3 (m/z) | Structures associated with MS3 |
|---|---|---|---|
| 2153 |

|
922 |
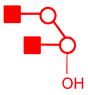
|
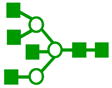
|
1167 |
|
|

|
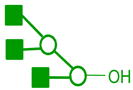
1227, 1412 |
1,5X3α,
|
|
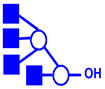
| 2071 |

|
– | – |
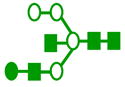
|
895 |

|
|
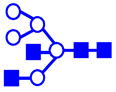
|
1085 |

|
|
| 1865 |

|
676 |

|

|
1009 | 2,4A4 | |

|
885 |

|
|
| 2356 |

|
922 |

|

|
1010 | 2,4A4 | |
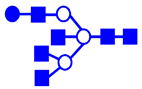
|
1023 | 3,5A4 |
Figure 2.
MS2 fragmentation spectra of [M+Na]+ permethylated glycan precursor m/z 2153 (from ovalbumin) obtained by (b) low energy CID in ion trap (c) high energy CID in MALDI-TOF/TOF (d) 157 nm photodissociation in ion trap. All fragments contain sodium. The inset demonstrates the formation of key product ions (denoted in different colors) during fragmentation. Non-specific product ions are labeled in black letters. Note changes in vertical scale within the spectrum.
■ → GlcNAc ○ → Man ● → Gal
The same analyte was subjected to high-energy CID in MALDI-TOF/TOF and the resulting spectrum is shown in Figure 2(b). Despite the strong signal upon isolation, the first MS/MS event did not completely fragment the precursor ion. The product ion at m/z 922, formed by two glycosidic cleavages B3/Y3α as depicted in the spectrum inset (usually denoted as D-ion)[64], is indicative of isomer I (red). Ions at m/z 1444 (Y3β) and 1718 (1,5X3β) also support the presence of isomer I. Product ion at m/z 1472 (1,5X3α) can be assigned to isomer II (green). Other fragment ions at m/z 1227 (1,5X3α) and 1412 (D-ion) in the spectrum point to structure III (blue). As shown in the pie chart more specific fragments for each of the three isomers were observed in this type of fragmentation. However, most of the fragment ions observed by high-energy CID were again non-specific. Although the information obtained from these fragments is better than low-energy CID (Figure 2(a)), they are not sufficient to thoroughly characterize all three isomers present in the mixture. Most importantly, further explorations of these ions through MS3 experiments are not possible by this technique.
The analogous ion-trap 157 nm photodissociation spectrum of m/z 2153 ion, obtained by 3 laser shots, is presented in Figure 2(c). Most strikingly there is a dramatic increase in the number of fragment ions distributed evenly across the mass range. Extensive cross-ring fragment ions appear along with characteristic features that provide critical information about the existence of the three isomers. Ions observed at m/z values of 922(D), 1037 (0,4A3) 1050 (2,4A3), 1094 (1,4A3), 1137 (0,4X2), 1255 (0,2X3α), 1442 (Y3α), 1558 (2,5A3) and 1717 (1,5X3β) were consistent with structure I (red), while ions observed at m/z values of 790 (0,4A3), 1065 (3,5A3), 1167 (D), 1369 (2,4X2), 1385 (0,4X3), 1472 (1,5X3α) and 1614 (1,3X2) account for isomer II (green). Ions originating from isomer III (blue) include those observed at m/z values of 906.5 (2,5A2α), 949 (1,5A2α), 993.5 (C2α), 1199 (Y3α), 1227 (1,5X3α), 1311 (3,5A3), 1327 (0,3A3), 1412 (D), 1542 (0,3X4α), 1600 (1,5A3), 1933 (Y3γ) and 1962(1,5X4 γ). Accordingly, these unique features in the MS2 experiments permit the three isobaric structures to be distinguished. As indicated in its pie chart, photofragmentation appears to generate more specific fragment ions for the three isomers relative to the other techniques. This being said, fragmentation in general is dependent on the structure; therefore, the percentages observed in Figure 2 could easily vary for different structures.
Although several other peaks appear in the spectrum, they do not provide adequate information about the conformational isomers. In particular, the distribution of numerous intense high mass photofragments separated by 14 and 16 Daltons is common to all photofragmentation spectra. We believe that these product ions originate from a series of facile losses of small neutral molecules. To identify these molecules we are currently studying the photofragmentation of the chitobiose core and this will be the subject of a future publication.
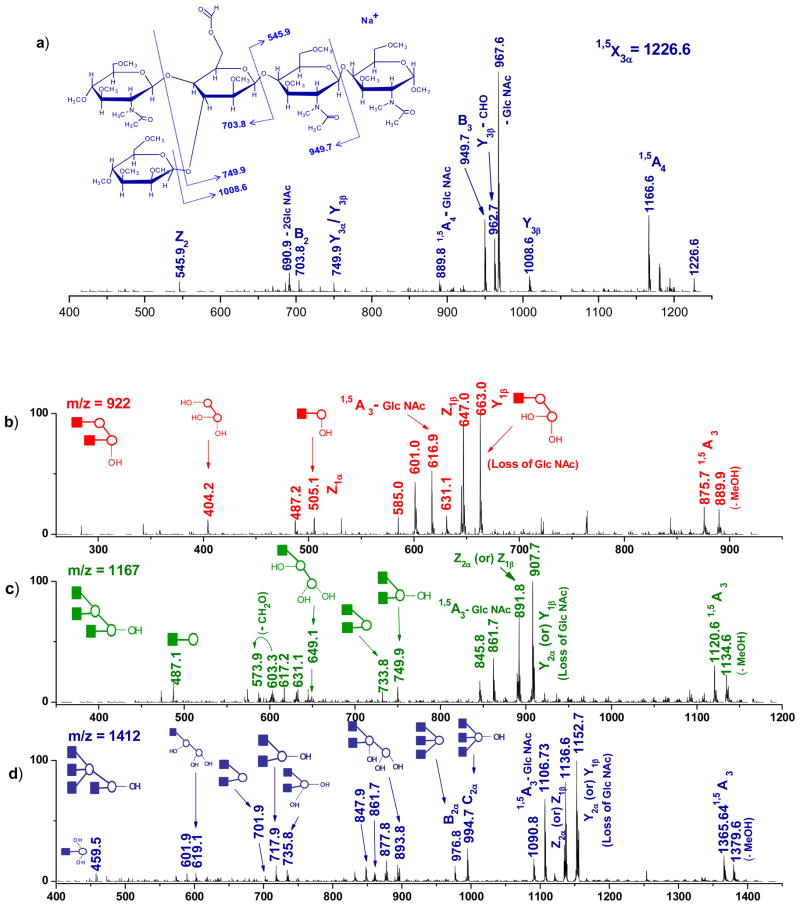
A major advantage of the ion-trap mass spectrometer is the ability to perform MSn CID experiments on any of the fragments including cross-ring product ions generated by photodissociation in order to confirm their identities. An example of this was obtained by the collisional activation of a cross-ring photofragment ion observed at m/z 1227 (1,5X3α) originating from isomer III. The spectrum along with the structure and assignments of the fragments observed are displayed in Figure 3(a). The detection of ions at m/z values of 1008.6 (Y3β), 949 (B3), 704 (B2) and 546 (Z2) allowed the assignment of the 1,5X3α cross-ring fragment ion and its structure is presented in the spectrum inset.
Figure 3.
(a) MS3 CID spectrum of the cross-ring photofragment m/z 1226 obtained from [M+Na]+ permethylated glycan precursor m/z 2153 after 157 nm photodissociation. MS3 CID spectra of photofragments m/z (b) 922 (c) 1167 (d) 1412 generated from ion m/z 2153. Ions generated from multiple cleavage sites are designated with a slash between the sites of cleavage. All fragments contain sodium.
The most important features at m/z 922, 1167 and 1412 in the MS2 photofragmentation spectrum (Figure 2(c)) are D ions that result from two glycosidc cleavages. They are attributable to structures I, II and III, respectively. Although there are higher mass fragments that are more abundant in the photofragmentation spectrum, they are not unique to specific isomers. Therefore subjecting these ions to high order tandem MS experiments does not provide the needed information to unambiguously discriminate among the different glycan isomers. In contrast, D-type photofragment ions that have lost the core GlcNAc residues and the 3- antenna are unique to each isomeric form and their identities can be confirmed by one stage of collisional fragmentation. To investigate the D ion structures, the m/z 922 photofragment ion was selected for MS3 CID analysis and the resulting spectrum is presented in Figure 3(b). Appearance of fragment ions at m/z values of 404, 505 and 663 could only come from isomer I. Similarly, MS3 experiments with photofragment ions m/z 1167 and 1412 are illustrated in Figures 3(c) and 3(d), respectively. The most informative ions at m/z values of 908, 750 and 734 in Figure 3(c) and 1153, 995, 977 and 894 in Figure 3(d) provide strong evidence for structures II and III, respectively. It is noteworthy that all the isomers were conclusively assigned in MS3 experiments.
Figure 4(a) is the MS2 photodissociation spectrum of the precursor ion at m/z 2071 [composition, (GlcNAc)4 (Man)5 and (GlcNAc)4 (Man)4 (Gal)1] derived from the permethylated ovalbumin glycan sample. The observed fragmentation pattern contains a variety of diagnostic fragment ions, suggesting the presence of three isomers I (Red), II (Green) and III (blue) as depicted in the inset of Figure 4. This is in agreement with the structures previously reported [62]. Ions at m/z values of 709, 778, 879, 895, 1009, 1314, 1384, 1431 and 1676, that originate from structure II (Green), correspond to C3α, 3,5A4, B4/Y3α, C4/Y3α, 2,4A4, 3,5X2, Z3α, 1,5X3α and 1,5X3β, respectively. Photofragmentation of isomer III (blue) generates ions at m/z values of 983 (3,5A3), 1069 (B3/Z3α), 1085 (C3/Z3α) and 1109 (3,5X2). The low abundance fragment ions at m/z values of 750 (C2α), 866 (1,5X3α- 2GlcNAc), 1040 (1,5X3α -1GlcNAc and N-acetyl group) and 1213 (1,5X3β - 1GlcNAc) detected in the photofragmentation spectrum were only consistent with isomer I. Other peaks at m/z 620 (1,5A2β), 666 (C2α, C2β) and 1472 (1,5X3β) were supporting the presence of isomers II and III. Unfortunately, compared with isomers II and III, the diagnostic product ions from structure I were not sufficiently intense to perform MS3 experiments. However, the presence of structure I is conclusive from the presence of the above mentioned ions. On the other hand, in order to validate that photofragment ions m/z 895 (C4/Y3α) and m/z 1085 (C3/Z3α) originate from structures II and III, respectively, they were isolated and collisionally dissociated in the trap. The resulting MS3 spectra are depicted in Figure 4(b) and 4(c). The fragmentation pattern observed in the spectrum depicted in Figure 4(b) provides strong evidence for the presence of isomer II. Specifically, strong features at m/z values of 678 (Y2α), 636 (Y1β) and 620 (Z1β) could only originate from structure II. Similarly, the most intense fragment ions at m/z 826 (Y1β), 810 (Z1β), 668.5 (C2α) and 650.6 (B2α) in Figure 4(c) are all consistent with the III structure and could not arise from the others.
Figure 4.
(a) MS2 photodissociation spectrum of [M+Na]+ permethylated glycan precursor m/z 2071 (from ovalbumin). MS3 CID spectra of photofragments m/z (b) 895 (c) 1085 generated from ion m/z 2071. Ions due to multiple cleavage sites are designated with a slash between the sites of cleavage. All fragments contain sodium. Note the changes in vertical scale within the spectrum.
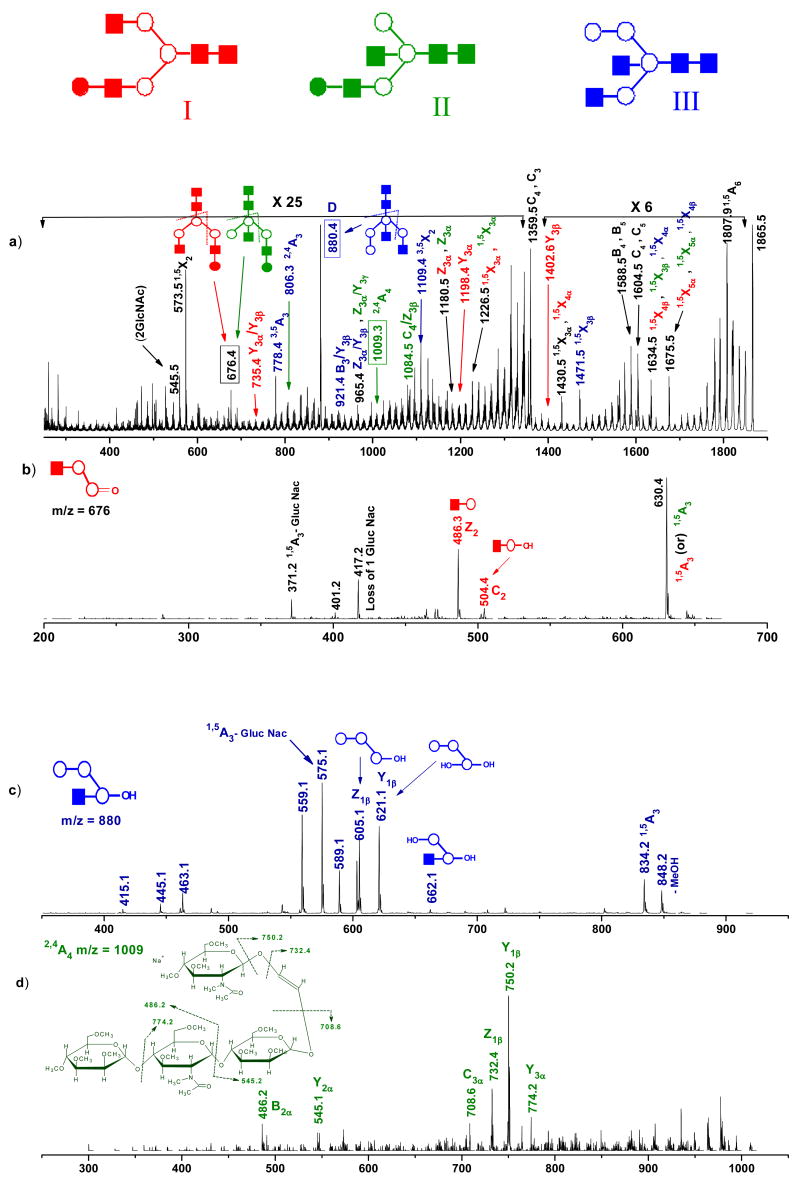
Next, we photofragmented the ion at m/z 1865 that could be associated with three possible isomeric structures containing (GlcNAc)4 (Man)4 and (GlcNAc)4 (Man)3 (Gal)1. The resulting photofragmentation MS/MS spectrum is shown in Figure 5(a), and the structures of the three isomers are depicted in the inset. Again, a significant number of diagnostic product ions appear in this MS2 spectrum that provide valuable information about the existence of the different isomers associated with this glycan. Fragment ions at m/z values of 735 (Y3α/ Y3β), 1198 (Y3α) and 1403 (Y3β) result from isomer I (Red). Ions at m/z values of 1009 (2,4A4) and 1084.5 (C4/ Z3β) indicate the presence of structure II (Green). Other ions at m/z values of 778 (3,5A3), 806 (2,4A3), 880 (Z3α/C3 or D), 921 (B3/Y3β), 1109 (3,5X2) and 1471 (1,5X3β) point to isomer III (Blue). Also detected in Figure 5(a) are the ions at m/z values of 676 (D), 1180 (Z3α) and 1226 (15X3α) that are consistent with both I and II isomers. The peak at m/z 965 (Z3α/Y3β, Z3α/Y3 γ) could be associated with structures II and III.
Figure 5.
(a) MS2 photodissociation spectrum of [M+Na]+ permethylated glycan precursor m/z 1865 (from ovalbumin). MS3 CID spectra of photofragments m/z (b) 676 (c) 880 (d) 1009 generated from ion m/z 1865. Ions due to multiple cleavage sites are designated with a slash between the sites of cleavage. All fragments contain sodium. Note the changes in vertical scale within the spectrum.
To further confirm the structures of these isomers MS3 experiments were performed on m/z 676 (D), 880 (D) and 1009 (2,4A4). Figure 5(b) was generated by the collisional activation of the diagnostic photofragment m/z 676 that appeared to be a mixture of isomers I and II. However, the presence of two features at m/z 504 (C2) and 486 (Z2) confirmed the presence of isomeric structure I. Figure 5(c) presents an MS3 CID spectrum of the photofragment ion observed at m/z 880. Unique features at m/z 662 (Y2α), 621(Y1β) and 605 (Z1β) are very helpful in identifying isomer III. Most noteworthy, these fragment ions enabled us to distinguish structure III from structures I and II. In the collisional dissociation of the photofragment observed at m/z 1009 (2,4A4), Figure 5(d), we see diagnostic fragments such as m/z 774 (Y3α), 750 (Y1β), 732 (Z1β) and 709 (C3α) that provide valuable information to successfully assign the cross-ring fragment ion structure 2,4A4 corresponding to isomer II. In summary, MS2 and MS3 analysis of these structures conclusively assigns all isomers.
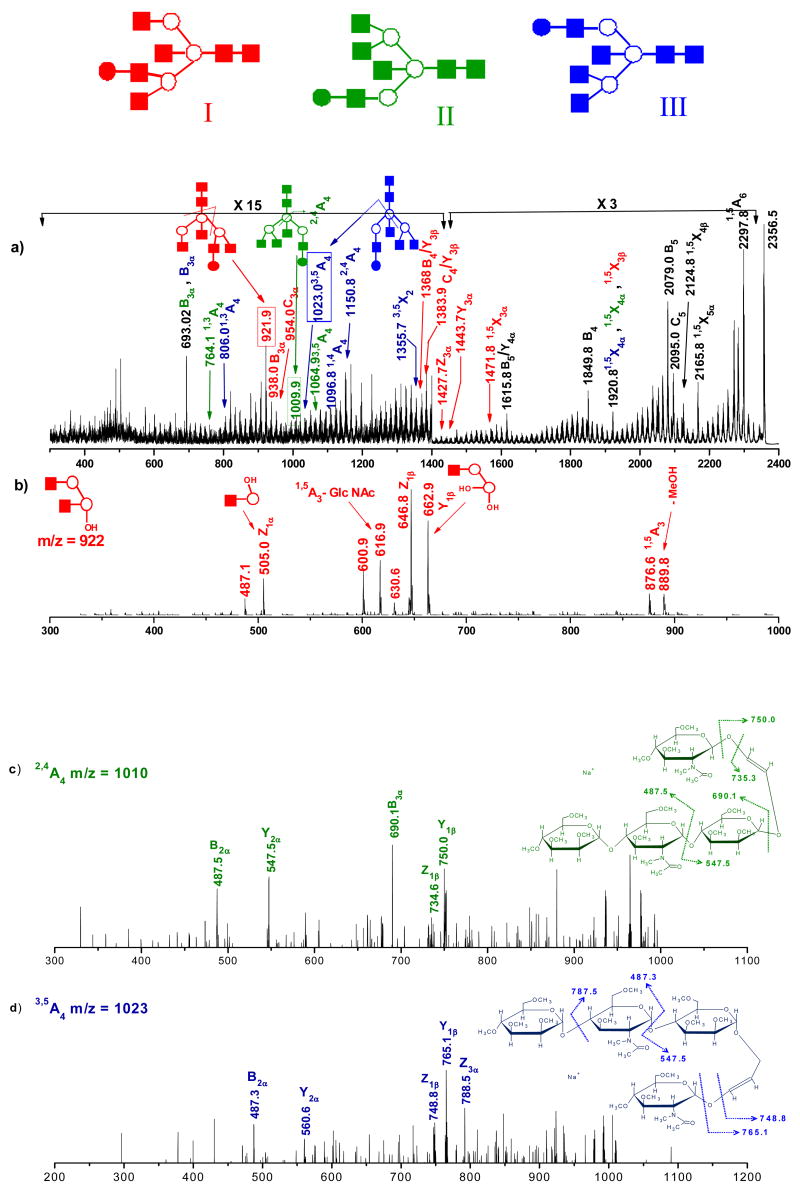
The effectiveness of photofragmentation in the structural characterization of glycans is also illustrated for the hybrid glycan consisting of (GlcNAc)6 (Man)3 (Gal), which generates singly charged ions at m/z 2356. The laser induced MS/MS spectrum of this precursor ion is shown in Figure 6(a). The observed fragmentation pattern is indicative of three possible isomers that are similar to those previously reported [62]. Although photodissociation provides informative MS2 fragment ions, the overall S/N ratio of the spectrum is noticeably lower than with the other examples discussed here, thus limiting our ability to perform high order tandem MS experiments. The lower S/N ratio is associated with the formation of multiply-charged precursor ions by electrospray ionization, which significantly limits the abundance of the singly charged precursors. Nevertheless, it is interesting to note in Figure 6(a) that a variety of characteristic fragment ions provide important information regarding three isomeric structures, including ions at m/z values of 922(C4/Z3α), 938 (B3α), 954 (C3α), 1368 (B4/Y3β), 1384 (C4/ Y3β), 1428 (Z3α), 1444 (Y3α) and 1472 (1,5X3α), reflecting the presence of isomer I (Red). The structure of the II (Green) isomer is supported by the appearance of diagnostic cross-ring fragment ions at m/z values of 764 (1,3A4), 1010 (2,4A4) and 1065 (3,5A4). Also present in Figure 6(a) are the features at m/z values of 806 (1,3A4), 1023 (3,5A4), 1097 (1,4A4), 1151 (2,4A4) and 1356 (3,5X2) that reveal the existence of isomeric structure III (Blue). To further confirm, we isolated certain specific photofragments and collisionally dissociated them inside the trap. The MS3 spectrum of the photofragment observed at m/z 922 (D ion- C4/Z3α) is displayed in Figure 6(b). The most important and intense fragment ions at m/z 663 (Y1β), 647 (Z1β) and 505 (Z1α) in the spectrum provide strong evidence for the isomeric structure I. Similarly, collisional dissociation of photofragment ion m/z 1010 yields the spectrum in Figure 6(c). Despite the low S/N ratio, this spectrum contains valuable product ions at m/z 750 (Y1β), 690 (B3α), 548 (Y2α) and 488 (B3α), pointing to the presence of structure II. The MS3 spectrum depicted in Figure 6(d) was obtained from photofragment ion m/z 1023 (3,5A4). It contains low-abundance product ions at m/z 788 (Z2α), 765 (Y1β), 749 (Z1β) and 487 (B2α), consistent with isomer III.
Figure 6.
(a) MS2 photodissociation spectrum of [M+Na]+ permethylated glycan precursor m/z 2356 (from ovalbumin). MS3 CID spectra of photofragments m/z (b) 921 (c) 1009 (d) 1023 generated from ion m/z 2356. Ions due to multiple cleavage sites are designated with a slash between the sites of cleavage. All fragments contain sodium. Note the changes in vertical scale within the spectrum.
Summary and Conclusions
Several examples of indigenous isomeric ovalbumin glycan structures could be identified from MS2 spectra alone. The ion trap MS3 experiments allow us to isolate diagnostic photofragments including cross-ring fragment ions and confirm the individual isomeric structures present in the mixture without prior chromatographic separation. Furthermore, these experiments confirmed that the product ions observed with photodissociation are similar to those generated during high-energy CID methods. Here, we demonstrated the ability to isolate and collisionally activate ions generated through photofragmentation. A challenging aspect, however, is to understand the distribution of product ions that are separated by 14 and 16 Daltons throughout the photodissociation spectra. These are currently under investigation.
In this work, we demonstrated the analytical merits of 157 nm photofragmentation to characterize the isomeric structures of high mass N-glycans from ovalbumin. Photodissociation yielded numerous product ions including extensive cross-ring fragment ions of analytical value such as those distinguishing between isomeric N-glycan structures. Another major advantage exploited in this study is the ability to perform MSn CID experiments on the characteristic fragments generated by photodissociation. The presence of individual isomers in a glycoform was confirmed in MS3 experiments. However, as mass increases the number of singly-charged ions generated by electrospray ionization drops. This makes high order MSn experiments on high mass ions more difficult. This problem could be addressed if MALDI ionization sources are coupled to the LTQ mass spectrometer. Currently, experiments with multiply-charged ions to investigate the influence of charge state on glycan photodissociation are also being pursued. It will be interesting to perform photodissociation experiments on the ions generated by either photofragmentation or CID.
Supplementary Figures
Supplemtal Figure 1. The calculated percent depletion (by photodissociation) for [M+Na]+ permethylated glycan ions m/z 2153 and 2071 derived from hen ovalbumin for 1, 2 and 3 laser shots.
Supplemtal Figure 2. Positive ion nano-ESI MS1 spectrum of permethylated glycan mixture released from ovalbumin. Glycan ions selected for this study are indicated with a circle over the peak label.
Acknowledgments
This work has been supported by the National Science Foundation grants CHE-0518234 & CHE-0431991 and by a grant from the National Center for Research Resources a component of the National Institute of Health (NIH-NCRR) for the National Center for Glycomics and Glycoproteomics (NCGG) (grant No - RR018942).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Varki A, et al. Essentials of Glycobiology. Cold Spring Harbor Laboratory Press; New York: 1999. [PubMed] [Google Scholar]
- 2.Campbell CT, Yarema KJ. Large-scale approaches for glycobiology. Genome Biology. 2005;6:236, 231–238. doi: 10.1186/gb-2005-6-11-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Helenius A, Aebi M. Intracellular Functions of N-Linked Glycans. Science. 2001;291:2364–2369. doi: 10.1126/science.291.5512.2364. [DOI] [PubMed] [Google Scholar]
- 4.Rudd PM, Elliott T, Cresswell P, Wilson IA, Dwek RA. Glycosylation and the Immune System. Science. 2001;291:2370–2376. doi: 10.1126/science.291.5512.2370. [DOI] [PubMed] [Google Scholar]
- 5.Shriver Z, Raguram S, Sasisekharan R. GLYCOMICS: A PATHWAY TO A CLASS OF NEW AND IMPROVED THERAPEUTICS. Nature Rev Drug Discov. 2004;3:863–873. doi: 10.1038/nrd1521. [DOI] [PubMed] [Google Scholar]
- 6.Bianco GA, Toscano MA, Ilarregui JM, Rabinovich GA. Impact of protein-glycan interactions in the regulation of autoimmunity and chronic inflammation. Autoimmun Rev. 2006;5:349–356. doi: 10.1016/j.autrev.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993;3:97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mechref Y, Novotny MV. Structural Investigations of Glycoconjugates at High Sensitivity. Chem Rev. 2002;102:321–369. doi: 10.1021/cr0103017. [DOI] [PubMed] [Google Scholar]
- 9.Reinhold VN, Reinhold BB, Costello CE. Carbohydrate Molecular Weight Profiling, Sequence, Linkage and Branching Data: ES-MS and CID. Anal Chem. 1995;67:1772–1784. doi: 10.1021/ac00107a005. [DOI] [PubMed] [Google Scholar]
- 10.Dommon B, Muller DR, Richter WJ. High performance tandem mass spectrometry for sequence, branching and interglycosidic linkage analysis of peracetylated oligosaccharides. Biomed Environ Mass Spectrom. 1990;19:390–402. [Google Scholar]
- 11.Dommon B, Muller DR, Richter WJ. Determination of interglycosidic linkages in disaccharides by high performance tandem mass spectrometry. Int J Mass Spectrom Ion Processes. 1990;100:301–311. [Google Scholar]
- 12.Garozzo D, Giuffrida M, Impallomeni G, Ballistreri A, Montaudo G. Determination of linkage position and identification of the reducing end in linear oligosaccharides by negative ion fast atom bombardment mass spectrometry. Anal Chem. 1990;62:279–286. [Google Scholar]
- 13.Spengler B, Dolce JW, Cotter RJ. Infrared laser desorption mass spectrometry of oligosaccharides: fragmentation mechanisms and isomer analysis. Anal Chem. 1990;62:1731–1737. [Google Scholar]
- 14.Carroll JA, Ngoka L, Mccullough S, Gard E, Jones AD, Lebrilla CB. Quadrupole Fourier transform mass spectrometry of oligosaccharides. Anal Chem. 1991;63:2526–2529. [Google Scholar]
- 15.Carroll JA, Ngoka L, Beggs CG, Lebrilla CB. Liquid secondary ion mass spectrometry/Fourier transform mass spectrometry of oligosaccharide anions. Anal Chem. 1993;65:1582–1587. doi: 10.1021/ac00059a017. [DOI] [PubMed] [Google Scholar]
- 16.Harvey DJ, Rudd PM, Bateman RH, Bordoli RS, Howes K, Hoyes JB, Vickers RG. Examination of complex oligosaccharides by matrix-assisted laser desorption/ionization mass spectrometry on time-of-flight and magnetic sector instruments. Org Mass Spectrom. 1994;29:753–765. [Google Scholar]
- 17.Harvey DJ, Naven TJP, Kuster B, Bateman RH, Green MR, Critchley G. Comparison of fragmentation modes for the structural determination of complex oligosaccharides ionized by matrix-assisted laser desorption/ionization mass spectrometry. Rapid Comm Mass Spectrom. 1995;9:1556–1561. doi: 10.1002/rcm.1290091517. [DOI] [PubMed] [Google Scholar]
- 18.Visuex N, deHoffmann E, Dommon B. Structural Analysis of Permethylated Oligosaccharides by Electrospray Tandem Mass Spectrometry. Anal Chem. 1997;69:3193–3198. doi: 10.1021/ac961285u. [DOI] [PubMed] [Google Scholar]
- 19.Weiskopf AS, Vouros P, Harvey DJ. Characterization of oligosaccharide composition and structure by quadrupole ion trap mass spectrometry. Rapid Comm Mass Spectrom. 1997;11:1493–1504. doi: 10.1002/(SICI)1097-0231(199709)11:14<1493::AID-RCM40>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 20.Naven TJP, Harvey DJ, Brown J, Critchley G. Fragmentation of complex carbohydrates following ionization by matrix-assisted laser desorption with an instrument fitted with time-lag focusing. Rapid Comm Mass Spectrom. 1997;11:1681–1686. doi: 10.1002/(SICI)1097-0231(19971015)11:15<1681::AID-RCM34>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 21.Harvey DJ. Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry of Carbohydrates. Mass Spectrom Rev. 1999;18:349–451. doi: 10.1002/(SICI)1098-2787(1999)18:6<349::AID-MAS1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 22.Mechref Y, Baker AG, Novotny MV. Matrix-assisted laser desorption/ionization mass spectrometry of neutral and acidic oligosaccharides with collision-induced dissociation. Carbohydr Res. 1998;313:145–155. doi: 10.1016/s0008-6215(98)00264-x. [DOI] [PubMed] [Google Scholar]
- 23.Weiskopf AS, Vouros P, Harvey DJ. Electrospray Ionization-Ion Trap Mass Spectrometry for Structural Analysis of Complex N-Linked Glycoprotein Oligosaccharides. Anal Chem. 1998;70:4441–4447. doi: 10.1021/ac980289r. [DOI] [PubMed] [Google Scholar]
- 24.Sheeley DM, Reinhold VN. Structural Characterization of Carbohydrate Sequence, Linkage, and Branching in a Quadrupole Ion Trap Mass Spectrometer: Neutral Oligosaccharides and N-Linked Glycans. Anal Chem. 1998;70:3053–3059. doi: 10.1021/ac9713058. [DOI] [PubMed] [Google Scholar]
- 25.Konig S, Leary JA. Evidence for Linkage Position Determination in Cobalt Co-ordinated Pentasaccharides Using Ion Trap Mass Spectrometry. J Am Soc Mass Spectrom. 1998;9:1125–1134. doi: 10.1016/S1044-0305(98)00096-8. [DOI] [PubMed] [Google Scholar]
- 26.Solouki T, Reinhold BB, Costello CE, O’Malley M, Guan SH, Marshall AG. Electrospray Ionization and Matrix-Assisted Laser Desorption/Ionization Fourier Transform Ion Cyclotron Resonance Mass Spectrometry of Permethylated Oligosaccharides. Anal Chem. 1998;70:857–864. doi: 10.1021/ac970562+. [DOI] [PubMed] [Google Scholar]
- 27.Harvey DJ. Electrospray mass spectrometry and fragmentation of N-linked carbohydrates derivatized at the reducing terminus. J Am Soc Mass Spectrom. 2000;11:900–915. doi: 10.1016/S1044-0305(00)00156-2. [DOI] [PubMed] [Google Scholar]
- 28.Harvey DJ. Collision-induced fragmentation of underivatized N-linked carbohydrates ionized by electrospray. J Mass Spectrom. 2000;35:1178–1190. doi: 10.1002/1096-9888(200010)35:10<1178::AID-JMS46>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 29.Harvey DJ, Bateman RH, Bordoli RS, Tyldesley R. Ionisation and fragmentation of complex glycans with a quadrupole time-of-flight mass spectrometer fitted with a matrix-assisted laser desorption/ionisation ion source. Rapid Comm Mass Spectrom. 2000;14:2135–2142. doi: 10.1002/1097-0231(20001130)14:22<2135::AID-RCM143>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 30.Hunnam V, Harvey DJ, Priestman DA, Bateman RH, Bordoli RS, Tyldesley R. Ionization and fragmentation of neutral and acidic glycosphingolipids with a Q-TOF mass spectrometer fitted with a MALDI ion source. J Am Soc Mass Spectrom. 2001;12:1220–1225. doi: 10.1016/S1044-0305(01)00309-9. [DOI] [PubMed] [Google Scholar]
- 31.Spengler B, Kirsch D, Kaufmann R, Lemoine J. Structural analysis of branched oligosaccharides using post-source decay in matrix-assisted laser desorption ionization mass spectrometry. Org Mass Spectrom. 1994;12:782–787. [Google Scholar]
- 32.Sato Y, Suzuki M, Nirasawa T, Suzuki A, Endo T. Microsequencing of Glycans Using 2-Aminobenzamide and MALDI-TOF Mass Spectrometry: Occurrence of Unique Linkage-Dependent Fragmentation. Anal Chem. 2000;72:1207–1216. doi: 10.1021/ac9908750. [DOI] [PubMed] [Google Scholar]
- 33.Rouse JC, Strang A-M, Yu W, Vath JE. Isomeric Differentiation of Asparagine-Linked Oligosaccharides by Matrix-assisted Laser Desorption-Ionization Postsource Decay Time-of-Flight Mass Spectrometry. Anal Biochem. 1998;256:33–46. doi: 10.1006/abio.1997.2450. [DOI] [PubMed] [Google Scholar]
- 34.Mechref Y, Novotny MV, Krishnan C. Structural Characterization of Oligosaccharides Using Maldi-TOF/TOF Tandem Mass Spectrometry. Anal Chem. 2003;75:4895–4903. doi: 10.1021/ac0341968. [DOI] [PubMed] [Google Scholar]
- 35.Mechref Y, Kang P, Novotny MV. Differentiating structural isomers of sialylated glycans by matrix-assisted laser desorption/ionization time-of-flight/time-of-flight tandem mass spectrometry. Rapid Comm Mass Spectrom. 2006;20:1381–1389. doi: 10.1002/rcm.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spina ESL, Romeo D, Impallomeni G, Garozzo D, Waidelich D, Glueckmann M. New fragmentation mechanisms in matrix-assisted laser desorption/ionization time-of-flight/time-of-flight tandem mass spectrometry of carbohydrates. Rapid Comm Mass Spectrom. 2004;18:392–398. doi: 10.1002/rcm.1350. [DOI] [PubMed] [Google Scholar]
- 37.Morelle W, Slomianny M-C, Diemer H, Schaeffer C, van Dorsselaer A, Michalski J-C. Fragmentation Characteristics of Neutral N-Linked Glycans using a MALDI-TOF/TOF Tandem Mass spectometer. Anal Chem. 2004;76:2343–2354. doi: 10.1021/ac030333p. [DOI] [PubMed] [Google Scholar]
- 38.Morelle W, Slomianny M-C, Diemer H, Schaeffer C, van Dorsselaer A, Michalski J-C. Fragmentation characteristics of permethylated oligosaccharides using a matrix-assisted laser desorption/ionization two-stage time-of-flight (TOF/TOF) tandem mass spectrometer. Rapid Comm Mass Spectrom. 2004;18:2637–2649. doi: 10.1002/rcm.1668. [DOI] [PubMed] [Google Scholar]
- 39.Ashline DJ, Lapadula AJ, Liu YH, Lin M, Grace M, Pramanik B, Reinhold VN. Carbohydrate Structural Isomers Analyzed by Sequential Mass Spectrometry. Anal Chem. 2007;79:3830–3842. doi: 10.1021/ac062383a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi SD-H, Hendrickson CL, Marshall AG, Seigel MM, Kong F, Carter GT. Structural validation of saccharomicins by high resolution and high mass accuracy fourier transform-ion cyclotron resonance-mass spectrometry and infrared multiphoton dissociation tandem mass spectrometry. J Am Soc Mass Spectrom. 1999;10:1285–1290. [Google Scholar]
- 41.Xie Y, Lebrilla CB. Infrared Multiphoton Dissociation of Alkali Metal-Coordinated Oligosaccharides. Anal Chem. 2003;75:1590–1598. doi: 10.1021/ac026009w. [DOI] [PubMed] [Google Scholar]
- 42.Zhang J, Schubothe K, Li B, Russell S, Lebrilla CB. Infrared Multiphoton Dissociation of O-Linked Mucin-Type Oligosaccharides. Anal Chem. 2005;77:208–214. doi: 10.1021/ac0489824. [DOI] [PubMed] [Google Scholar]
- 43.Lancaster KS, An HJ, Li B, Lebrilla CB. Interrogation of N-Linked Oligosaccharides Using Infrared Multiphoton Dissociation in FT-ICR Mass Spectrometry. Anal Chem. 2006;78:4990–4997. doi: 10.1021/ac0600656. [DOI] [PubMed] [Google Scholar]
- 44.Budnik BA, Haselmann KF, Elkin Y, Gorbach VI, Zuberev RA. Applications of Electron-Ion Dissociation Reactions for Analysis of Polycationic Chitooligosaccharides in Fourier Transform Mass Spectrometry. Anal Chem. 2003;75:5994–6001. doi: 10.1021/ac034477f. [DOI] [PubMed] [Google Scholar]
- 45.Adamson JT, Hakansson K. Electron Capture Dissociation of Oligosaccharides Ionized with Alkali, Alkaline Earth, and Transition Metals. Anal Chem. 2007;79:2901–2910. doi: 10.1021/ac0621423. [DOI] [PubMed] [Google Scholar]
- 46.Thompson MS, Cui W, Reilly JP. Fragmentation of singly-charged peptides by photodissociation at 157 nm. Angew Chem Int. 2004;43:4791–4794. doi: 10.1002/anie.200460788. [DOI] [PubMed] [Google Scholar]
- 47.Cui W, Thompson MS, Reilly JP. Pathways of Peptide Ion Fragmentation Induced by Vacuum Ultraviolet Light. J Am Soc Mass Spectrom. 2005;16:1384–1398. doi: 10.1016/j.jasms.2005.03.050. [DOI] [PubMed] [Google Scholar]
- 48.Kim TY, Thompson MS, Reilly JP. Peptide photodissociation at 157 nm in a linear ion trap mass spectrometer. Rapid Comm Mass Spectrom. 2005;19:1657–1665. doi: 10.1002/rcm.1969. [DOI] [PubMed] [Google Scholar]
- 49.Devakumar A, Thompson MS, Reilly JP. Fragmentation of oligosaccharide ions with 157 nm vacuum ultraviolet light. Rapid Comm Mass Spectrom. 2005;19:2313–2320. doi: 10.1002/rcm.2058. [DOI] [PubMed] [Google Scholar]
- 50.Devakumar A, Mechref Y, Kang P, Novotny MV, Reilly JP. Laser-induced photofragmentation of neutral and acidic glycans inside an ion-trap mass spectrometer. Rapid Comm Mass Spectrom. 2007;21:1452–1460. doi: 10.1002/rcm.2981. [DOI] [PubMed] [Google Scholar]
- 51.Mechref Y, Novotny MV. Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry of Acidic Glycoconjugates Facilitated by the Use of Spermine as a Co-matrix. J Am Soc Mass Spectrom. 1998;9:1293–1302. doi: 10.1016/S1044-0305(98)00106-8. [DOI] [PubMed] [Google Scholar]
- 52.Kang P, Mechref Y, Klouckova I, Novotny MV. Solid-phase permethylation of glycans for mass spectrometric analysis. Rapid Comm Mass Spectrom. 2005;19:3421–3428. doi: 10.1002/rcm.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Devakumar A, O’Dell DK, Walker JM, Reilly JP. Structural Analysis of Leukotriene C4 isomers using Collisional Activation and 157 nm Photodissociation. J Am Soc Mass Spectrom. 2007;19:14–26. doi: 10.1016/j.jasms.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 54.Payne AH, Glish GL. Thermally Assisted Infrared Multiphoton Photodissociation in a Quadrupole Ion Trap. Anal Chem. 2001;73:3542–3548. doi: 10.1021/ac010245+. [DOI] [PubMed] [Google Scholar]
- 55.Wilson JJ, Brodbelt JS. MS/MS Simplification by 355 nm Ultraviolet Photodissociation of Chromophore-Derivatized Peptides in a Quadrupole Ion Trap. Anal Chem. 2007;79:7883–7892. doi: 10.1021/ac071241t. [DOI] [PubMed] [Google Scholar]
- 56.Dommon B, Costello CE. A Systematic Nomenclature for Carbohydrate Fragmentations in FAB-MS/MS Spectra of Glycoconjugates. Glycoconjugate J. 1988;5:397–409. [Google Scholar]
- 57.Spina ESL, Romeo D, Impallomeni G, Garozzo D, Waidelich D, Glueckmann M. New fragmentation mechanisms in matrix-assisted laser desorption/ionization time-of-flight/time-of-flight tandem mass spectrometry of carbohydrates. Rapid Comm Mass Spectrom. 2004;18:392–398. doi: 10.1002/rcm.1350. [DOI] [PubMed] [Google Scholar]
- 58.Stephens E, Maslen SL, Green LG, Williams DH. Fragmentation Characteristics of Neutral N-linked Glycans Using a MALDI-TOF/TOF Tandem Mass Spectrometer. Anal Chem. 2004;76:2343–2354. doi: 10.1021/ac030333p. [DOI] [PubMed] [Google Scholar]
- 59.Silva MLCD, Stubbs HJ, Tamura T, Rice KG. 1H NMR Characterization of a Hen Ovalbumin Tyrosinamide N-Linked Oligosaccharide Library. Arch Biochem Biophys. 1995;318:465–475. doi: 10.1006/abbi.1995.1255. [DOI] [PubMed] [Google Scholar]
- 60.Kuster B, Naven TJP, Harvey DJ. Rapid Approach for Sequencing Neutral Oligosaccharides by Exoglycosidase Digestion and Matrix-assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry. J Mass Spectrom. 1996;31:1131–1140. doi: 10.1002/(SICI)1096-9888(199610)31:10<1131::AID-JMS401>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 61.North S, Birrell H, Camilleri P. Postive and Negative Ion Matrix-assisted Laser Desorption/Ionization Time-of-flight Mass Spectrometric Analysis of Complex Glycans Released from Hen Ovalbumin and Derivatized with 2-Aminoacridone. Rapid Comm Mass Spectrom. 1998;12:349–356. [Google Scholar]
- 62.Lattova E, Perreault H, Krokhin O. Matrix-Assisted Laser Desorption/Ionization Tandem Mass Spectrometry and Post-Source Decay Fragmentation Study of Phenylhydrazones of N-Linked Oligosaccharides from Ovalbumin. J Am Soc Mass Spectrom. 2004;15:725–735. doi: 10.1016/j.jasms.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 63.Lattova E, Perreault H. Profilling of N-Linked Oligosaccharides using phenylhydrazine derivatization and mass spectrometry. J Chromatogr A. 2003;1016:71–87. doi: 10.1016/s0021-9673(03)01297-4. [DOI] [PubMed] [Google Scholar]
- 64.Harvey DJ. Fragmentation of Negative Ions from Carbohydrates: Part 3. Fragmentation of Hybrid and Complex N-Linked Glycans. J Am Soc Mass Spectrom. 2005;16:647–659. doi: 10.1016/j.jasms.2005.01.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemtal Figure 1. The calculated percent depletion (by photodissociation) for [M+Na]+ permethylated glycan ions m/z 2153 and 2071 derived from hen ovalbumin for 1, 2 and 3 laser shots.
Supplemtal Figure 2. Positive ion nano-ESI MS1 spectrum of permethylated glycan mixture released from ovalbumin. Glycan ions selected for this study are indicated with a circle over the peak label.