Abstract
An efficient method has been developed to synthesize zapotin (5,6,2′,6′-tetramethoxyflavone), a component of the edible fruit Casimiroa edulis, on multi-gram scale. The synthesis utilizes a regioselective C-acylation of a dilithium dianion derived from a substituted o-hydroxyactophenone to afford a β-diketone intermediate that can be cyclized to zapotin in good overall yield, thus avoiding the inefficient Baker-Venkataraman rearrangement pathway. Zapotin was found to induce both cell differentiation and apoptosis with cultured human promyelocytic leukemia cells (HL-60 cells). In addition, the compound inhibits 12-O-tetradecanoylphorbol 13-acetate (TPAc)-induced ornithine decarboxylase (ODC) activity with human bladder carcinoma cells (T24 cells), and TPA-induced nuclear factor-kappa B (NF-κB) activity with human hepatocellular liver carcinoma cells (HepG2 cells). These data suggest that zapotin merits further investigation as a potential cancer chemopreventive agent.
Introduction
Cancer is currently the second most common cause of death in the United States, and it is likely to become the most common in the near future.1 Chemoprevention is the use of either synthetic drugs or natural products to inhibit, reverse, or suppress the development of invasive malignant cancer, either by blocking the DNA damage that initiates carcinogenesis or by arresting or reversing the progression of premalignant cells in which DNA damage has already started. Chemoprevention is one of the most direct ways to reduce cancer-related morbidity and mortality.2 Several food-based chemopreventive agents have shown promise in clinical trials.3 The medicinal value of zapote blanco, a fruit of Casimiroa edulis Llave & Lex (Rutaceae) that is consumed in many parts of the world, was first discovered by Aztecs, and crude plant extracts of the seeds or leaves of Casimiroa edulis were later found to affect blood pressure,4–6 cardiac activity,4–6 aortic muscular tone,7 and to possess anticonvulsant activity.8,9 Recently, zapotin (1), a polymethoxylated flavonoid isolated from zapote blanco seeds, has been found to be a non-toxic inducer of cellular differentiation with cultured HL-60 promyelocytic cells.10 Zapotin (1), therefore, has the potential to inhibit carcinogenesis. The goals of the present investigation were to evaluate the potential of zapotin (1) as a chemopreventive agent in various biological systems, and to devise and execute a practical synthesis of zapotin (1) that would afford gram quantities for more advanced biological testing.
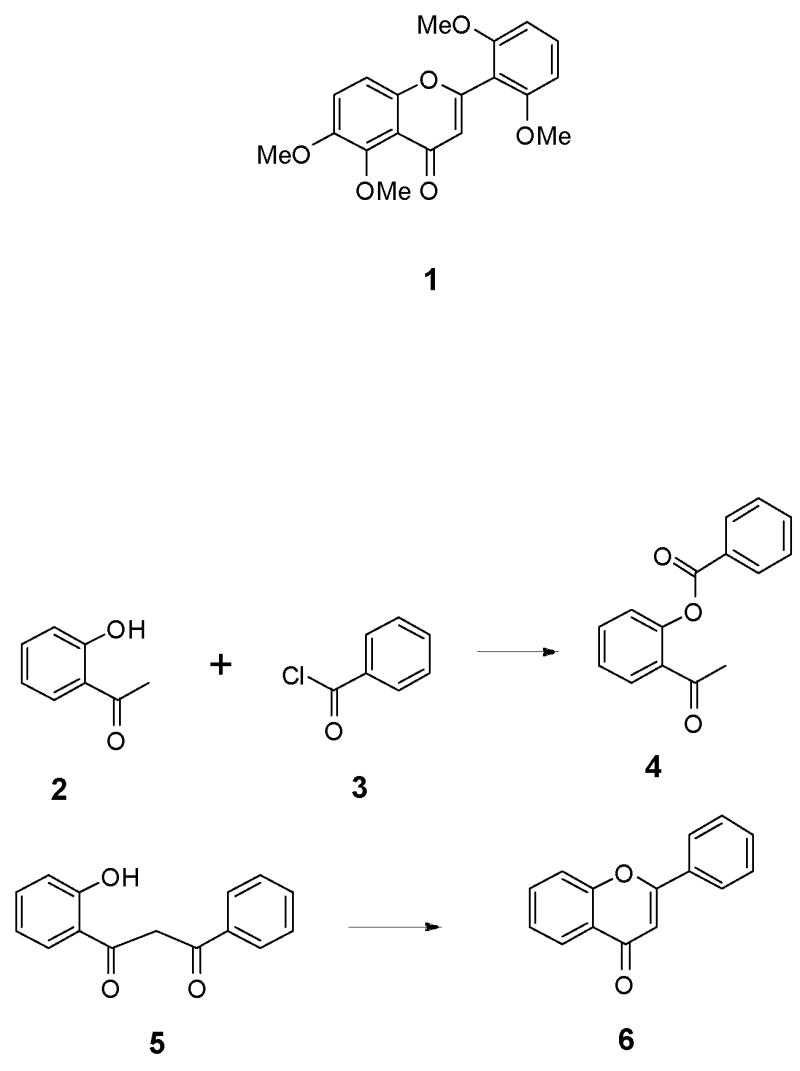
Limited quantities of zapotin (1) are available from natural sources as well as from several poor-yielding syntheses that are based on the Baker-Venkataraman rearrangement pathway.11–14 In general, the Baker-Venkataraman flavone synthesis (Scheme 1) involves the O-acylation of o-hydroxyacetophenone 2 with a benzoyl chloride 3 to afford an ester intermediate 4, which rearranges to a β-diketone 5 under basic conditions. Cyclization of the β-diketone 5 then affords the desired flavone 6. The main disadvantages of this method of flavone synthesis are that it involves an indirect rearrangement pathway to yield the β-diketone intermediate 5, and it often results in the formation of 3-aroylflavones instead of the desired 3-unsubstituted flavones.15 In order to circumvent these difficulties, a direct synthesis of the required β-diketone was investigated in which a dilithium dianion of an appropriately substituted o-hydroxyacetophenone would be directly acylated regioselectively on carbon, and the resulting β-diketone would be cyclized to afford zapotin (1).16–18
Scheme 1.
Chemistry
In practice, the present synthesis of zapotin (1) was executed in a straightforward manner without any unexpected difficulties as outlined in Scheme 2. Commercially available 2-hydroxy-6-methoxyacetophone (7) was subjected to Elbs oxidation19 using sodium persulfate and aqueous sodium hydroxide to yield the substituted acetophenone 8, followed by regioselective methylation using anhydrous potassium carbonate and dimethyl sulfate in acetone to afford 6-hydroxy-2,3-dimethoxyacetophenone (9) in 53% yield in two steps. Intermediate 9 is a known compound that has been synthesized previously,19 but the reported reaction conditions were modified as reported in the Experimental Section. The yields of both steps increase significantly when the mixtures are stirred for a prolonged period at room temperature; however, over-oxidation of the intermediate hydroquinone 8 was detected if the oxidation was carried out for more than one week. The generation of the dilithium dianion 10 of the acetophenone 9 was ensured by treatment with four equivalents of lithium hexamethyldisilylazide in THF. Treatment of dilithium dianion 10 with commercially available 2,6-dimethoxybenzoyl chloride (11), followed by acidification, afforded the β-diketone intermediate 12, which was used without purification for cyclization to zapotin (1). The crude intermediate 12 was heated at 100 °C in the presence of glacial acetic acid containing 0.5% sulfuric acid for 3.5 h to provide zapotin (1) on multi-gram scale and also in high yield (82%). Thus, a short and economical synthesis of zapotin (1) can be executed in 44% overall yield from a commercially available starting material, which compares favorably with the previously reported zapotin (1) syntheses that rely on the Baker-Venkataraman rearrangement.11–14
Scheme 2.
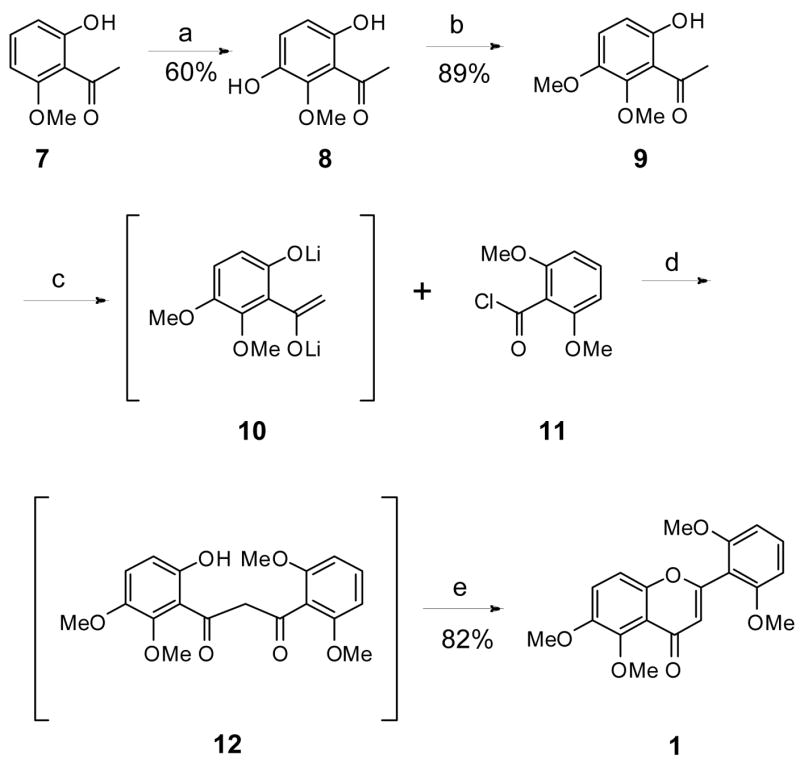
aReagents and conditions: (a) (1) NaOH, K2S2O8, 20°C (7d), (2) aq HCl; (b) K2CO3, Me2SO4, Me2CO (7d); (c) Compound 9, LiHMDS, THF, −78°C to −10°C, (3h), (1.5h); (d) Compound 11, −78°C to 23°C (25h), (2) aq HCl; (e) H2SO4, AcOH, 95°C to 100°C (3.5h)
Biological Results and Discussion
As a part of a comprehensive program for discovery of cancer chemopreventive agents from natural sources, synthetic zapotin (1) was tested in several in vitro systems to demonstrate potential chemopreventive activity.
Inhibition of TPA-Induced ODC Activity in T24 Cells
Ornithine decarboxylase (ODC) is the first and apparently the rate-limiting enzyme for the biosynthesis of polyamines in mammalian cells and is highly inducible by growth-promoting stimuli including growth factors, steroid hormones, cAMP-elevating agents, and tumor promoters.20,21 A number of factors finely tune ODC activity, including the expression, stability, and transcription rate of ODC messenger RNA, the stability and translation rate of the ODC enzyme, and also the post-translational modification of the enzyme.22 Since ODC activity is essential for proliferation of normal cells, and, on the other hand, is overexpressed in various cancer cell lines, it is now recognized that inhibition of ODC may be a good strategy for cancer chemoprevention and chemotherapy.23,24 Zapotin (1) was therefore tested for inhibition of the induction of ODC activity by TPA (12-O-tetradecanoylphorbol-13-acetate) using human bladder carcinoma T24 cells and was found to be active, with an IC50 of 3.4 ± 1.7 μM. This activity is comparable to standard inhibitors of TPA-induced ODC activity, such as apigenin (IC50 6.0 μM), menadione (IC50 8.3 μM), and deguelin (IC50 0.1 μM).
Inhibition of TPA-induced NF-κB Activity in HepG2 Cells
Nuclear factor-κB (NF-κB) is an inducible transcription factor for genes involved in cell survival, cell adhesion, inflammation, differentiation, and growth.25–28 In normal cells, NF-κB present in the cytoplasm binds to the inhibitory IκB proteins, which blocks the nuclear localization sequences of NF-κB.29 Activation of NF-κB by a variety of stimuli such as carcinogens, inflammatory agents, tumor promoters including cigarette smoke, phorbol esters, and okadaic acid, promote degradation of IκBα and thus unmasks the nuclear localization sequences, permitting NF-κB to enter the nucleus and bind to a specific sequence in DNA, which in turn results in transcription of targeted genes. Various genes that are involved in tumor cell invasion and angiogenesis have been found to be regulated by NF-κB. During the past few years, several reports have shown that activation of NF-κB promotes cell survival and proliferation, and down regulation of NF-κB sensitizes the cell to apoptosis. Thus, agents that suppress NF-κB activation can abrogate carcinogenesis. This has encouraged a search for specific inhibitors of NF-κB activation from natural sources, which might lead to a good candidate for cancer chemoprevention. Zapotin (1) was tested and it displayed promising inhibition of TPA-induced NF-κB activity in HepG2 cells stably transfected with NF-κB-luciferase plasmid with an IC50 value of 7.6 ± 3.3 μM.
Induction of Differentiation by Zapotin (1) in HL-60 Cell Line
The HL-60 cell line is used as a model system to investigate the mechanism of cell differentiation, proliferation and cell death by inducers. Differentiation-inducing agents suppress cancer cell self-renewal selectivity from normal stem cell renewal by inducing terminal differentiation followed by apoptosis. Inducers of terminal differentiation have shown promising chemopreventive activity as suppressing agents that act during the promotion-progression stages of carcinogenesis. Previous studies performed in our laboratories demonstrated that zapotin (1) was able to induce differentiation of HL-60 cells in a concentration-dependent fashion without cytotoxicity.10 Zapotin (1) induced 50% of the cells to differentiate at 0.2 μg/mL (ED50 0.5 μM), compared to 10 μM required by apigenin and 30 μM by genistein to exert the same activity, representing a 20–60 fold increase in potency. In the current study, cells were treated with various concentrations of zapotin (1) for 24, 48, 72, or 96 h and harvested after 4 days for evaluation of enzymatic and cell membrane markers of differentiation.
Analysis of NBT (nitroblue tetrazolium)-reduction for evaluation of superoxide formation demonstrated myeloid maturation in HL-60 cells. The irreversibility of zapotin (1) effects on growth and differentiation of HL-60 cells was tested using withdrawal assays during a 4-day experiment. Withdrawal of zapotin (1) after 24 h of exposure resulted in the differentiation of a similar percentage of cells as without withdrawal (Figure 1), while maintaining a higher cellular viability and density. This indicates that there is no further need for the presence of the compound after 24 h, at which time cells have become committed to differentiate. However, cell density was reduced by increasing the time of exposure to the drug.
Figure 1.
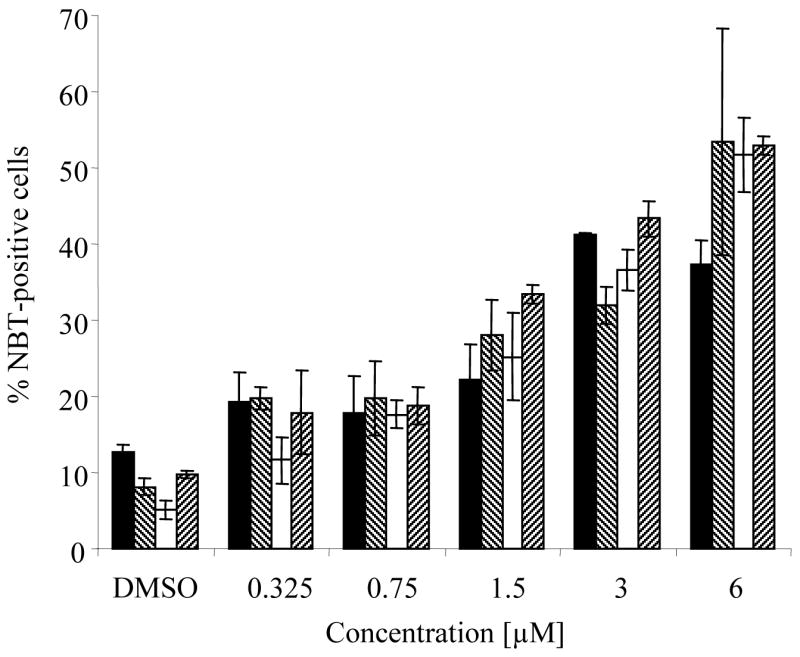
Commitment toward differentiation of HL-60 cells is obtained at 24 h of exposure to zapotin (1). In each case, total incubation time was 4 days (96 h), and then cells were analyzed for differentiation marker. Cells were treated with the specified concentrations of zapotin (1), which was withdrawn after 24 (■), 48 (▧), 72 (□) or 96 (▨) h. For the 24, 48, and 72 h exposures, cells were resuspended in fresh complete media for the remaining time. Results are shown as the mean of duplicate samples.
Membrane phenotype was also analyzed using flow cytometry with a set of four myeloid markers (CD11b, CD13, CD14 and CD15). Zapotin (1) up-regulated CD11b, CD13 and CD14, and down-regulated CD15 in HL-60 cells (Figure 2). Thus, zapotin (1) induced a pattern of expression similar to that produced by macrophage inducers, with down-regulation of CD15 (granulocytic marker) and up-regulation of CD13 and CD11b (granulocytic/monocytic markers).30
Figure 2.
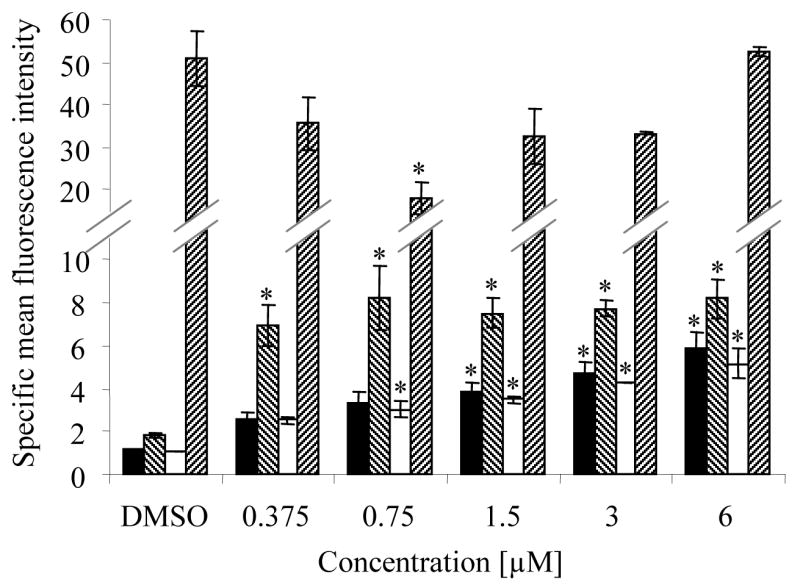
Effect of zapotin (1) on the membrane phenotype of HL-60 cells. As described in the Experimental Section, cells were induced to differentiate with the indicated concentrations of zapotin (1) using a 4-day protocol, and then analyzed for the following membrane markers of differentiation: CD11b (■), CD13 (▧), CD14 (□), and CD15 (▨). Results are expressed as the specific mean fluorescence intensity (ratio of antigen antibody fluorescence over isotype antibody fluorescence), and represent the mean of two independent studies.
* Significantly different from control values (p < 0.05).
Induction of Apoptosis by Zapotin (1) in HL-60 Cell Line
After a 24 h treatment period, zapotin (1) induced dose-dependent increases in apoptosis, as judged by the formation of apoptotic bodies observed with DAPI (4′,6-diamidino-2-phenylindole) staining (Figure 3), which were significant with doses of 3 μM and higher (p < 0.01). A time-dependent increase of apoptosis was also observed when cells were treated with 12 μM zapotin (1) (data not shown).
Figure 3.
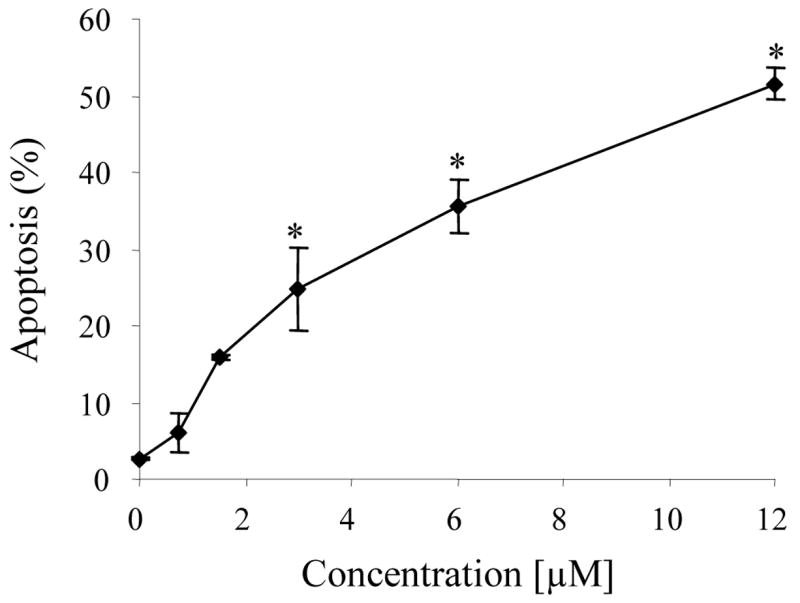
Dose-dependent induction of apoptosis in HL-60 cells treated with zapotin (1). Cells were treated with the indicated concentrations for 24 h. Apoptosis was quantified by counting nuclei stained with 4′,6-diamidino-2-phenylindole (DAPI). Results represent the mean of two independent studies.
* Significantly different from control values (p < 0.01).
Zapotin (1) Causes Accumulation of HL-60 Cells in S Phase
The impact of zapotin (1) on the cell cycle progression of HL-60 cells was determined by flow cytometry. Cell cycle analysis performed after 24-hour incubations of HL-60 cells with increasing concentrations of zapotin (1) showed that this agent arrested the cells in the S phase of the cell cycle in a dose-dependent manner (Figure 4). In the cells treated with 3 μM zapotin (1), 61% of the cells were in S-phase as compared with 36% in the control population. A complete suppression of cells in the G2/M phase of the cycle could be noted with concentrations of zapotin (1) as low as 0.75 μM. Also, a dose-dependent increase of the sub G1 peak, characteristic of apoptosis, was evident.
Figure 4.
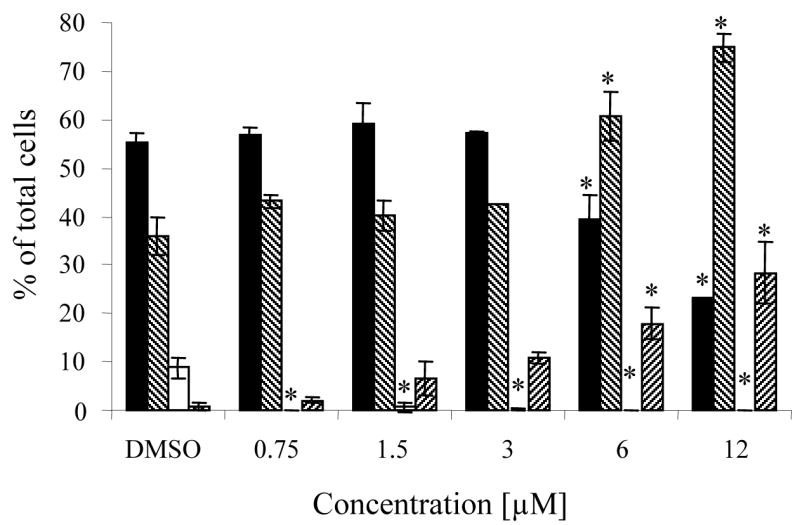
Cell cycle effects of zapotin (1) in HL-60 cells. Cells were treated with the indicated concentrations for 24 h, fixed in ethanol and stained with PI (propidium iodide) for flow cytometric analysis, as described in the Experimental Section. Values are expressed as percentage of total cells and represent the mean ± SD of three determinations, for the following compartments of the cell cycle: G1 (■), S (▧), G2/M (□) and apoptotic peak sub G1 (▨).
* Significantly different from control values (p < 0.01).
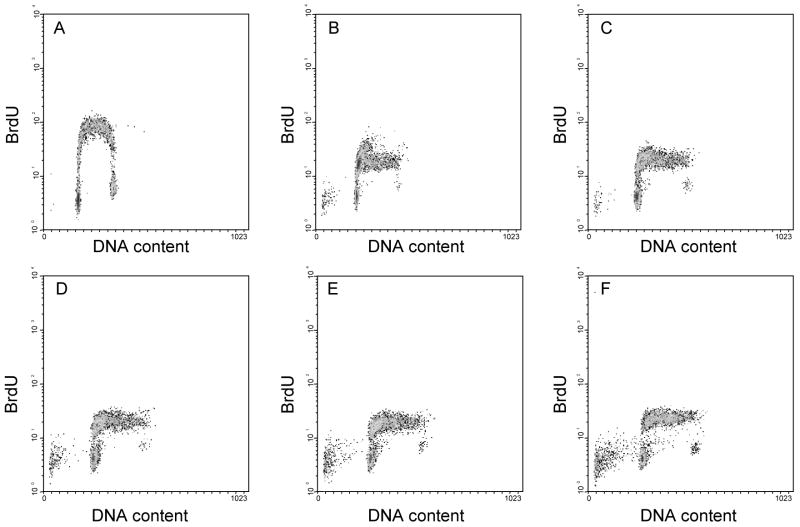
To firmly establish this point, control and zapotin (1)-treated cells were allowed to incorporate BrdU for 30 minutes prior to harvest, and the level of incorporation due to new DNA synthesis was determined by staining with fluorescently labeled antibody to BrdU prior to analysis by flow cytometry. In Figure 5, total DNA content is presented along the x-axis and BrdU staining along the y-axis. After treatment with zapotin (1), cells did continue to enter the early stages of S phase confirming that zapotin (1) induces an S phase arrest.
Figure 5.
Effects of zapotin (1) on BrdU incorporation in HL-60 cells. Cells were treated with DMSO (A), 0.75 μM (B), 1.5 μM (C), 3 μM (D), 6 μM (E), or 12 μM (F) of zapotin (1), and harvested for cell cycle analysis 24 h later. For 30 min prior to harvest, cells synthesizing DNA were allowed to incorporate BrdU. Cells synthesizing DNA during this period were then labeled using fluorescein-modified antibody to BrdU and were analyzed by flow cytometry.
Conclusion
Zapotin (1) was tested for various biological activities and found to be capable of mediating responses of sufficient magnitude or selectivity to demonstrate preliminary chemopreventive activity. To help facilitate more advanced biological investigations, a novel, efficient, multi-gram synthesis of zapotin (1) has been devised, which relies of the regioselective C-acylation of a dilithium dianion generated from an o-hydroxyacetophenone.
Experimental Section
NMR spectra were obtained at 300 MHz (1H) and 75 MHz (13C) in CDCl3 using CHCl3 as internal standard. Flash chromatography was performed with 230–400 mesh silica gel. TLC was carried out using commercially available precoated glass silica gel plates of 2.5 mm thickness. Melting points are uncorrected. Unless otherwise stated, chemicals and solvents were of reagent grade and used as obtained from commercial sources without further purification. Tetrahydrofuran (THF) was freshly distilled from sodium/benzophenone ketyl radical prior to use. Acetone was freshly distilled from potassium carbonate prior to use.
3,6-Dihydroxy-2-methoxyacetophenone (8)
A solution of 2-hydroxy-6-methoxy-acetophenone (5.0 g, 30.1 mmol) in 10% sodium hydroxide (57.5 g in 575 mL H2O) was added dropwise at room temperature to an aqueous solution of potassium persulfate (8.2 g, 30.3 mmol) in water (350 mL) with stirring for 7 d at 20 °C. The mixture was cooled in an ice bath, acidified to pH 5–6 with concd HCl and left overnight at room temperature. The unreacted starting material was removed by extraction with ethyl acetate and the aqueous solution was further acidified to pH 2, and then heated for 4 h on a water bath after addition of solid sodium sulfite (5.8 g, 46.0 mmol). The cooled solution was extracted with chloroform (3 × 100 mL). The organic extract was evaporated under reduced pressure and the residue was purified by chromatography on silica gel (eluent: CHCl3) to give 3,6-dihydroxy-2-methoxyacetophenone (8) as a pale greenish-yellow solid (3.2 g, 60%): mp 95 °C (lit 94–97 °C).19 Rf = 0.25 (SiO2, CHCl3); 1H NMR (300 MHz, CDCl3) δ 12.0 (s, 1 H), 7.12 (d, J = 9.2 Hz, 1 H), 6.67 (d, J = 9.2 Hz, 1 H), 5.08 (s, 1 H), 3.81 (s, 3 H), 2.70 (s, 3 H). All other data are the same as reported.19
6-Hydroxy-2,3-dimethoxyacetophenone (9)
A mixture of 3,6-dihydroxy-2-methoxyacetophenone (1.3 g, 7.1 mmol) and oven-dried potassium carbonate (1.0 g, 7.6 mmol) in dry acetone was stirred for 10 min. Dimethyl sulfate (0.6 mL, 7.1 mmol) was added dropwise to the reaction mixture and the mixture was stirred for 7 d at room temperature. The solvent was evaporated under reduced pressure and water (25 mL) was added to the residue. The mixture was extracted with chloroform (3 × 100 mL) and the organic phase was dried over Na2SO4 and evaporated under reduced pressure. The residue was column chromatographed on silica gel eluting with CHCl3 to afford compound 9 (1.26 g, 89% yield). Rf = 0.75 (SiO2, CHCl3); 1H NMR (300 MHz, CDCl3) δ 7.10 (d, J = 9.3 Hz, 1 H), 6.66 (d, J = 9.3 Hz, 1 H), 3.92 (s, 3 H), 3.81 (s, 3 H), 2.70 (s, 3 H). All other data are the same as reported.19
Zapotin (1)
A solution of LiHMDS in THF (1 M, 20 mL, 20 mmol) was added to a well-stirred solution of acetophenone 9 (1.0 g, 5.1 mmol) in THF (10 mL) under argon at −78 °C over 15 min. The reaction mixture was stirred at −78 °C for 1 h and at −10 °C for 2 h and was cooled again to −78 °C, and a solution of 2,6-dimethoxybenzoyl chloride 11 (1.38 g, 90% tech. grade, 6.2 mmol) in THF (10 mL) was added in one portion. Stirring was continued at −78 °C for 1 h and at room temperature for 24 h (until the disappearance of the starting material by TLC). The reaction mixture was poured into a mixture of ice (50 g) and concd HCl (5.4 mL) and extracted with CHCl3 (3 × 100 mL). The solvent was evaporated from the dried (Na2SO4) extracts and the residue was dried under vacuum for 24 h. A small portion of the crude product was taken out and purified by column chromatography on silica gel (eluent EtOAc-hexanes 3:1) to give compound 12: mp 119–120 °C. Rf = 0.55 (SiO2, EtOAc-hexanes 3:1); IR (neat) 2939, 2838, 1610, 1593, 1574, 1474, 1269, 1254, 1112 cm−1; 1H NMR (300 MHz, CDCl3 ) δ 7.30 (t, J = 8.7 Hz, 1 H), 7.02 (d, J = 8.7 Hz, 1 H), 6.87 (s, 1 H), 6.67 (d, J = 8.7 Hz, 1 H), 6.58 (d, J = 8.4 Hz, 2 H), 3.80 (s, 6 H), 3.78 (s, 6 H); 13C NMR (75 MHz, CDCl3) δ 193.6, 178.2, 158.0, 155.9, 145.5, 131.5, 120.4, 114.1, 112.7, 105.8, 104.0, 61.5, 57.1, 56.0; EIMS (m/z, relative intensity) 360 (M+, 6), 329 (3), 222 (2), 180 (7), 165 (100), 150 (9), 137 (5), 122 (5), 107 (7); HRMS m/z calcd for (C19H20O7) 360.1209, found 360.1213. Anal. (C19H22O7) C, H. The rest of the residue was mixed with glacial acetic acid (20.0 mL) and sulfuric acid (0.1 mL) and heated at 95–100 °C under argon atmosphere for 3.5 h. Solvent was removed under reduced pressure and the residue was poured into water (100 mL). The mixture was extracted with chloroform (3 × 100 mL), dried with Na2SO4 and the residue was chromatographed with silica gel (eluent ethyl acetate-hexane, 3:1) to yield pure zapotin (1, 1.4 g, 82%): mp 146–147 °C (lit31 147–148 °C). Rf = 0.25 (SiO2, EtOAc-hexane 3:1); IR (neat) 2939, 2840, 1650, 1592, 1475, 1417, 1357, 1281, 1255, 1111 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.35 (t, J = 8.7 Hz, 1 H), 7.25 (d, J = 9.3 Hz, 1 H), 7.16 (d, J = 9.3 Hz, 1 H), 6.59 (d, J = 8.4 Hz, 2 H), 6.26 (s, 1 H), 3.94 (s, 3 H), 3.88 (s, 3 H), 3.75 (s, 6 H); 13C NMR (75 MHz, CDCl3) δ 177.9, 158.7, 158.2, 152.2, 149.3, 147.4, 131.8, 119.0, 118.5, 114.9, 113.5, 110.9, 103.6, 61.5, 56.8, 55.7; EIMS (m/z, relative intensity) 342 (M+, 50), 327 (100), 311 (7), 283 (5), 253 (8), 237 (3), 197 (3), 182 (5), 165 (37), 137 (83), 109 (26), 91 (18), 69 (19), 53 (14); HRMS m/z calcd for (C19H18O6) 342.1103, found 342.1107. Anal. (C19H18O6) C, H.
Cell Culture
T24 and HL-60 cells were obtained from the American Type Culture Collection (Rockville, MD). T24 cells were cultured in MEM medium (Invitrogen, Carlsbad, CA) containing 10% heat-inactivated fetal bovine serum, non-essential amino acids, 1 mM sodium pyruvate (BioWhittaker, Walkersville, MD), 100 units of penicillin/mL, 100 μg streptomycin/mL and 250 ng amphotericin B/mL (Gibco Invitrogen, Grand Island, NY). The HL-60 cell line was maintained in suspension culture using RPMI 1640 medium (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum, 100 units of penicillin/mL and 100 μg of streptomycin/mL. HepG2 human hepatoma cells stably transfected with NF-κB-luciferase plasmid32 were maintained in Ham’s F12 supplemented with 10% heat-inactivated fetal bovine serum, 100 units/mL penicillin G sodium, 100 μg/mL streptomycin sulfate, 1% MEM amino acid and 0.1% insulin. The cell lines were maintained in a 5% CO2 atmosphere at 37 °C and were routinely tested for mycoplasma contamination.
Determination of TPA-Induced ODC Activity in T24 Cells
T24 cells were treated with various concentrations of zapotin (1) and determination of ODC activity was performed as described previously.33 In brief, cells were plated at an initial density of 2 × 105 cells per well in 24-well plates. After an 18 h pre-incubation, a solution of zapotin (1) in DMSO was added in duplicate (5 μL, 0.5% final concentration) before the induction of ODC activity with TPA (200 nM final concentration). After an additional 6 h incubation, plates were washed twice with PBS and kept at −85 °C until tested. ODC activity was directly assayed by measuring the release of [14C]CO2 from L-[1-14C]-ornithine HCl in the presence of 190 μM nonradioactive ornithine HCl. The amount of radioactivity captured in NaOH-impregnated filter discs was determined by scintillation counting in 24-well plates using a Wallac 1450 Microbeta liquid scintillation counter. Protein was determined according to the Lowry procedure. Interfering dithiothreitol contained in the reaction mixture was destroyed by adding chloramine T (50 μL, 8 mg/mL) to each well (30 min incubation at RT), followed by NaOH (50 μM, 5.7 M) to solubilize the protein. The protein was measured in 96-well plates using an aliquot of the reaction mixture and bovine serum albumin as a standard. The optical density was measured at 660 nm using a BT2000 Microkinetic Reader. The results were calculated as nmol [14C]CO2/h/mg protein and expressed as a percentage in comparison with a control treated with DMSO and TPA. Dose-response curves were prepared and the results were expressed as IC50 values in micromolar concentrations. IC50 values were generated from the results of four serial dilutions of zapotin (1) tested in duplicate.
Determination of TPA-Induced NF-κB Activity in HepG2 Cells
HepG2 cells stably transfected with NF-κB-luciferase plasmid were treated with various concentrations of zapotin (1) and determination of luciferase activity was performed as described previously.34 In brief, transfected cells were incubated for 48 h in 96-well plates. After 6 h incubation with TPA (100 nM) and zapotin (1), cells were analyzed for luciferase activity. Cells were washed with PBS, lysed using 50 μL 1X Reporter Lysis Buffer (Promega, Madison, WI) for 10 min, and the luciferase determination was performed according to the manufacturer’s protocol. Data were expressed as the concentration required to inhibit activation by 50% (IC50 value). Tumor necrosis factor (TNF)-α was used as a standard inhibitor (IC50 15–25 ng/mL).33 IC50 values were generated from the results of four serial dilutions of zapotin (1) tested in duplicate. With the experimental conditions used, no signs of overt cellular toxicity were observed.
Cell Differentiation Assays
HL-60 cells were tested using a 4-day protocol.35 In brief, cells in log phase (approximately 106 cells/mL) were diluted to 105 cells/mL and preincubated overnight (18 h) in 24-well plates to allow cell growth recovery. Then, samples dissolved in DMSO were added, keeping the final DMSO concentration at 0.1% (v/v). Control cultures were treated with the same concentration of DMSO. After four days of incubation, cells were analyzed to determine the percentage exhibiting functional nitroblue tetrazolium (NBT) reduction, and cell surface markers of differentiated cells, as described below.
Nitroblue Tetrazolium (NBT) Reduction
Evaluation of NBT reduction was used to assess the ability of sample-treated cells to produce superoxide when challenged with TPA. A 1:1 (v/v) mixture of a cell suspension (106 cells) and TPA/NBT solution [2 mg/mL NBT and 1 μg/mL TPA in phosphate buffer saline (PBS)] was incubated for 1 h at 37 °C. Then, cells were smeared on glass slides, and counterstained with 0.3% (w/v) safranin O in methanol. Positive cells reduce NBT yielding intracellular black-blue formazan deposits and were quantified by microscopic examination of >200 cells. Results were expressed as a percentage of positive cells.
Determination of Cell Surface Antigens
Cells (106), prewashed with PBS, were resuspended in 100 μL diluent (PBS with 0.1% sodium azide and 1% BSA) and incubated for 30 min at room temperature with the monoclonal antibodies anti-CD-11b (Sigma, St. Louis, MO), anti-CD13 (Caltag, Burlingame, CA), anti-CD14 (Sigma), and anti-CD15 (Caltag), conjugated with FITC. Cells were washed with 20 volumes of diluent, and resuspended in 0.5 mL of 2% paraformaldehyde for flow cytometry evaluation. Identical samples were prepared using isotype antibodies to correct fluorescence due to non-specific binding.
Quantification of Apoptosis
Cells were treated with various concentrations of zapotin (1) for 24 h, or with 12 μM zapotin (1) for various time intervals, washed with PBS, and fixed with methanol-acetic acid 1:1 for 30 min at room temperature. Cells were then treated with 4′,6-diamidino-2-phenylindole (DAPI, 1 μg/mL) for 15 min at room temperature. DAPI staining of the nucleus was observed by fluorescence microscopy. At least 100 cells were counted for each sample. Dose-response curves showing the percentage of apoptosis at different doses and times were constructed.
Cell Cycle Analysis
Cells (3 × 106) were treated with various concentrations of zapotin (1) for 24 h, and washed with PBS. Cells were resuspended in 1 mL PBS + 9 mL ice-cold 70% EtOH and stored at −20 °C. Just before analysis, samples were centrifuged and cell pellets were resuspended in 2 mL of propidium iodide solution (2 μg/mL propidium iodide, 100 μg/mL ribonuclease A in PBS). Solutions were incubated at 37 °C for 1 h, placed on ice and analyzed by flow cytometry. At least 10,000 cells were counted for each sample. The percentage of apoptotic cells was calculated by measuring the area under the subdiploid (DNA <2 N) peak in the plot of cell number against cellular DNA content.
Alternatively, cells were exposed to 5-bromo-2-deoxyuridine (BrdU) for 30 min prior to trypsinization to specifically label S-phase cells. After fixation, cells were stained with fluorescein-conjugated antibody to BrdU and counter stained with propidium iodide following the manufacturer’s protocol (Phoenix Flow Systems, San Diego, CA). Cell suspensions were analyzed by flow cytometry, and data were collected using appropriate electronic gating to remove background debris and aggregates.
Statistical analysis
Data were expressed as means ± SD and analyzed through one-way analysis of variance (ANOVA), followed by pairwise comparisons made with Dunnett’s test, using the SAS statistical package (SAS Institute, Cary, NC). All of the tests were two-sided, and, unless otherwise specified, a p value of less than 0.01 was considered to be significant.
Acknowledgments
This work was supported by program project P01 CA48112 awarded by the National Cancer Institute.
Footnotes
Abbreviations: TPA, 12-O-tetradecanoylphorbol 13-acetate; ODC, Ornithine decarboxylase; T24 cells, Human bladder carcinoma cells; NF-κB, Nuclear factor-kappa B; HepG2 cells, Human hepatocellular liver carcinoma cells, HL-60 cells, Promyelocytic leukemia cells; DAPI, 4′,6-Diamidino-2-phenylindole; LiHMDS, Lithium hexamethyldisilazide; BrdU, 5-bromo-2-deoxyuridine.
Supporting Information Available. 1H NMR, 13C NMR and elemental analyses for compound 12 and zapotin (1). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Varmus H. The New Era in Cancer Research. Science. 2006;312:1162–1165. doi: 10.1126/science.1126758. [DOI] [PubMed] [Google Scholar]
- 2.Hong WK, Sporn MB. Recent Advances in Chemoprevention of Cancer. Science. 1997;278:1073–1077. doi: 10.1126/science.278.5340.1073. [DOI] [PubMed] [Google Scholar]
- 3.Decensi A, Serrano D, Bonanni B, Cazzaniga M, Guerrieri-Gonzales A. Breast Cancer Prevention Trials Using Retinoids. J Mammary Gland Biol Neoplasia. 2003;8:19–30. doi: 10.1023/a:1025779120649. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Gonzalez M, Freer BE, Morales MO. Effects of Casimiroa edulis (Rutacea) on Blood Pressure and Heart Rate in Albino Rats. Rev Biol Trop. 1994;42:115–119. [PubMed] [Google Scholar]
- 5.Vidrio H, Magos GA. Pharmacology of Casimiroa edulis; II. Cardiovascular Effects in the Anesthesized Dog. Planta Med. 1991;57:217–220. doi: 10.1055/s-2006-960077. [DOI] [PubMed] [Google Scholar]
- 6.Magos GA, Vidrio H. Pharmacology of Casimiroa edulis; Part I. Blood Pressure and Heart Rate Effects in the Anesthetized Rat. Planta Med. 1991;57:20–24. doi: 10.1055/s-2006-960008. [DOI] [PubMed] [Google Scholar]
- 7.Magos GA, Vidrio H, Enriquez R. Pharmacology of Casimiroa edulis; III. Relaxant and Contractile Effects in Rat Aortic Rings. J Ethnopharmacol. 1995;47:1–8. doi: 10.1016/0378-8741(95)01247-b. [DOI] [PubMed] [Google Scholar]
- 8.Garzon-De la Mora P, Garcia-Lopez PM, Garcia-Estrada J, Navarro-Ruiz A, Villanueva-Michel T, Villarreal-de Puga LM, Casillass-Ochoa J. Casimiroa edulis Seed Extracts Show Anticonvulsive Properties in Rats. J Ethnopharmacol. 1999;68:275–282. doi: 10.1016/s0378-8741(99)00125-7. [DOI] [PubMed] [Google Scholar]
- 9.Navarro RA, Bastidas RBE, Garcia EJ, Garcia LP, Garzon P. Anticonvulsant Activity of Casimiroa edulis in Comparison to Phenytoin and Phenobarbital. J Ethnopharmacol. 1995;45:199–206. doi: 10.1016/0378-8741(94)01216-m. [DOI] [PubMed] [Google Scholar]
- 10.Mata-Greenwood E, Ito A, Westenburg H, Cui B, Mehta RG, Kinghorn AD, Pezzuto JM. Discovery of Novel Inducers of Cellular Differentiation Using HL-60 Promyelocytic Cells. Anticancer Res. 2001;21:1763–1770. [PubMed] [Google Scholar]
- 11.Farkas L, Gottsegen A, Nogradi M. Structure of Zapotin and Zapotinin. II. Synthesis of Zapotin. Magyar Kemiai Folyoirat. 1969;75:377–378. [Google Scholar]
- 12.Datta SC, Murti VVS, Seshadri TR. New Synthesis of Zapotin, Zapotinin, and Related Flavones. Indian J Chem. 1969;7:746–750. [Google Scholar]
- 13.Phadke PS, Rao AV, Rama VS, Venkataraman K. Synthetic Experiments in the Chromone Group. XXXVII. A Synthesis of Zapotin (5,6,2′,6-Tetramethoxyflavone) and Zapotinin (5-Hydroxy-6,2′,6-trimethoxyflavone) Indian J Chem. 1968;6:177–179. [Google Scholar]
- 14.Farkas L, Gottsegen A, Nogradi M. On the Structure of Zapotin and Zapotinin. II. The Synthesis of Zapotin. Tetrahedron Lett. 1968:3993–3996. [Google Scholar]
- 15.Looker JH, Hanneman WW. Physical and Chemical Properties of Hydroxyflavones. 11. 3-Aroyl-5-hydroxyflavones. Synthetic and Infrared Spectral Studies. J Org Chem. 1962;27:3261–3263. [Google Scholar]
- 16.Cushman M, Nagarathnam D. A Method for the Facile Synthesis of Ring-A Hydroxylated Flavones. Tetrahedron Lett. 1990;31:6497–6500. [Google Scholar]
- 17.Nagarathnam D, Cushman M. A Short and Facile Synthetic Route of Hydroxylated Flavones. New Syntheses of Apigenin, Tricin, and Luteolin. J Org Chem. 1991;56:4884–4887. [Google Scholar]
- 18.Cushman M, Zhu H, Geahlen RL, Kraker A. Synthesis and Biochemical Evaluation of a Series of Aminoflavones as Potential Inhibitors of Protein-Tyrosine Kinases p56lck, EGFr, and p60v-src. J Med Chem. 1994;37:3353–3362. doi: 10.1021/jm00046a020. [DOI] [PubMed] [Google Scholar]
- 19.Wollenweber E, Iinuma M, Tanaka T, Mizuno M. 5-Hydroxy-6,2′-dimethoxyflavone from Primula denticulata. Phytochemistry. 1990;29:633–637. [Google Scholar]
- 20.Pena A, Reddy CD, Wu SJ, Hickok NJ, Reddy EP, Yumet G, Soprano DR, Soprano KJ. Regulation of Human Ornithine Decarboxylase Expression by the c-Myc Center Max Protein Complex. J Biol Chem. 1993;268:27277–27285. [PubMed] [Google Scholar]
- 21.McCann PP, Pegg AE. Ornithine Decarboxylase as an Enzyme Target for Therapy. Pharmacol Ther. 1992;54:195–215. doi: 10.1016/0163-7258(92)90032-u. [DOI] [PubMed] [Google Scholar]
- 22.Wetters TV, Brabant M, Coffino P. Regulation of Mouse Ornithine Decarboxylase Activity by Cell-Growth, Serum and Tetradecanoyl Phorbol Acetate Is Governed Primarily by Sequences within the Coding Region of the Gene. Nucleic Acids Res. 1989;17:9843–9860. doi: 10.1093/nar/17.23.9843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelloff GJ, Boone CW, Crowell JA, Steele VE, Lubet R, Sigman CC. Chemopreventive Drug Development - Perspectives and Progress. Cancer Epidemiol Biomarkers Prev. 1994;3:85–98. [PubMed] [Google Scholar]
- 24.Szarka CE, Grana G, Engstrom P. Chemoprevention of Cancer. Curr Probl Cancer. 1994;18:1–78. doi: 10.1016/0147-0272(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 25.Bharti AC, Aggarwal BB. Nuclear Factor-Kappa B and Cancer: Its Role in Prevention and Therapy. Biochem Pharmacol. 2002;64:883–888. doi: 10.1016/s0006-2952(02)01154-1. [DOI] [PubMed] [Google Scholar]
- 26.Shishodia S, Aggarwal BB. Nuclear Factor-Kappa B Activation: A Question of Life or Death. J Biochem Mol Biol. 2002;35:28–40. doi: 10.5483/bmbrep.2002.35.1.028. [DOI] [PubMed] [Google Scholar]
- 27.Chen F, Castranova V, Shi XL. New Insights into the Role of Nuclear Factor-Kappa B in Cell Growth Regulation. Am J Pathol. 2001;159:387–397. doi: 10.1016/s0002-9440(10)61708-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baeuerle PA, Baltimore D. NF-Kappa B: Ten Years After. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 29.Dorai T, Aggarwal BB. Role of Chemopreventive Agents in Cancer Therapy. Cancer Lett. 2004;215:129–140. doi: 10.1016/j.canlet.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 30.Trayner ID, Bustorff T, Etches AE, Mufti GJ, Foss Y, Farzaneh F. Changes, in Antigen Expression on Differentiating HL-60 Cells treated with Dimethylsulfoxide, all-trans Retinoic Acid, 1α,25-Dihydroxyvitamin D3 or 12-O-Tetradecanoyl phorbol 13-acetate. Leuk Res. 1998;22:537–547. doi: 10.1016/s0145-2126(98)00041-1. [DOI] [PubMed] [Google Scholar]
- 31.Dreyer DL. The Structure of Zapotin. Tetrahedron. 1967;23:4607–4612. [Google Scholar]
- 32.Pezzuto JM, Kosmeder J, Park EJ, Lee SK, Cuendet M, Gills JJ, Bhat K, Grubjesic S, Park HS, Mata-Greenwood E, Tan Y, Yu R, Lantvit DD, Kinghorn AD. Characterization of Natural Product Chemopreventive Agents. Vol. 2. Humana Press, Inc.; Totowa, NJ: 2005. pp. 3–37. [Google Scholar]
- 33.Gerhauser C, Mar W, Lee SK, Suh N, Luo Y, Kosmeder J, Luyengi L, Fong HHS, Kinghorn AD, Moriarty RM, Mehta RG, Constantinou A, Moon RC, Pezzuto JM. Rotenoids Mediate Potent Cancer Chemopreventive Activity through Transcriptional Regulation of Ornithine Decarboxylase. Nature Med. 1995;1:598–598. doi: 10.1038/nm0395-260. [DOI] [PubMed] [Google Scholar]
- 34.Homhual S, Zhang HJ, Bunyapraphatsara N, Kondratyuk TP, Santarsiero BD, Mesecar AD, Herunsalee A, Chaukul W, Pezzuto JM, Fong HHS. Bruguiesulfurol, a New Sulfur Compound from Bruguiera gymnorrhiza. Planta Med. 2006;72:255–260. doi: 10.1055/s-2005-873171. [DOI] [PubMed] [Google Scholar]
- 35.Suh N, Luyengi L, Fong HHS, Kinghorn AD, Pezzuto JM. Discovery of Natural Product Chemopreventive Agents Utilizing HL-60 Cell Differentiation as a Model. Anticancer Res. 1995;15:233–240. [PubMed] [Google Scholar]