Abstract
Casein kinase 1 protein kinases are ubiquitous and abundant Ser/Thr-specific protein kinases with activity on acidic substrates. In yeast, the products of the redundant YCK1 and YCK2 genes are together essential for cell viability. Mutants deficient for these proteins display defects in cellular morphogenesis, cytokinesis, and endocytosis. Yck1p and Yck2p are peripheral plasma membrane proteins, and we report here that the localization of Yck2p within the membrane is dynamic through the cell cycle. Using a functional green fluorescent protein (GFP) fusion, we have observed that Yck2p is concentrated at sites of polarized growth during bud morphogenesis. At cytokinesis, GFP–Yck2p becomes associated with a ring at the bud neck and then appears as a patch of fluorescence, apparently coincident with the dividing membranes. The bud neck association of Yck2p at cytokinesis does not require an intact septin ring, and septin assembly is altered in a Yck-deficient mutant. The sites of GFP–Yck2p concentration and the defects observed for Yck-deficient cells together suggest that Yck plays distinct roles in morphogenesis and cytokinesis that are effected by differential localization.
INTRODUCTION
The casein kinase 1 (CK1) family of structurally conserved Ser/Thr-specific protein kinases is abundant in all eukaryotic cell types (Tuazon and Traugh, 1991). CK1 activities have been characterized for decades, but the cloning of CK1 genes from various organisms led to the discovery that these kinases can be grouped into multiple subfamilies (Rowles et al., 1991; Robinson et al., 1992; Wang et al., 1992; Graves et al., 1993; Klimczak and Cashmore, 1993; Hoekstra et al., 1994; Zhai et al., 1995). All CK1 enzymes act as monomers and recognize acidic substrate sites; however, members of each subfamily differ with regard to substrate selectivity, inhibition by ATP analogs, and subcellular location. Thus, each is likely to recognize unique substrates.
CK1 protein kinases are active in the absence of second messengers and do not appear to associate with regulatory subunits (Tuazon and Traugh, 1991). The in vivo modes of regulation of CK1 protein kinases are unclear, but several mechanisms have been proposed to control their activity. One isoform that may act on secretory vesicle proteins is inhibited by an increased molar ratio of phosphatidylinositol 4,5-bisphosphate phospholipid in the membrane (Brockman and Anderson, 1991; Gross et al., 1995). One isoform in liver cells was found to recognize a substrate site on glycogen synthase that is created by phosphorylation of an upstream serine by the cAMP-dependent protein kinase (Flotow and Roach, 1989). This result, and observations that affinity of this isoform for a peptide containing phosphoserine is significantly higher than its affinity for a peptide containing the classical -Asp/Glu-Asp/Glu-X-Ser- CK1 site, suggested that CK1 could be regulated by signal-dependent phosphorylation (Flotow et al., 1990; Roach, 1990; Flotow and Roach, 1991; Meggio et al., 1991). Finally, immunofluorescence studies with a mammalian CK1α isoform revealed that localization of this protein changes during the cell cycle, suggesting that localization could regulate activity toward specific substrates (Brockman et al., 1992).
The essential CK1 proteins encoded by the duplicate YCK1 and YCK2 genes are two of four Saccharomyces cerevisiae CK1 proteins (Hoekstra et al., 1991; Robinson et al., 1992; Wang et al., 1992, 1996). The Yck1 and Yck2 proteins are peripherally but tightly associated with the plasma membrane (Vancura et al., 1993, 1994) and carry a carboxy-terminal (C-terminal) -Cys-Cys signal for geranylgeranylation (Robinson et al., 1992; Wang et al., 1992). This modification on Yck1p and Yck2p is likely required for plasma membrane localization, because deletion or mutation of the signal abolishes plasma membrane cofractionation and significantly impairs function (Robinson et al., 1993; Vancura et al., 1994). How the activities of Yck1p and Yck2p are regulated is not yet clear, although both lipid association and the C-terminal domain have been proposed to modulate catalytic function (Vancura et al., 1993; Nickels, Robinson, and Broach, unpublished results).
Phenotypic analysis of yck mutants demonstrated that Yck-mediated phosphorylation is required for processes including morphogenesis and cytokinesis. Cells lacking all Yck activity arrest after several aberrant rounds of cell division with multiple elongated buds containing multiple nuclei (Robinson et al., 1992, 1993). The same phenotype is observed for a temperature-sensitive yck mutant (yck1-Δ1::ura3 yck2-2ts; hereafter referred to as yckts) after a shift to its restrictive temperature. The Yck− terminal phenotype is reminiscent of that exhibited by mutants lacking any of the four septin proteins. These proteins are presumed structural components of a 10-nm filament ring (Byers and Goetsch, 1976a,b) that assembles at the bud site about the time of bud emergence and remains at the mother–bud neck through most of the cell cycle. The four septins are encoded by the CDC3, CDC10, CDC11, and CDC12 genes (Haarer and Pringle, 1987; Ford and Pringle, 1991; Kim et al., 1991). These proteins are conserved through evolution, and in all cases examined are involved in cytokinesis, although their mechanism of action is unclear (Cooper and Kiehart, 1996; Longtine et al., 1996). Loss of septin function results in failure to assemble the 10-nm filament ring structure and in the associated terminal phenotype. The similarity of the terminal phenotype attributable to loss of Yck kinase activity to that resulting from loss of septin function suggests a possible relationship between Yck activity and septin ring structure or function.
Most proteins required for polarized growth, cytokinesis, and bud site selection require the septin proteins for localization to the mother–bud neck (Chant et al., 1995; Halme et al., 1996; Roemer et al., 1996; Sanders and Herskowitz, 1996; DeMarini et al., 1997; Lippincott and Li, 1998). One proposed function for the septin ring is to provide a structure on which assembly of other proteins can take place at appropriate times to promote bud site selection and to direct cytokinesis (Longtine et al., 1996). Overexpressed epitope-tagged Yck protein was detected in a ring at the neck of some budded cells (Vancura et al., 1994), supporting a relationship of Yck protein localization or function with septin proteins.
In this study, we have defined the localization of Yck protein and examined its relationship with the actin cytoskeleton and septin proteins, using a functional fusion of Yck2p to a bright variant of the Aequorea victoria GFP (Chalfie et al., 1994). This fusion allowed us to monitor Yck2 protein distribution in living cells. Our results, together with the defects associated with loss of Yck function, indicate that Yck activity is required at distinct times and sites within the membrane to regulate polarization of growth, cytokinesis/septation, and selection of bud sites. This regulation may be mediated in part through effects on septin organization, which is altered in yckts mutant cells.
MATERIALS AND METHODS
DNA Manipulation
The bacterial strain DH5α was used for all recombinant DNA manipulation except for recovery of GFP PCR (Saiki et al., 1988) products in the pRSETB vector (Clontech, Palo Alto, CA). For this purpose, the strain BL21(DE3) (Clontech) was used to allow visual assessment of GFP expression. Standard methods were used for DNA manipulation (Maniatis et al., 1982), except for the use of kits, which were used according to manufacturers’ instructions. Plasmid DNA was purified either by alkali lysis or a rapid boiling protocol depending on the Escherichia coli strain used (Taylor et al., 1993). For PCR and DNA sequence analysis, plasmid DNA was further purified by treatment with RNase and precipitation from PEG8000.
PCR amplification was carried out using the Stratagene (La Jolla, CA) Optiprime buffer system and Taq polymerase as recommended by the manufacturer, in a Perkin Elmer-Cetus (Norwalk, CT) 9600 thermocycler. Synthetic oligonucleotide primers were obtained from Oligos Etc. (Redding Center, CT) and Integrated DNA Technologies (Coralville, IA). DNA sequence analysis of cloned PCR products was performed by the dideoxy chain termination method (Sanger et al., 1977) using Sequenase reagents (US Biochemical, Cleveland, OH) and synthetic oligonucleotide primers.
Construction of GFP Fusions
The bright S65T GFP variant (Heim et al., 1995) in the T7 expression vector pRSETB was obtained from R. Tsien (Howard Hughes Medical Institute, University of California, San Diego, CA). The GFP gene is inserted into the vector such that an EcoRI site lies upstream of the GFP coding sequence. Synthetic oligonucleotide primers were designed to amplify by PCR a fragment containing a second EcoRI site at the 3′ end of the GFP coding sequence: GFP–p1: 5′-CGACGATGACGATAAGG-3′; GFP–P2: 5′-ATTGAATTCTTTGTATAGTTCATC-3′. This EcoRI site separates the final Lys codon from the stop codon, allowing amino (N)-terminal fusion of the GFP gene. The linear PCR product corresponding to the GFP coding sequence with EcoRI ends was cloned into pRSETB. Expression of GFP was monitored in BL21(DE3) by examining colonies for green fluorescence with a hand-held UV lamp. One colony showed significantly brighter fluorescence than the rest, and sequence analysis of the clone (pTII6) expressed in this colony showed that another mutation was present, causing substitution of Leu for Phe at position 64. The additional F64L mutation results in at least eightfold brighter fluorescence than the parent protein with excitation and emission spectra similar to those of the parent (our unpublished results; described by Cormack et al., 1996). The new variant was used for fusion with YCK2.
For fusion of GFP to the N-terminus of Yck2p, an EcoRI site was introduced following the YCK2 ATG. Full-length YCK2, carried on a 2.6-kb XbaI–SacI fragment, was excised from pLS2.3 (Robinson et al., 1992) and cloned into pUC19 deleted for the EcoRI site (pUC19ΔE). This vector was prepared by treatment of EcoRI-linearized pUC19 with S1 nuclease (Boehringer Mannheim, Indianapolis, IN) followed by religation of the blunt-ended product and determination of the sequence of the multiple cloning site to confirm loss of only the EcoRI site. The pUC19ΔE:YCK2 construct, pL2.99, was used as the PCR template with primers containing an EcoRI site following the YCK2 ATG, to amplify the entire pL2.99 sequence: YCK2epi7: 5′-CTTGAATTCTCTCAAGTGCAAAGTC-3′; YCK2epi8: 5′-CTTGAATTCCATTTTTGGAAAACTATTTTC-3′. The resulting product was digested with EcoRI and religated. After sequence analysis to confirm addition of the site and the correct sequence of the YCK2 gene, the GFP EcoRI fragment was ligated into this YCK2 plasmid. Products of ligation were examined for insertion of the GFP gene in the appropriate orientation. Plasmid pL2.991 was one such product. The fusion gene from pL2.991, carried on a 3.3-kb XbaI–SacI fragment, was cloned into the low-copy vector pRS315 (CEN/ARS) (Sikorski and Hieter, 1989) for expression in yeast, creating pL2.992. To assess the ability of fusion proteins to substitute for Yck, plasmids were introduced into strain LRB756, and the resulting transformants were crossed to the yck2::HIS3 strain LRB344. Diploid cells were sporulated, and tetrad analysis was performed. The presence of yck1-Δ1::ura3 yck2::HIS3 pGFP–YCK2 spore clones was inferred from segregation of temperature-sensitive growth attributable to yck1-Δ1::ura3 yck2-2ts and was confirmed by lack of mitotic segregation of the GFP:YCK2 plasmid.
To generate the Cys545,546Ser mutant lacking the C-terminal geranylgeranylation signal sequence, plasmid pL2.99 was mutagenized using the QuikChange oligonucleotide-directed mutagenesis system (Stratagene) with primers designed to introduce one point mutation into each of the two 3′ Cys codons, resulting in substitution of Ser codons at these positions. Primers were YCK2CS-F (5′-CAGTAAGCTAGGAAGCTCTTAGAATAGAAAACG-3′) and YCK2CS-R (5′-CGTTTTCTATTCTAAGAGCTTCCTAGCTTACTG-3′). The presence of the mutations and otherwise correct sequence of the resulting products were confirmed by complete sequence analysis of the YCK2 open reading frame. The pGal:GFP:YCK2Cys545,546Ser plasmid pJB2 was constructed using the pGal:GFP:YCK2 plasmid pJB1, swapping in the 3′ 350 bp of the mutant gene using the HindIII site in YCK2 and the SalI plasmid cloning site. pJB1 was constructed in several steps. A PCR product of the YCK2 plasmid pL2.99 was generated with primers Y2ORF1 (5′-GCAGGATCCATGGAATTCTCTCAAG-3′) and Y2ORF2X (5′-GCTCGAGGTCGACCTAACAGCATCCTAG-3′) to introduce 5′ BamHI and 3′ SalI sites (underlined). These primers also contain an EcoRI site following the YCK2 initiating ATG. The PCR product was digested with BamHI and SalI, and the resulting fragment was cloned into pUC19ΔE. The GFP gene on an EcoRI fragment was cloned into the unique EcoRI site of this plasmid. The BamHI–SalI fragment of the resulting plasmid, containing the entire fusion gene, was introduced into a YCp50-based (CEN) pGal1 promoter vector provided by C. Wittenberg (Scripps Research Institute, La Jolla, CA) to generate pJB1.
To induce synthesis of Yck2 fusion protein from pJB2, cells were grown in selective medium containing raffinose, and then galactose was added to 2%. Cells were examined for GFP–Yck2p fluorescence at 90 min after induction, when immunoblot analysis showed that fusion protein levels are comparable to the level observed at steady state in cells expressing the fusion from a low-copy plasmid (our unpublished results).
The Cdc12–GFP-expressing plasmid pTD150-CDC12 was generated using PCR amplification to introduce restriction sites upstream of the CDC12 coding region and replacing the stop codon. PCR primers CDC12–GFP1 (5′-GTGAGTGCGGATCCGACATGATGCAG-3′) and CDC12–GFP2 (5′-GACATTAATTAATCTAGATTTTAAATGGG-3′) were used with YEp(CDC12)N′ (provided by S. Lillie, University of Michigan, Ann Arbor, MI) as template. Primer 1 introduces a BamHI site (underlined) approximately 270 bp upstream of the CDC12 coding sequence, and primer 2 replaces the CDC12 stop codon with an XbaI site (underlined). The PCR product was digested with BamHI and XbaI, and the resulting 1.4-kb fragment was cloned into the BamHI and XbaI sites of pTD150 (CEN, URA3 vector containing a short polylinker between the ACT1 promoter and the GFP coding sequence; provided by T. Doyle and D. Botstein, Stanford University, Stanford, CA). This produced a functional (our unpublished results) in-frame fusion between the two coding regions, with the fusion gene under the control of the CDC12 promoter (although we cannot rule out effects of the ACT1 promoter on expression). The GFP gene in plasmid pTD150 is the GFPm2 mutant (Cormack et al., 1996), encoding S65A, V68L, and S72A amino acid changes.
Yeast Strains
Yeast strains used for this work are listed in Table 1. All LRB strains are closely related or differ only at the YCK loci. Yeast transformation was performed using the LiAc procedure (Ito et al., 1983), and standard culture media and conditions and genetic techniques were used (Sherman et al., 1986). The integration of GFP:YCK2 at the YCK2 chromosomal locus was performed by one-step gene replacement (Rothstein, 1983). The XbaI–SacI fragment (from pL2.991) carrying the GFP:YCK2 fusion with flanking sequences was cotransformed into the yckts strain LRB756 with vector pRS315, and Leu+ transformants were screened for the ability to grow at 37°C and for green fluorescence. Strain LRB829 was among the progeny of a cross of one such transformant by LRB757 (yck1-Δ1::ura3 yck2-2ts) and was used to generate LRB854 and LRB855 by a cross to LRB759. The YCK1 genotype of these strains was confirmed by analyzing the segregation of the yckts phenotype in meiotic progeny of subsequent crosses to LRB756 or LRB757. To construct the diploid rho− GFP:YCK2 strain LRB859, LRB834 cells were grown in the presence of 10 μg/ml ethidium bromide (Sherman et al., 1986). Colonies unable to grow using ethanol as the sole carbon source were examined by DAPI staining without fixation to ensure that little or no staining similar to mitochondrial signal was present.
Table 1.
Yeast strains used
| Strain | Genotype | Source/reference |
|---|---|---|
| LRB344 | MATα his3 leu2 ura3-52 yck2::HIS3 | (Robinson et al., 1993) |
| LRB756 | MATa his3 leu2 ura3-52 yck1-Δ1::ura3 yck2-2ts | (Panek et al., 1997) |
| LRB757 | MATα his3 leu2 ura3-52 yck1-Δ1::ura3 yck2-2ts | (Panek et al., 1997) |
| LRB758 | MATa his3 leu2 ura3-52 | (Panek et al., 1997) |
| LRB759 | MATα his3 leu2 ura3-52 | (Panek et al., 1997) |
| LRB829 | MATa his3 leu2 ura3-52 yck1-Δ1::ura3 GFP:YCK2 | This study |
| LRB834 | MATa/MATα his3/his3 leu2/leu2 ura3-52/ura3-52 yck1-Δ1::ura3/yck1-Δ1::ura3 | Cross of LRB854 by LRB855 |
| GFP:YCK2/GFP:YCK2 | ||
| LRB852 | MATα ade1 his3 leu2 ura3-52 cdc15-2 yck1-Δ1::ura3 GFP:YCK2 | This study |
| LRB854 | MATa his3 leu2 ura3-52 yck1-Δ1::ura3 GFP:YCK2 | This study |
| LRB855 | MATα his3 leu2 ura3-52 yck1-Δ1::ura3 GFP:YCK2 | This study |
| LRB859 | MATa/MATα his3/his3 leu2/leu2 ura3-52/ura3-52 yck1-Δ1::ura3/yck1-Δ1::ura3 | This study |
| GFP:YCK2/GFP:YCK2 rho− | ||
| LRB860 | MATa/MATα his3/his3 leu2/leu2 ura3-52/ura3-52 yck1-Δ1::ura3/yck1-Δ1::ura3 yck2-2ts/yck2-2ts | Cross of LRB756 by LRB757 |
| LRB861 | MATa/MATα his3/his3 leu2/leu2 ura3-52/ura3-52 | Cross of LRB758 by LRB759 |
| DSY885 | MATα ade1 his2 leu2-3,112 trp1-1 ura3-Δns cdc15-2 bar1 | D. Stuart and C. Wittenberg; congenic with 15DaubΔ (Richardson et al., 1989) |
| YAB47 | MATa his3 leu2 trp1 ura3-52 cdc3-1 | A. Bloecher and K. Tatchell; segregant from the third serial backcross of SLTD3-6B into the JC482 genetic background (Cannon and Tatchell, 1987) |
| SLTD3-6B | MATa his3 leu2 trp1 gal2 cdc3-1 | (Johnson et al., 1987) |
Microscopy
Expression of GFP–Yck2p was monitored using an Olympus (Lake Success, NY) AX70 Provis microscope equipped for differential interference contrast (DIC) optics and epifluorescence. Rhodamine and DAPI fluorescence were monitored using standard filter sets (U-MNG and U-MWU, respectively; Olympus America, Lake Success, NY). GFP fluorescence was monitored using a filter set optimized for the spectral characteristics of S65T, #41001 (Chroma Technology, Brattleboro, VT).
To observe GFP fluorescence in asynchronous populations, cells were grown to log phase and observed either directly in synthetic complete liquid medium on precleaned slides or supported on a selective medium agarose pad as described for observation of actin structures (Waddle et al., 1996). For observation of synchronous cells, the thermosensitive cdc15-2 mutation was used to arrest the cell cycle. Mutations in CDC15 cause temperature-sensitive cell-cycle arrest with large buds and anaphase spindle morphology (Culotti and Hartwell, 1971; Schweitzer and Philippsen, 1991). A strain containing cdc15-2 and GFP:YCK2 was generated by a cross of the GFP:YCK2 strain LRB854 by DSY885. Four serial backcrosses of this strain to the GFP:YCK2 parent strain resulted in strain LRB852. Cells of this strain were arrested by incubation at 37°C for 3 h, at which time 71% showed the large-budded morphology characteristic of this mutant. Further incubation did not result in significant increases in the fraction of large-budded cells. Cells were then released from arrest by shift to the permissive temperature of 24°C and monitored for GFP fluorescence at intervals over 110 min. Cells with different patterns of fluorescence were quantitated at each time point. Also at each interval, an aliquot was removed and formaldehyde was added to a final concentration of 4%. After 2 h of fixation, cells were pelleted, resuspended in fixative (Pringle et al., 1989), and then stored at 4°C for up to 12 h. Fixed cells were then stained for filamentous actin structures using rhodamine-conjugated phalloidin (Molecular Probes, Eugene, OR) as described by Pringle et al. (1989). Cells were assessed for actin structures, and cells with distinct patterns were quantitated in multiple fields at each time point.
DAPI staining to reveal nuclei was performed with live cells of strain LRB859. Cells were grown to log phase in synthetic medium and harvested by slow centrifugation. After resuspension to 108 cells/ml in 0.1 M HEPES, pH 7, staining was performed for 15 min in 0.5 μg/ml DAPI in 0.1 M HEPES, pH 7. Bud scars on cells grown to late log phase were visualized by staining with Calcofluor White (Sigma Chemical, St. Louis, MO) (Pringle, 1991) as described by Lippincott and Li (1998). Only cells with three or more easily distinguished scars were counted.
DIC and fluorescence images were acquired using a Photometrics Ltd. (Tucson, AZ) Series 200 cooled charge-coupled device camera. Charge-coupled device images were transferred to a Macintosh 7100 computer and processed using IPLab Spectrum software (Scanalytics, Fairfax, VA). Image composites were constructed using Photoshop v.3.0 software (Adobe Systems, Mountain View, CA), and photographic prints were made using a Codonics (Middleburg Heights, OH) NP1600 color photographic network printer.
RESULTS
Fusion of a Bright GFP Variant to the Amino Terminus of Yck2p Results in a Functional Yck Protein
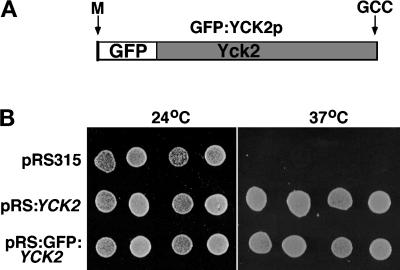
A bright GFP variant was fused in-frame to the YCK2 coding sequence following the initiating Met codon (Figure 1A) (see MATERIALS AND METHODS), and the fusion protein was expressed in yeast under control of the YCK2 promoter, from a low-copy vector. This plasmid restores growth and wild-type morphology to a yck1-Δ1::ura3 yck2-2ts (yckts) strain at 37°C (Figure 1B), and supports growth of yck1-Δ1::ura3 yck2::HIS3 cells (see MATERIALS AND METHODS; our unpublished results). We also constructed a YCK2 Cys545,546Ser GFP fusion (GFP:YCK2 Cys545,546Ser), which lacks the putative geranylgeranylation signal (see MATERIALS AND METHODS). This fusion protein does not support growth of yck1-Δ1::ura3 yck2::HIS3 cells even when expressed at high levels from the galactose-inducible GAL1 promoter (our unpublished results). We have observed previously that the YCK2Δ82 C-terminal truncation allele lacking the prenylation signal and 70 additional amino acids fails to substitute for the YCK genes (Robinson et al., 1993).
Figure 1.
Fusion of GFP to the amino terminus of Yck2p results in a functional Yck2 protein. (A) The GFP fusion construction is diagrammed, with the Yck2 portion shaded. The positions of the Yck2 initiating Met (M) and the -Cys-Cys isoprenylation motif (CC) that is altered in pJB2 are indicated. (B) The wild-type GFP–Yck2p fusion protein supports growth of yckts cells at their restrictive temperature for growth as does the intact Yck2 protein. Haploid yckts cells (strain LRB756) carrying the indicated low-copy (CEN) plasmids were grown to a density of 2 × 107 cells/ml in selective medium at 24°C. Undiluted (right circle of each pair) and 10-fold diluted (left circle of each pair) cultures of two independent transformants for each plasmid were plated on solid synthetic medium and incubated at the indicated temperature for 24 h before they were photographed.
Introduction of the fusion gene into the chromosomal YCK2 locus of the yckts strain LRB756 by gene replacement restored temperature-independent growth to the resulting strains, including LRB829 (our unpublished results). To confirm replacement, putative GFP:YCK2 strains were crossed to the yck2::HIS3 strain LRB344, and tetrad analysis was performed. The green fluorescence phenotype segregated in opposition to the yck2::HIS3 allele in 12 of 12 tetrads, and no temperature-sensitive spore clones were recovered. Strains carrying the GFP:YCK2 allele as the only YCK copy (yck1-Δ1::ura3 GFP:YCK2) show growth rates similar to wild-type cells (our unpublished results) and normal morphology (Figure 2).
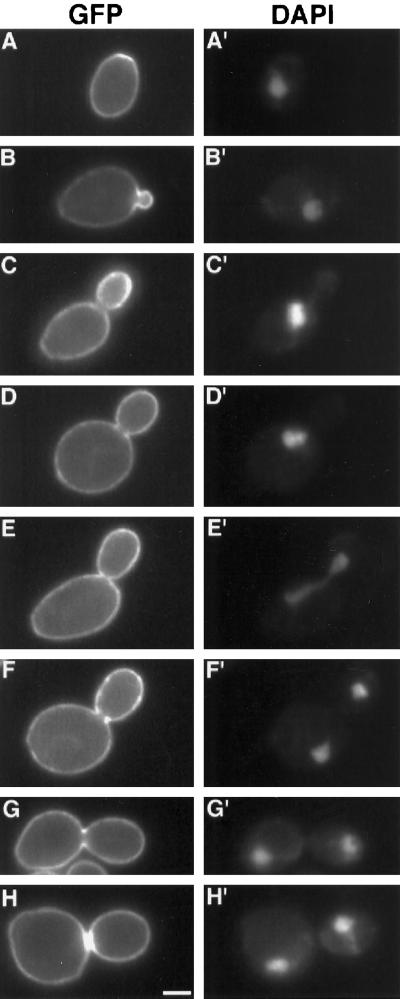
Figure 2.
GFP–Yck2p fluorescence is plasma membrane-associated and is dynamic during the cell cycle. GFP–Yck2p fluorescence (A–H) and corresponding DAPI staining (A′–H′) were observed for cells of strain LRB859 (diploid GFP:YCK2 rho− strain) grown at 30°C in synthetic medium to a density of 2 × 107 cells/ml. Cells were observed in 10 mM HEPES, pH 7, containing 5% glucose. The cell shown in each pair of panels represents a different cell cycle position, but each pair depicts a different cell. Bar, 2 μm.
GFP–Yck2p Shows Plasma Membrane Localization That Is Dynamic during the Cell Cycle
GFP–Yck2 protein expressed from a single chromosomal copy in haploid or diploid strains localizes to the plasma membrane at all stages of the cell cycle (diploid cells are shown in Figure 2). This pattern was not observed in cells expressing a Yck2 fusion protein lacking the C-terminal prenylation signal, which is known to be required for membrane association (Vancura et al., 1994). Cells expressing a GFP:YCK2Cys545,546Ser fusion gene (MATERIALS AND METHODS) showed both cytoplasmic and nuclear fluorescence but no plasma membrane signal (Figure 3).
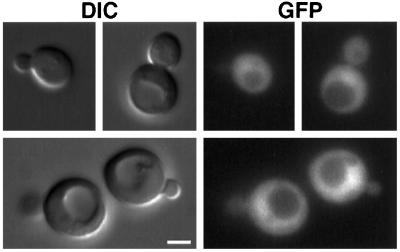
Figure 3.
Mutation of the C-terminal Cys codons of GFP–Yck2p abolishes plasma membrane localization. Cells of strain LRB758 (YCK+) carrying pJB2 (pGal1: GFP:YCK2Cys545,546Ser) were grown in selective medium containing raffinose to log phase. Galactose was added to the culture to induce synthesis of the mutant Yck2 protein, and cells were observed in synthetic medium directly on slides after 90-min incubation. Bar, 2 μm.
Enrichment of Yck2 fusion protein in specific locations within the plasma membrane occurs at two intervals during the cell cycle. In cells with emerging and small buds, the fusion protein is evenly distributed within the membrane but is heavily concentrated in bud as compared with mother cell membranes (Figure 2, A–C). Labeling is not restricted to the bud tip at any point. This enrichment persists until the bud is greater than half the size of the mother cell, after which bud and mother cell show more similar levels of plasma membrane fluorescence (Figure 2, D–H). In addition, in 48% of large-budded cells for which bud and mother cell membrane fluorescence were similar (17% of the total population; n = 144), a ring or thin bright bar was visible at the mother–bud neck. The ring structure (Figure 2, F and G) is perpendicular to the mother–bud axis and appears to be symmetrical between mother and bud. This structure was visibly a ring in cross-section in most focal planes. The bar structures (Figure 2H) probably represent patches of protein, because fluorescence of these structures was uniformly bright in all focal planes. Concentration at the bud neck is observed only in cells with two DAPI-staining regions, suggesting that the concentration occurs around the time of cytokinesis. Neck enrichment was never observed in cells earlier in the cell cycle. Separating cells often show patches of increased fluorescence on both mother and bud membranes at the site of separation (for example, Figure 2H; see also Figure 6B, bottom panels), suggesting that the fusion protein is partitioned between mother and daughter membranes at division.
Figure 6.
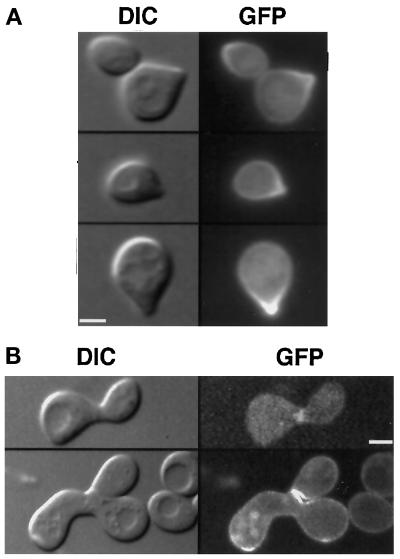
GFP–Yck2p becomes concentrated at shmoo tips in pheromone-treated cells and is localized to the site of fusion in mating cells. (A) Cells of strain LRB854 (MATa yck1-Δ1::ura3 GFP:YCK2) were grown to 2 × 107 cells/ml in synthetic medium at 30°C and exposed to alpha mating pheromone in the same medium at a final concentration of 6 μg/ml for 2 h. Cells were observed as described in the legend to Figure 2. (B) GFP:YCK2 haploid cells of strains LRB854 and LRB855 were mixed on solid rich medium and incubated for 3 h at 30°C. Cells were then suspended in synthetic medium and placed on slides for observation by DIC and fluorescence optics. Bar, 2 μm.
Concentration of GFP–Yck2 in Bud Membranes Parallels Actin Cytoskeletal Polarization
The pattern of Yck2p distribution and the timing of the changes in distribution are reminiscent of both polarized secretion and rearrangements in the actin cytoskeleton, which appear to be functionally connected (reviewed by Botstein et al., 1997). The actin cytoskeleton is polarized into the bud through most of the cell cycle, with cortical patches concentrated in the bud and cables extending from mother to bud (Adams and Pringle, 1984; Kilmartin and Adams, 1984). Late in the cell cycle, when bud expansion is basically complete, cortical patches are evenly distributed between mother cell and bud. The actin cytoskeleton is perturbed in the yckts mutant such that these cells fail to depolarize the actin cytoskeleton during mitosis when grown at the restrictive temperature (Robinson et al., 1993).
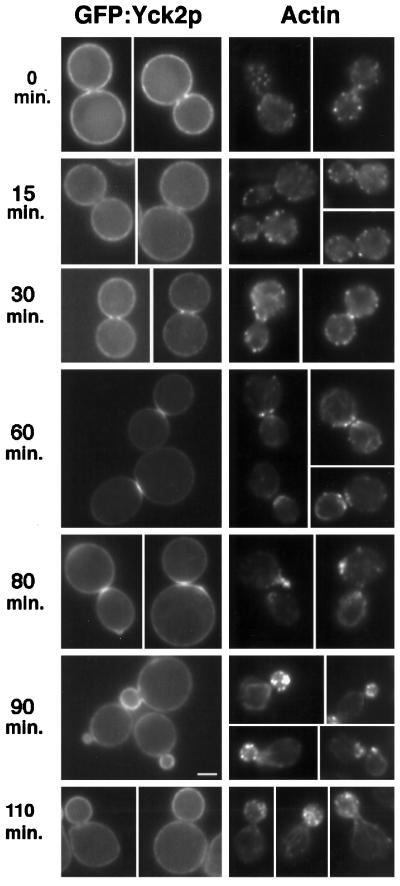
We tested whether changes in Yck2p distribution coincide temporally with the major rearrangements of the actin cytoskeleton during mitosis, and thus, the changes in polarized secretion, by monitoring the actin cytoskeleton along with GFP–Yck2p over time in cells synchronized in anaphase using the cdc15-2 mutation (MATERIALS AND METHODS). Cells containing the cdc15-2 mutation and expressing a chromosomal GFP:YCK2 allele (LRB852) show wild-type localization of the Yck2 fusion protein at 24°C. Cells were arrested by incubation at 37°C and then released from arrest by a shift to 24°C. In this experiment, arrest was maximal at 3 h, with 71% of the cells showing characteristic large-budded morphology (n = 170). After the shift back to 24°C, cells were monitored at intervals for GFP fluorescence and for filamentous actin organization (Figure 4). We first examined whether the change in GFP–Yck2p distribution between bud and mother cell membranes coincides with redistribution of actin structures. At the time of arrest (t = 0 min), and up to 60 min after release from arrest, GFP fluorescence levels in mother cell and bud membranes are similar (although neck enrichment is obvious in cells at 30- and 60-min time points, as discussed below). Actin distribution between mother cell and bud is also uniform (with the same exception as for GFP–Yck2p). Emerging buds are observed at the 80-min time point, and both GFP–Yck2p and actin are heavily concentrated in the bud. The GFP concentration and cortical actin concentration in bud membranes are also obvious 90 min after release from arrest. At the next time point, 110 min after release, bud membranes show only slightly higher levels of GFP–Yck2p fluorescence than do mother cell membranes. At this time, the actin cytoskeleton is still clearly polarized into the bud. Thus, the GFP concentration in bud membranes clearly parallels the distribution of actin, although the concentration of GFP–Yck2p in bud membranes appears to decrease before depolarization of the actin cytoskeleton occurs at nuclear division.
Figure 4.
Polarization of GFP–Yck2p to the bud neck and to sites of polarized growth occurs concomitant with polarization of the actin cytoskeleton. Cells of strain LRB852 (cdc15-2 GFP:YCK2) were grown to log phase in complete synthetic medium supplemented with adenine. The culture was shifted to 37°C and incubated for 3 h. The culture was then released from arrest by shift to 24°C. Samples were taken after release at the indicated intervals for both direct observation of GFP fluorescence (GFP–Yck2p; left panels) and fixation and staining with rhodamine-phalloidin (Actin; right panels). Bar, 2 μm.
The Bud Neck Localization of Yck2 Begins as a Ring at the End of Mitosis and Becomes a Patch under the Septum
As mentioned previously, bud and mother cell membranes of all large-budded cells with two DAPI-staining regions are equally fluorescent, but a bright ring or thin bright bar is often visible at the bud neck (Figure 2, F–H). The observation of a septum by Nomarski optics that lies above the bright bar (as shown for a diploid cell in Figure 6B, bottom panel) supports the idea that GFP–Yck2p becomes enriched in the membrane that underlies the growing septum. This change in Yck2p distribution could also parallel the timing of a major change in actin cytoskeletal organization (Adams and Pringle, 1984; Kilmartin and Adams, 1984; Botstein et al., 1997). Before cytokinesis, actin becomes concentrated in a contractile ring at the bud neck (Epp and Chant, 1997; Bi et al., 1998; Lippincott and Li, 1998). As cytokinesis and secretion of new cell wall material to the neck region occur, the cortical actin becomes distributed in patches underlying mother and bud sides of the division site.
We used the time course of release from cdc15-2-induced anaphase arrest (Figure 4) to establish when the bud neck enrichment of GFP–Yck2p occurs relative to actin cytoskeletal organization. None of the cells arrested in anaphase (0 min) showed strong concentration of GFP–Yck2p fluorescence at the bud neck. Therefore, the neck concentration occurs after the cdc15 arrest point. Bright GFP–Yck2p rings at bud necks were first observed at 30 min after release for 67% of cells examined. In cells fixed at this time point, the actin cytoskeleton was concentrated in a ring at the bud neck in 65% of cells examined. Cells with bright GFP fluorescence in bar or patch structures were prevalent at 60 min after release (65% of cells examined), indicating that the ring structure temporally precedes the bar structure. At this time point, actin staining of mother and daughter cells, clusters of patches on each side of the bud neck, was most similar to that described for cells at cytokinesis (Adams and Pringle, 1984; Botstein et al., 1997). The GFP–Yck2p patch appears to be located between the mother and bud sites of cortical actin staining. These results support the idea that the Yck2 kinase is localized to the site of cytokinesis in a ring and maintains this localization through the process of cytokinesis. The ring could expand and fill in to form a patch as cytokinesis and septation occur. Both mother and daughter cells show a patch at the division site as separation occurs, and for some cells a patch is present at the time when a new bud is forming (for example, see Figure 4, 80 min).
Yck Activity Is Required for Accurate Bud Site Selection in Haploid and Diploid Cells
The observation of a patch of Yck2 protein at the previous division site after emergence of a new bud in some cells, coupled with the requirement for Yck proteins for regulation of growth polarity, suggested that the Yck2 protein could act in the selection of a new bud site. The two patterns of bud site selection, axial in haploid cells and bipolar in diploid cells, appear to be determined by the activities of two different sets of proteins (Chant and Herskowitz, 1991; Zahner et al., 1996; Yang et al., 1997). Proteins that are required for both patterns are thought to communicate signals to the cytoskeleton (Bender and Pringle, 1989; Chant and Herskowitz, 1991; Chant and Pringle, 1995).
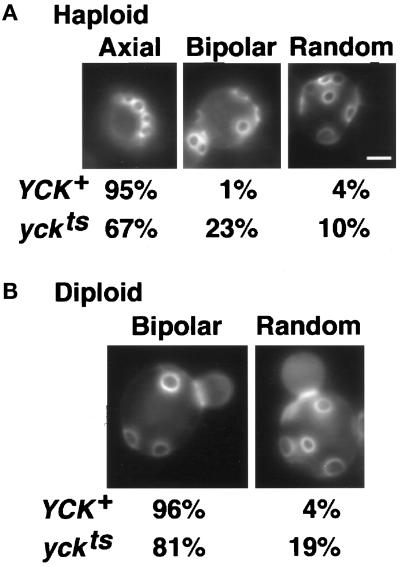
To determine whether Yck activity is important for proper bud site selection, we examined bud scars on yckts mutant cells grown at 24°C, visualizing these with the fluorescent dye Calcofluor (Pringle, 1991) (see MATERIALS AND METHODS). At this permissive temperature, the activity of the yck2ts kinase is reduced at least 40% from wild type, although cells are morphologically normal (Robinson et al., 1993). Bud scars on cells of the haploid strains LRB758 (YCK+) and LRB756 (yck1-Δ1::ura3 yck2-2ts) were categorized as showing either the normal axial pattern, a random pattern, or the bipolar pattern. Although 95% of haploid cells of our wild-type genetic background selected the axial sites preferred by haploid cells, only 67% of haploid yckts cells selected only axial sites (Figure 5A). The remainder of the cells included 23% that showed a bipolar pattern and 10% that appeared to be fully random. Thus, the activity of the Yck proteins is required for accurate selection of axial bud sites in haploid cells. The retention of normal budding pattern in more than half of the cells could reflect the fact that 60% of wild-type Yck activity is retained at this temperature.
Figure 5.
Yck activity is required for accurate bud site selection in haploid and diploid cells. (A) Haploid strains were YCK+, LRB758 (n = 80) and yckts, LRB756 (n = 172). (B) Diploid strains were YCK+, LRB861 (n = 317) and yckts, LRB860 (n = 395). Cells grown at 24°C to a density of 108 cells/ml in rich medium were stained with Calcofluor as in MATERIALS AND METHODS and were observed in 0.1 M potassium phosphate buffer, pH 7. Bar, 2 μm.
We also examined the effect of decreased Yck kinase activity on the bipolar budding pattern of diploid cells by staining diploid wild-type cells (LRB861) and homozygous yckts cells (LRB860) with Calcofluor. More than 95% of wild-type diploid cells showed bipolar budding, with the remaining cells appearing to select bud sites at random (Figure 5B); however, diploid yckts cells showed a significant increase in random budding, to 19% of cells examined. Again, the magnitude of the change could reflect the level of Yck2 activity retained at the permissive temperature for growth. Overall, the results of these experiments show that budding patterns in both haploid and diploid cells with reduced Yck activity are perturbed, suggesting that Yck activity could play a role in communication of spatial information to the cytoskeleton. The retention of a concentrated patch of Yck2 at the site of cell separation until bud emergence could provide the means for such communication.
Localization of GFP–Yck2p Occurs at Shmoo Tips and in Mating Cells
If the polarized distribution of GFP–Yck2p to emerging and small buds reflects association of the Yck2 protein with regions of polarized growth, haploid cells treated with mating pheromone could also show concentration of the Yck2 protein at the region of growth polarization. Therefore, we examined the localization of GFP–Yck2p in haploid cells (LRB854) treated with alpha mating pheromone. As shown in Figure 6A, cells with a mating projection (100% where n > 200 cells) show strong concentration of the fusion protein in the plasma membrane at the tip of the shmoo. This localization is similar to that of actin-based cytoskeletal proteins and other proteins required for polarized secretion (Snyder, 1989; Liu and Bretscher, 1992; Lillie and Brown, 1994; TerBush and Novick, 1995; Santos and Snyder, 1997; Finger and Novick, 1998; Finger et al., 1998). We also monitored the distribution of GFP–Yck2p during the mating process and observed that fluorescence is enriched at the site of cell juxtaposition and subsequent fusion and that fully formed zygotes briefly retain a bar of fluorescence at the site of fusion (Figure 6B, top panels). After fusion, localization is that of vegetative cells (Figure 6B, bottom panels).
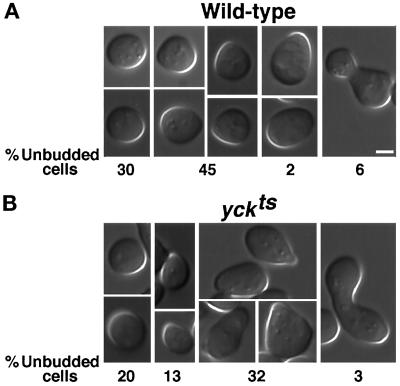
To determine whether the enrichments in the membrane of the pheromone-induced mating projection and the site of cell fusion are functionally significant, we examined the morphology of cells with low Yck activity (yckts cells) during mating. These cells form hyperpolarized structures during mating at the permissive temperature of 24°C, although yckts cells grow vegetatively with normal morphology at this temperature. Shown in Figure 7 are representative examples of cells from 2-h mating mixtures of two wild-type strains (A) and of two yckts strains (B), together with the frequency with which each morphological class was observed. The percentage of cells in the mating mixtures that were recognizably responding to pheromone, as judged by growth polarization, was similar between wild-type and yckts matings: 47% and 45%, respectively. The responding yckts cells, however, included a large proportion of more elongated and enlarged shmoos relative to responding cells from a mating mixture of wild-type strains (Figure 7) (3% of wild-type cells visibly responding to mating pheromone were enlarged and elongated versus 71% of yckts shmoos). The morphological abnormalities become exaggerated over time, eventually resulting in morphologies resembling vegetative cells lacking Yck activity, but we have not observed a significantly different mating efficiency of yckts cells at 24°C than wild-type cells (our unpublished results). The hyperpolarization during the mating process parallels that in mitotic cells deficient for Yck activity, consistent with the idea that Yck activity plays a general role in the control of polarized growth.
Figure 7.
Yck-deficient cells show a hyperpolarized mating response. Cells of strains LRB758 and LRB759 (A; MATa YCK+ and MATα YCK+, respectively) and cells of strains LRB756 and LRB757 (B; MATa yckts and MATα yckts, respectively) were mixed and incubated for 2 h at 24°C on rich medium. Mating mixtures were suspended in rich medium and placed on slides for photography using DIC optics. Panels show representative cell morphologies that were quantitated to yield percentages of different morphological types among the unbudded cells in the mixture (n = 130, YCK+; n = 79, yckts). Bar, 2 μm (magnification in all panels is identical).
Concentration of GFP–Yck2p at the Bud Neck or the Emerging Bud Does Not Require Septin Function
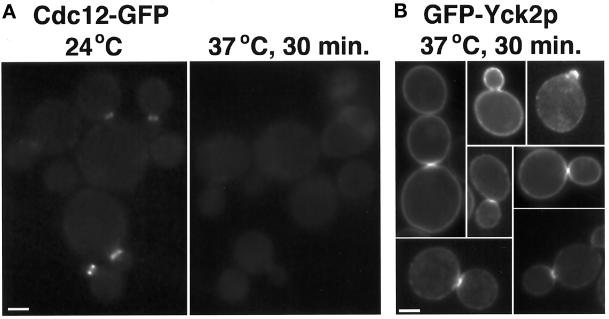
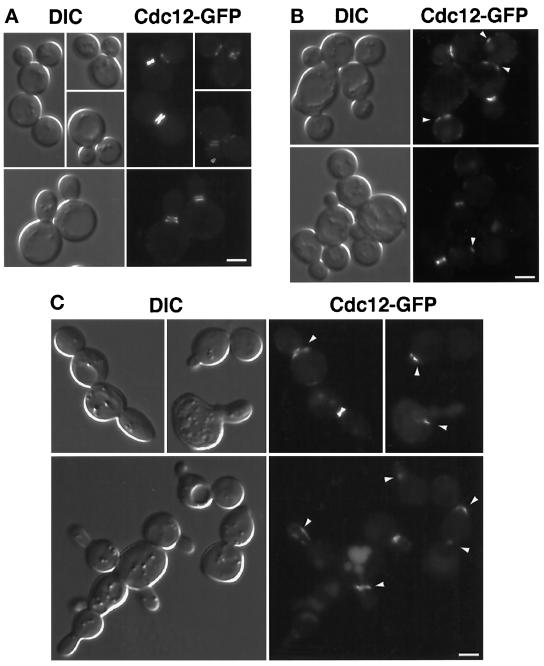
The concentration of GFP–Yck2p in a ring at the bud neck at the time of cytokinesis could reflect association with the septin neck ring. We tested whether GFP–Yck2p shows normal localization in cells defective for septin function by comparing GFP:YCK2 cells carrying a temperature-sensitive cdc3-1 or cdc10-1 mutation after growth at permissive and restrictive temperatures. To monitor septin function, we examined localization of a functional fusion of GFP to Cdc12 (MATERIALS AND METHODS) in cdc3-1 and cdc10-1 cells incubated under the same conditions.
At the permissive temperature, bud morphology, GFP–Yck2p localization, and Cdc12–GFP localization in the septin mutants were identical to those in wild-type cells. The Cdc12–GFP fusion protein expressed in the cdc3-1 strain decorated a ring at the neck of cells grown at 24°C (Figure 8A). After 30 min at 37°C, the septin ring was no longer assembled in cdc3-1 cells, as shown by the lack of Cdc12–GFP-labeled neck structures (Figure 8A, right panels). In contrast, at the same 30-min time point, the distribution of GFP–Yck2p was similar to that at permissive temperature in cdc3-1 cells. GFP signal was observed at small bud membranes and at the neck of dividing cells (Figure 8B), although the patch-like concentration at the division site was more often seen than a ring in dividing cells. Similar results were obtained using cdc10-1 cells (our unpublished results). At longer times after the temperature shift, only localization to ends of elongating buds was observed (our unpublished results). Because GFP–Yck2p localization to the bud neck normally occurs around the time of cytokinesis, and cytokinesis does not occur in the septin mutants, localization to bud neck rings was not expected at these late times. Thus, GFP–Yck2p concentration at the bud neck at the time of division may require septin function to occur but does not require septin function to be maintained; however, it does not distinguish whether the kinase can be recruited to the neck in the absence of septins, because any concentration observed could remain from events before the shift. Furthermore, concentration in the membrane of the growing bud does not require septin function.
Figure 8.
GFP–Yck2p localization to sites of polarized growth and the division site occurs in the absence of septin function. Cells of strain YAB47, carrying the cdc3-1 temperature-sensitive septin mutation, and carrying either episomal CDC12: GFP (A) or the GFP:YCK2 plasmid pL2.992 (B) were grown to log phase in synthetic medium at 24°C. Cells were observed at permissive temperature and at intervals after the shift of the culture to 37°C. At 30 min after shift, <2% of cells examined showed Cdc12–GFP neck ring staining (A, right panel; n > 200); localization of the GFP–Yck2p was evaluated at this time point (B). Bar, 2 μm.
Localization of Septin Proteins Is Altered in the yckts Mutant
One possible explanation for the similar phenotype of septin and yckts mutants but a lack of requirement for septins for Yck2p localization is that Yck activity promotes assembly or function of the septin ring. It has been suggested that posttranslational modification might affect both assembly and localization of the septins, although there is no direct evidence for this idea (Longtine et al., 1996). We examined the possibility that Yck activity is required to direct or restrict the location of sites of septin assembly using the Cdc12–GFP fusion. This fusion was introduced into wild-type and yckts strains on a low-copy plasmid, and transformants were examined for green fluorescence at the permissive temperature (24°C) and after shifting to semipermissive (30°C) and restrictive (37°C) temperatures. In wild-type cells grown at 24° or at 37°C (Figure 9A), the only obvious fluorescent signal was a ring at the neck of small-budded cells or a double ring at the necks of cells with larger buds. yckts cells grown at 24°C showed the same pattern, identical to that observed by immunofluorescence detection of septin proteins (Haarer and Pringle, 1987; Ford and Pringle, 1991; Kim et al., 1991).
Figure 9.
Cdc12–GFP is localized abnormally in Yck-deficient cells. (A) Wild-type cells (strain LRB758) carrying pTD150-CDC12 (pRS316:CDC12: GFP) were grown to log phase in synthetic medium at 37°C. (B) yckts cells (strain LRB756) carrying pTD150-CDC12 were grown to log phase in synthetic medium at 24°C, and then a portion of the culture was shifted to 30°C for 3 h. Note the presence of thick neck rings in approximately half of the cells and the lack of clear separation of bright rings in large-budded cells. Cells with abnormal septin fluorescence (most morphologically normal) are marked by arrowheads. (C) The cells shown are from the same original culture as those shown in B, but the cells in C were shifted from 24° to 37°C for 3 h before observation. Note the lack of normal neck structures in both severely misshapen and relatively normal-appearing cells. Arrowheads mark abnormal septin structures. In all cases, cells were placed on agar pads for observation and photography by DIC and fluorescence optics. Bar, 2 μm.
At 30°C, the yckts cells displayed defects in septin–GFP localization (Figure 9B). Although most cells were morphologically normal, 44% either lacked neck staining completely or showed abbreviated or discontinuous neck staining (n = 230). Ectopic cortical staining was also observed (marked by arrowheads in Figure 9B). Even in the 56% of the cells that displayed neck ring fluorescence, we never observed thin bright rings or well separated double rings. Neck staining in these cells was generally thick, resembling the aberrant pattern observed for cells lacking the protein phosphatase type 2A regulatory subunit Cdc55p (Healy et al., 1991). Aberrant septin staining showed no correlation with abnormal morphology.
Cdc12–GFP in yckts cells incubated at 37°C for 3 h (Figure 9C) was present at the bud neck in <40% of cells examined, but again, such staining was thicker than in wild-type cells, and widely separated rings were never observed. Discontinuous neck rings and ectopic sites of bright fluorescence were more frequently observed (marked by arrowheads in Figure 9C) in cells grown at this restrictive temperature. These results suggest that Yck activity could positively regulate the site of assembly and/or the process of assembly of the septins at the bud neck.
DISCUSSION
Loss of Yck kinase activity results in formation of abnormal, elongated buds and in inefficient cytokinesis (Robinson et al., 1993). On the basis of this phenotype, Yck activity was proposed to control polarized growth and to positively regulate cytokinesis. We and others have also demonstrated that Yck activity is required for constitutive and regulated internalization of some membrane proteins (Panek et al., 1997; Hicke et al., 1998), which suggested that the kinase could regulate polarized growth by modulating membrane flow and/or membrane remodeling. That Yck2 is located at the plasma membrane was previously established by fractionation and immunofluorescence studies (Wang et al., 1992; Vancura et al., 1993, 1994). Furthermore, immunofluorescence data indicated that Yck2p was enriched in a ring at the bud neck in some cells (Vancura et al., 1994). To more fully characterize the localization of Yck2p, we examined its in vivo localization using a functional fusion of the Aequora victoria GFP to Yck2p. The fusion protein complements loss of Yck activity when expressed from its own promoter in single copy and is readily detectable. GFP–Yck2 protein is located throughout the plasma membrane, but its distribution changes during the cell cycle. The geranylgeranylation signal at the C-terminus of Yck2p is required for peripheral membrane localization of the fusion protein as it is for the native Yck2 protein (Robinson et al., 1993; Vancura et al., 1994).
Localization of Yck2 Could Provide Temporal Regulation of Its Activity
CK1 protein kinases have not been thought to be regulated by second messengers or regulatory proteins, although evidence is accumulating that lipids can regulate CK1 activities (Brockman and Anderson, 1991; Nickels, Robinson, and Broach, unpublished results). Alternatively, CK1 activity could be directed toward specific substrates via generation of an acidic recognition site by sequential (hierarchal) phosphorylation (Roach, 1990) or by accumulation of the protein kinase at the appropriate site of activity. Mammalian CK1α is thought to be regulated in the latter manner. This CK1 isoform, associated with vesicular compartments in the cytosol at most times in the cell cycle, is transiently localized to the spindle at mitosis (Brockman et al., 1992). Thus, the kinase would have access to its substrates in a cell cycle-dependent manner. The Yck2 protein is found associated with the plasma membrane at all times but is transiently concentrated at areas of polarized growth and at the site of cytokinesis. Therefore, localization could provide a means to regulate Yck activity at different sites in the plasma membrane, and thus, toward specific substrates, in a temporally specific manner.
Localization of Yck2p to Sites of Polarized Growth Correlates with Its Regulatory Role in This Process
Cells lacking Yck activity are hyperpolarized, suggesting a role for Yck in the regulation of growth polarization. Consistent with this proposed function, the Yck2 fusion protein is heavily concentrated in the membrane of the bud during the polarized growth of bud morphogenesis. The concentration in the bud membrane decreases steadily through the cell cycle until just before the time of actin depolarization that precedes cytokinesis, suggesting a functional connection between actin polarization and Yck2p localization. GFP–Yck2p is also concentrated at the shmoo tip in cells responding to mating pheromone, and this localization correlates with an exaggerated morphological response during mating by cells with reduced Yck activity.
Taken together, the localization and phenotypic data indicate that the kinase is in the right place at the right time to have a fundamental role in controlling growth polarization. Yck activity could regulate the extent or timing of growth polarization by transmitting or propagating a signal for the shift in growth pattern. Yck kinase activity could regulate, directly or indirectly, the association of the actin cytoskeleton with proteins at the plasma membrane. An effect of Yck-mediated phosphorylation on actin cytoskeleton organization could also explain the effects of Yck deficiency on endocytic processes, because one group of end mutants includes mutants in actin and actin-associated proteins (Munn et al., 1995).
Enrichment of Yck2 protein in the membranes of growing buds could reflect the trafficking pattern of Yck2 to the plasma membrane. Secretory traffic to the plasma membrane is polarized during these specific times in the cell cycle, dependent on polarization of the actin cytoskeleton (reviewed in Botstein et al., 1997 and Kaiser et al., 1997). The Yck2 protein is a peripheral membrane protein that is thought to be modified by geranylgeranylation, attributable to its requirement for a C-terminal GGTase type II signal sequence for membrane association (Vancura et al., 1994; and see above). The idea that a prenylated protein could transit to the plasma membrane on the cytosolic face of secretory vesicles was proposed recently for the prenylated Ras2 protein (Boyartchuk et al., 1997). Transport of Yck2p to the plasma membrane in association with polarized secretory vesicles would result in concentration at sites of polarized growth. Preliminary results indicate that this in fact may be the case, because secretory pathway function is required for Yck2p plasma membrane localization (our unpublished results).
Yck2p Activity Is Required for Accurate Bud Site Selection
A number of proteins with polarized distribution during budding play regulatory roles in the selection of budding pattern. The axial pattern of budding in haploid cells requires the action of the septin proteins (Flescher et al., 1993; Chant et al., 1995), Bud3p and Bud4p (Chant and Herskowitz, 1991), and the Axl1 and Axl2/Bud10 proteins (Fujita et al., 1994; Halme et al., 1996; Roemer et al., 1996). The diploid bipolar pattern is disturbed in mutants affecting the actin cytoskeleton (Schweitzer and Philippsen, 1991; Bauer et al., 1993; Amberg et al., 1997; Yang et al., 1997) as well as in Bud7p, Bud8p, Bud9p, Spa2p, and Bni1p (Zahner et al., 1996). Both patterns are disturbed in cells mutant for any of several other proteins, including Rsr1p, Bud2p, and Bud5p (Bender and Pringle, 1989; Chant et al., 1991; Chant and Herskowitz, 1991). These latter proteins may be required to communicate spatial information for organization of cytoskeletal proteins.
Normal levels of Yck2p activity are also required for this process. A significant change in bud site selection pattern was observed at 24°C for yckts cells, which contain 50–60% of wild-type activity at this permissive temperature. The inaccurate bud site selection observed for both haploid and diploid cell types deficient for Yck activity suggests a role(s) in both processes. It could be argued that this bud site selection defect simply reflects the requirement for Yck activity for proper septin organization (see below); however, no role for the septins in bipolar budding has been described, although mutants affecting bipolar budding interact genetically with septin mutants (Flescher et al., 1993; Longtine et al., 1996). The observation in some cells of a patch of GFP–Yck2p fluorescence at the site of previous cell separation at the time of new bud emergence suggests a role in determining or orienting toward the selected site. Alternatively, Yck activity could be required for accurate delivery of material to the selected bud site in response to the selection signal. The possibility that Yck activity could be involved in cytoskeletal communication would provide a basis for either model.
Yck2p May Regulate Septin Organization at the Site of Cytokinesis and Cell Separation
Cytokinesis and cell separation do not occur in cells lacking Yck activity, suggesting a positive role for the kinase in these processes. GFP–Yck2p is isotropically distributed at G2/M but becomes concentrated at the mother–bud neck at cytokinesis, suggesting that specific association with the neck region allows the action that promotes cytokinesis. The timing of GFP–Yck2p ring appearance is close to the time of assembly of the actomyosin contractile ring (Epp and Chant, 1997; Bi et al., 1998; Lippincott and Li, 1998), and the pattern of Yck2p distribution changes during cytokinesis and cell separation, such that it appears as a patch during division and a pair of patches after division. The patch of Yck2p appears to lie at and then between the separating membranes, as defined by the actin cortical patches that redistribute during division, and likely underlies the position of the septum. This localization places Yck2p at the site of cytokinesis and septum deposition, resembling the pattern observed for the Sec3 protein, which is required for polarized secretion that is mediated by the Exocyst complex (Finger et al., 1998).
The structures observed using a functional septin–GFP fusion are abnormal in the yckts mutant. In addition to an increase in ectopic assembly, both truncated and discontinuous septin ring structures were observed. Thus, the effects of loss of Yck activity on cytokinesis and septum formation could reflect a primary defect in septin assembly or localization. This is a novel observation for a mutant defective in cytokinesis. Although the septin ring often appears thickened in cdc55 mutants, which lack a protein phosphatase type 2A regulatory subunit (Healy et al., 1991), the defect did not include ectopic localization and abnormal ring structures. The defects reported here have not been observed with other polarity protein mutants, and septin mutants themselves cause complete loss of the ring structure (Haarer and Pringle, 1987; Ford and Pringle, 1991; Kim et al., 1991). One simple model to explain our results is that the Yck kinases could promote site specificity of septin assembly at the plasma membrane by modifying septin proteins themselves or a plasma membrane protein that directs their membrane association. No factors promoting association of the septins with the plasma membrane have been described.
Alternatively, Yck activity on septin proteins could regulate higher order assembly of the neck ring structure. The axial alignment model of septin assembly proposed by Field et al. (1996) provides an interesting way to think about the effects of posttranslational modification on septin ring structure. This model for septin ring structure in the yeast mother–bud neck is based on structural observations of purified Drosophila septins, which are structurally conserved with the yeast proteins. In the axial alignment model, the septin filaments composed of three dimers are arranged side-to-side, parallel to the mother–bud axis. Lateral interactions between filaments result in the ordered structure around the bud neck. Different states of assembly could account in part for the recruitment of different polarity proteins onto the ring at specific cell cycle times. Such different states of assembly have been proposed to account for the different times of appearance of the 10-nm filament ring and the septin ring during the cell cycle (Longtine et al., 1996). Modification of one or more of the septin proteins in principle could affect the lateral interactions between septins, causing alterations to the assembly state and thus to the fine-structure appearance of the ring. The Drosophila Pnut septin appears to be posttranslationally modified (Field et al., 1996), but phosphorylation of yeast septin proteins has not been reported.
A defect in septin organization could affect both the assembly of the actomyosin contractile ring and the recruitment of the septum forming proteins to the bud neck. The actin/Myo1p ring does not form in septin mutants (Epp and Chant, 1997; Bi et al., 1998; Lippincott and Li, 1998), which together with the aberrant septin localization could explain the cytokinesis defect in Yck-deficient cells. Septin function is also required for localization of both Chs2p, which forms the primary septum, and Chs3p, which forms the chitin ring at the presumptive bud site and participates in septum synthesis (DeMarini et al., 1997; Orlean, 1997; Santos and Snyder, 1997). Chs4p, the activator of Chs3p, also requires septin function for localization and appears to interact directly with the septin ring (Roncero et al., 1988; Bulawa, 1992; Bulawa, 1993; DeMarini et al., 1997). In cells mutant for the septin proteins, diffuse chitin accumulation occurs on cell surfaces, and neck deposition does not occur (Longtine et al., 1996). Loss of Yck activity also results in abnormal deposition of chitin on cell surfaces, but in a manner different from loss of septin function. Calcofluor staining of bud scars on yckts cells incubated at restrictive temperature showed accumulation of stained material over cell surfaces but also showed heavy accumulation in broad rings at bud necks and at constrictions along the long buds (Robinson et al., 1993). Bud scars also appear thickened in yckts cells grown at semipermissive temperature (our unpublished results). The yckts chitin mislocalization is more like that of the cdc55 mutant, which affects protein phosphatase type 2A (Healy et al., 1991). This pattern suggests the presence of the enzymes and activator at the cell surface but suggests mislocalization and possibly temporally inappropriate activity. In neither case is the primary defect resulting in the terminal phenotype known, but our results with Yck-deficient cells are consistent with inappropriate septin assembly rather than loss of septin function.
Another possible contributing factor to the mislocalization of chitin in Yck-deficient cells is the endocytic defect of these cells. The catalytic unit of chitin synthase III, Chs3p, and its targeting protein Chs5p both reside in an intracellular compartment through much of the cell cycle (Chuang and Schekman, 1996; DeMarini et al., 1997; Santos and Snyder, 1997). This compartment also contains endocytic markers (Chuang and Schekman, 1996) and could represent an endocytic intermediate or a point of intersection of biosynthetic and endocytic pathways. If Yck2 activity is required for normal clearance of Chs3p from the membrane, or for accurate targeting of enzyme pools to the membrane, the effects of losing Yck activity would be predicted to include aberrant deposition of chitin.
Association with the Bud Neck May Not Require the Septin Ring
How is Yck2 protein recruited to the site of cytokinesis and cell separation? As discussed above, most proteins that act in these processes, including the contractile ring components actin and Myo1p, are recruited to the bud neck late in the cell cycle in a septin-dependent manner. The Sec3 protein appears to be an exception, because it was reported to localize to a neck ring independent of septin function (Finger et al., 1998). Some of these proteins are also found at other areas of polarized growth, and this localization is not generally dependent on the septin ring (Adams and Pringle, 1984; Halme et al., 1996; Roemer et al., 1996). Similarly, Yck2p becomes concentrated in membranes of growing buds in the absence of septin function; however, we show here that association of the Yck2 kinase with the bud neck can be maintained in the absence of the septin ring. Our results do not rule out the possibility that recruitment to the bud neck at cytokinesis involves the septin proteins, because GFP–Yck2p rings were seldom observed. The patches that were frequently observed could represent fusion protein recruited to these sites before loss of the septin ring. Whether the Yck2p at the bud neck is recruited from elsewhere in the membrane or is newly synthesized protein targeted to the neck by polarized secretion remains to be determined, but the latter alternative could explain a lack of dependence on septin function for localization.
ACKNOWLEDGMENTS
We thank K. Tatchell for help with microscopy and discussion of results, R. Tsien for providing the S65T GFP plasmid, J. McMillan, D. Stuart, C. Wittenberg, A. Bloecher, and K. Tatchell for providing yeast strains, and B. Haarer and J. R. Pringle for helpful discussions. We especially thank the reviewers for their efforts to improve this manuscript. This work was supported by National Science Foundation grant MCB-9601294 to L.C.R and by the Department of Biochemistry and Molecular Biology at Louisiana State University Medical Center–Shreveport. H.R.P. was supported by Louisiana Educational Quality Support Fund doctoral student training program award GF11 to D. J. O’Callaghan and R. E. Rhoads.
REFERENCES
- Adams AEM, Pringle JR. Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic mutant Saccharomyces cerevisiae. J Cell Biol. 1984;98:934–945. doi: 10.1083/jcb.98.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amberg DC, Zahner JE, Mulholland JW, Pringle JR, Botstein D. Aip3p/Bud6p, a yeast actin-interacting protein that is involved in morphogenesis and the selection of bipolar budding sites. Mol Biol Cell. 1997;8:729–753. doi: 10.1091/mbc.8.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer F, Urdaci M, Aigle M, Crouzet M. Alteration of a yeast SH3 protein leads to conditional viability with defects in cytoskeletal and budding patterns. Mol Cell Biol. 1993;13:5070–5084. doi: 10.1128/mcb.13.8.5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender A, Pringle JR. Multicopy suppression of the cdc24 budding defect in yeast by CDC42 and three newly identified genes including the ras-related gene RSR1. Proc Natl Acad Sci USA. 1989;86:9976–9980. doi: 10.1073/pnas.86.24.9976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi E, Maddox P, Lew DJ, Salmon ED, McMillan JN, Yeh E, Pringle JR. Involvement of an actomyosin contractile ring in Saccharomyces cerevisiae cytokinesis. J Cell Biol. 1998;142:1301–1312. doi: 10.1083/jcb.142.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botstein D, Amberg D, Mulholland J, Huffaker T, Adams A, Drubin D, Stearns T. The yeast cytoskeleton. In: Pringle JR, Broach JR, Jones EW, editors. The Molecular and Cellular Biology of the Yeast Saccharomyces, Cell Cycle and Cell Biology. Plainview, NY: Cold Spring Harbor Laboratory; 1997. pp. 1–90. [Google Scholar]
- Boyartchuk VL, Ashby MN, Rine J. Modulation of Ras and a-factor function by carboxy-terminal proteolysis. Science. 1997;275:1796–1800. doi: 10.1126/science.275.5307.1796. [DOI] [PubMed] [Google Scholar]
- Brockman JL, Anderson RA. Casein kinase I is regulated by phosphatidylinositol 4,5-bisphosphate in native membranes. J Biol Chem. 1991;266:2508–2512. [PubMed] [Google Scholar]
- Brockman JL, Gross SD, Sussman MR, Anderson RA. Cell cycle-dependent localization of casein kinase I to mitotic spindles. Proc Natl Acad Sci USA. 1992;89:9454–9458. doi: 10.1073/pnas.89.20.9454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulawa CE. CSD2, CSD3, and CSD4, genes required for chitin synthesis in Saccharomyces cerevisiae: the CSD2 gene product is related to chitin synthases and to developmentally regulated proteins in Rhizobium species and Xenopus laevis. Mol Cell Biol. 1992;12:1764–1776. doi: 10.1128/mcb.12.4.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulawa CE. Genetics and molecular biology of chitin synthesis in fungi. Annu Rev Microbiol. 1993;47:505–534. doi: 10.1146/annurev.mi.47.100193.002445. [DOI] [PubMed] [Google Scholar]
- Byers B, Goetsch L. Loss of the filamentous ring in cytokinesis-defective mutants of budding yeast. J Cell Biol. 1976a;70:35a. doi: 10.1083/jcb.69.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers B, Goetsch L. A highly ordered ring of membrane-associated filaments in budding yeast. J Cell Biol. 1976b;69:717–721. doi: 10.1083/jcb.69.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon JF, Tatchell K. Characterization of Saccharomyces cerevisiae genes encoding subunits of cyclic AMP-dependent protein kinase. Mol Cell Biol. 1987;7:2653–2663. doi: 10.1128/mcb.7.8.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- Chant J, Corrado K, Pringle JR, Herskowitz I. Yeast BUD5, encoding a putative GDP-GTP exchange factor, is necessary for bud site selection and interacts with bud formation gene BEM1. Cell. 1991;65:1213–1224. doi: 10.1016/0092-8674(91)90016-r. [DOI] [PubMed] [Google Scholar]
- Chant J, Herskowitz I. Genetic control of bud site selection in yeast by a set of gene products that constitute a morphogenetic pathway. Cell. 1991;65:1203–1212. doi: 10.1016/0092-8674(91)90015-q. [DOI] [PubMed] [Google Scholar]
- Chant J, Mischke M, Mitchell E, Herskowitz I, Pringle JR. Role of Bud3p in producing the axial budding pattern of yeast. J Cell Biol. 1995;129:767–778. doi: 10.1083/jcb.129.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chant J, Pringle JR. Patterns of bud-site selection in the yeast Saccharomyces cerevisiae. J Cell Biol. 1995;129:751–765. doi: 10.1083/jcb.129.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang JS, Schekman RW. Differential trafficking and timed localization of two chitin synthase proteins, Chs2p and Chs3p. J Cell Biol. 1996;135:597–610. doi: 10.1083/jcb.135.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JA, Kiehart DP. Septins may form a ubiquitous family of cytoskeletal filaments. J Cell Biol. 1996;134:1345–1348. doi: 10.1083/jcb.134.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormack BP, Valdivia RH, Falkow S. FACS-optimized mutants of the green fluorescent protein (GFP) Gene. 1996;173:33–38. doi: 10.1016/0378-1119(95)00685-0. [DOI] [PubMed] [Google Scholar]
- Culotti J, Hartwell LH. Genetic control of the cell division cycle in yeast. III. Seven genes controlling nuclear division. Exp Cell Res. 1971;67:389–401. doi: 10.1016/0014-4827(71)90424-1. [DOI] [PubMed] [Google Scholar]
- DeMarini DJ, Adams AEM, Fares H, De Virgilio C, Valle G, Chuang JS, Pringle JR. A septin-based hierarchy of proteins required for localized deposition of chitin in the Saccharomyces cerevisiae cell wall. J Cell Biol. 1997;139:75–93. doi: 10.1083/jcb.139.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epp JA, Chant J. An IQGAP-related protein controls actin ring formation and cytokinesis in yeast. Curr Biol. 1997;7:921–929. doi: 10.1016/s0960-9822(06)00411-8. [DOI] [PubMed] [Google Scholar]
- Field CM, Al-Awar O, Rosenblatt J, Wong ML, Alberts B, Mitchison TJ. A purified Drosophila septin complex forms filaments and exhibits GTPase activity. J Cell Biol. 1996;133:605–616. doi: 10.1083/jcb.133.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger FP, Hughes TE, Novick P. Sec3p is a spatial landmark for polarized secretion in budding yeast. Cell. 1998;92:559–571. doi: 10.1016/s0092-8674(00)80948-4. [DOI] [PubMed] [Google Scholar]
- Finger FP, Novick P. Spatial regulation of exocytosis: lessons from yeast. J Cell Biol. 1998;142:609–612. doi: 10.1083/jcb.142.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flescher EG, Madden K, Snyder M. Components required for cytokinesis are important for bud site selection in yeast. J Cell Biol. 1993;122:373–386. doi: 10.1083/jcb.122.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flotow H, Graves PR, Wang A, Fiol CJ, Roeske RW, Roach PJ. Phosphate groups as substrate determinants for casein kinase I action. J Biol Chem. 1990;265:14264–14269. [PubMed] [Google Scholar]
- Flotow H, Roach PJ. Synergistic phosphorylation of rabbit muscle glycogen synthase by cyclic AMP-dependent protein kinase and casein kinase I: implications for hormonal regulation of glycogen synthase. J Biol Chem. 1989;264:9126–9128. [PubMed] [Google Scholar]
- Flotow H, Roach PJ. Role of acidic residues as substrate determinants for casein kinase I. J Biol Chem. 1991;266:3724–3727. [PubMed] [Google Scholar]
- Ford SK, Pringle JR. Cellular morphogenesis in the Saccharomyces cerevisiae cell cycle: localization of the CDC11 gene product and the timing of events at the budding site. Dev Genet. 1991;12:281–292. doi: 10.1002/dvg.1020120405. [DOI] [PubMed] [Google Scholar]
- Fujita A, Oka C, Arikawa Y, Katagai T, Tonouchi A, Kuhara S, Misumi Y. A yeast gene necessary for bud site selection encodes a protein similar to insulin-degrading enzymes. Nature. 1994;372:567–570. doi: 10.1038/372567a0. [DOI] [PubMed] [Google Scholar]
- Graves PR, Haas DW, Hagedorn CH, DePaoli-Roach AA, Roach PJ. Molecular cloning, expression, and characterization of a 49-kilodalton casein kinase I isoform from rat testis. J Biol Chem. 1993;268:6394–6401. [PubMed] [Google Scholar]
- Gross SD, Hoffman DP, Fisette PL, Baas P, Anderson RA. A phosphatidylinositol 4,5 bis phosphate-sensitive casein kinase Iα associates with synaptic vesicles and phosphorylates a subset of vesicle proteins. J Cell Biol. 1995;130:711–724. doi: 10.1083/jcb.130.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haarer BK, Pringle JR. Immunofluorescence localization of the Saccharomyces cerevisiae CDC12 gene product to the vicinity of the 10 nm filaments in the mother-bud neck. Mol Cell Biol. 1987;7:3678–3687. doi: 10.1128/mcb.7.10.3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halme A, Michelitch M, Mitchell EL, Chant J. Bud10p directs axial cell polarization in budding yeast and resembles a transmembrane receptor. Curr Biol. 1996;6:570–579. doi: 10.1016/s0960-9822(02)00543-2. [DOI] [PubMed] [Google Scholar]
- Healy AM, Zolnierowicz S, Stapleton AE, Goebl M, DePaoli-Roach AA, Pringle JR. CDC55, a Saccharomyces cerevisiae gene involved in cellular morphogenesis: identification, characterization, and homology to the B subunit of mammalian type 2A protein phosphatase. Mol Cell Biol. 1991;11:5767–5780. doi: 10.1128/mcb.11.11.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim R, Cubitt AB, Tsien RY. Improved green fluorescence. Nature. 1995;373:663–664. doi: 10.1038/373663b0. [DOI] [PubMed] [Google Scholar]
- Hicke L, Zanolari B, Riezman H. Cytoplasmic tail phosphorylation of the α-factor receptor is required for its ubiquitination and internalization. J Cell Biol. 1998;141:349–358. doi: 10.1083/jcb.141.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra MF, Dhillon N, Carmel G, DeMaggio AJ, Lindberg RA, Hunter T, Kuret J. Budding and fission yeast casein kinase I isoforms have dual-specificity protein kinase activity. Mol Biol Cell. 1994;5:877–886. doi: 10.1091/mbc.5.8.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra MF, Liskay RM, Ou AC, DeMaggio AJ, Burbee DJ, Heffron F. HRR25, a putative protein kinase from budding yeast: association with repair of damaged DNA. Science. 1991;253:1031–1034. doi: 10.1126/science.1887218. [DOI] [PubMed] [Google Scholar]
- Ito H, Fukuda Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DI, Jacobs CW, Pringle JR, Robinson LC, Carle GF, Olson MV. Mapping of the Saccharomyces cerevisiae CDC3, CDC25, and CDC42 genes to chromosome XII by chromosome blotting and tetrad analysis. Yeast. 1987;3:243–253. doi: 10.1002/yea.320030405. [DOI] [PubMed] [Google Scholar]
- Kaiser CA, Gimeno RE, Shaywitz DA. Protein secretion, membrane biogenesis, and endocytosis. In: Pringle JR, Broach JR, Jones EW, editors. The Molecular and Cellular Biology of the Yeast Saccharomyces, Cell Cycle and Cell Biology. Plainview, NY: Cold Spring Harbor Laboratory; 1997. pp. 91–227. [Google Scholar]
- Kilmartin JV, Adams AEM. Structural rearrangements of tubulin and actin during the cell cycle of the yeast Saccharomyces. J Cell Biol. 1984;98:922–933. doi: 10.1083/jcb.98.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HB, Haarer BK, Pringle JR. Cellular morphogenesis in the Saccharomyces cerevisiae cell cycle: localization of the CDC3 gene product and the timing of events at the budding site. J Cell Biol. 1991;112:535–544. doi: 10.1083/jcb.112.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimczak LJ, Cashmore AR. Purification and characterization of casein kinase I from broccoli. Biochem J. 1993;293:283–288. doi: 10.1042/bj2930283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie SH, Brown SS. Immunofluorescence localization of the unconventional myosin, Myo2p, and the putative kinesin-related protein, Smy1p, to the same regions of polarized growth in Saccharomyces cerevisiae. J Cell Biol. 1994;125:825–842. doi: 10.1083/jcb.125.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott J, Li R. Sequential assembly of myosin II, an IQGAP-like protein, and filamentous actin to a ring structure involved in budding yeast cytokinesis. J Cell Biol. 1998;140:355–366. doi: 10.1083/jcb.140.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HP, Bretscher A. Characterization of TPM1 disrupted yeast cells indicates an involvement of tropomyosin in directed vesicular transport. J Cell Biol. 1992;118:285–299. doi: 10.1083/jcb.118.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine MS, DeMarini DJ, Valencik ML, Al-Awar OS, Fares H, De Virgilio C, Pringle JR. The septins: roles in cytokinesis and other processes. Curr Opin Cell Biol. 1996;8:106–119. doi: 10.1016/s0955-0674(96)80054-8. [DOI] [PubMed] [Google Scholar]
- Maniatis T, Fritsch EF, Sambrook J. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- Meggio F, Perich JW, Reynolds EC, Pinna LA. A synthetic β-casein phosphopeptide and analogues as model substrates for casein kinase-1, a ubiquitous, phosphate-directed protein kinase. FEBS Lett. 1991;283:303–306. doi: 10.1016/0014-5793(91)80614-9. [DOI] [PubMed] [Google Scholar]
- Munn AL, Stevenson BJ, Geli MI, Riezman H. end5, end6, and end7: mutations that cause actin delocalization and block the internalization step of endocytosis in Saccharomyces cerevisiae. Mol Biol Cell. 1995;6:1721–1742. doi: 10.1091/mbc.6.12.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlean P. Biogenesis of yeast cell wall and surface components. In: Pringle JR, Broach JR, Jones EW, editors. The Molecular and Cellular Biology of the Yeast Saccharomyces, Cell Cycle and Cell Biology. Plainview, NY: Cold Spring Harbor Laboratory; 1997. pp. 229–362. [Google Scholar]
- Panek HR, Stepp JD, Engle HM, Marks KM, Tan PK, Lemmon SK, Robinson LC. Suppressors of YCK-encoded yeast casein kinase 1 deficiency define the four subunits of a novel clathrin AP-like complex. EMBO J. 1997;16:4194–4204. doi: 10.1093/emboj/16.14.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle JR. Staining of bud scars and other cell wall chitin with calcofluor. Methods Enzymol. 1991;194:732–735. doi: 10.1016/0076-6879(91)94055-h. [DOI] [PubMed] [Google Scholar]
- Pringle JR, Preston RA, Adams AEM, Stearns T, Drubin DG, Haarer BK, Jones EW. Fluorescence microscopy methods for yeast. Methods Cell Biol. 1989;31:357–435. doi: 10.1016/s0091-679x(08)61620-9. [DOI] [PubMed] [Google Scholar]
- Richardson HE, Wittenberg C, Cross FR, Reed SI. An essential G1 function for cyclin-like proteins in yeast. Cell. 1989;59:1127–1133. doi: 10.1016/0092-8674(89)90768-x. [DOI] [PubMed] [Google Scholar]
- Roach PJ. Control of glycogen synthase by hierarchal protein phosphorylation. FASEB J. 1990;4:2961–2968. [PubMed] [Google Scholar]
- Robinson LC, et al. Yeast casein kinase I homologues: an essential gene pair. Proc Natl Acad Sci USA. 1992;89:28–32. doi: 10.1073/pnas.89.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson LC, Menold MM, Garrett S, Culbertson MR. Casein kinase I-like protein kinases encoded by YCK1 and YCK2 are required for yeast morphogenesis. Mol Cell Biol. 1993;13:2870–2881. doi: 10.1128/mcb.13.5.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roemer T, Madden K, Chang J, Snyder M. Selection of axial growth sites in yeast requires Axl2p, a novel plasma membrane glycoprotein. Genes Dev. 1996;10:777–793. doi: 10.1101/gad.10.7.777. [DOI] [PubMed] [Google Scholar]
- Roncero C, Valdivieso MH, Ribas JC, Durán A. Isolation and characterization of Saccharomyces cerevisiae mutants resistant to Calcofluor White. J Bacteriol. 1988;170:1950–1954. doi: 10.1128/jb.170.4.1950-1954.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein RJ. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–210. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Rowles J, Slaughter C, Moomaw C, Hsu J, Cobb MH. Purification of casein kinase I and isolation of cDNAs encoding multiple casein kinase I-like enzymes. Proc Natl Acad Sci USA. 1991;88:9548–9552. doi: 10.1073/pnas.88.21.9548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki RK, Gelfand DH, Stoffel S, Scharf SJ, Higuchi R, Horn GT, Mullis KB, Erlich HA. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988;239:487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanders SL, Herskowitz I. The Bud4 protein of yeast, required for axial budding, is localized to the mother/bud neck in a cell cycle-dependent manner. J Cell Biol. 1996;134:413–427. doi: 10.1083/jcb.134.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos B, Snyder M. Targeting of chitin synthase 3 to polarized growth sites in yeast requires Chs5p and Myo2p. J Cell Biol. 1997;136:95–110. doi: 10.1083/jcb.136.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer B, Philippsen P. CDC15, an essential cell cycle gene in Saccharomyces cerevisiae, encodes a protein kinase domain. Yeast. 1991;7:265–273. doi: 10.1002/yea.320070308. [DOI] [PubMed] [Google Scholar]
- Sherman F, Fink GR, Hicks JB. Methods in Yeast Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1986. [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M. The SPA2 protein of yeast localizes to sites of cell growth. J Cell Biol. 1989;108:1419–1429. doi: 10.1083/jcb.108.4.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RG, Walker DC, McInnes RR. E. coli host strains significantly affect the quality of small scale plasmid DNA preparations used for sequencing. Nucleic Acids Res. 1993;21:1677–1678. doi: 10.1093/nar/21.7.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TerBush DR, Novick P. Sec6, Sec8 and Sec15 are components of a multisubunit complex which localizes to small bud tips in Saccharomyces cerevisiae. J Cell Biol. 1995;130:299–312. doi: 10.1083/jcb.130.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuazon PT, Traugh JA. Casein kinase I and II-multipotential serine kinases: structure, function and regulation. Adv Second Messenger Phosphoprotein Res. 1991;23:123–164. [PubMed] [Google Scholar]
- Vancura A, O’Connor A, Patterson SD, Mirza U, Chait BT, Kuret J. Isolation and properties of YCK2, a Saccharomyces cerevisiae homolog of casein kinase I. Arch Biochem Biophys. 1993;305:47–53. doi: 10.1006/abbi.1993.1391. [DOI] [PubMed] [Google Scholar]
- Vancura A, Sessler A, Leichus B, Kuret J. A prenylation motif is required for plasma membrane localization and biochemical function of casein kinase I in budding yeast. J Biol Chem. 1994;269:19271–19278. [PubMed] [Google Scholar]
- Waddle JA, Karpova TS, Waterston RH, Cooper JA. Movement of cortical actin patches in yeast. J Cell Biol. 1996;132:861–870. doi: 10.1083/jcb.132.5.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P-C, Vancura A, Mitcheson TG, Kuret J. Two genes in Saccharomyces cerevisiae encode a membrane bound form of casein kinase-1. Mol Biol Cell. 1992;3:275–286. doi: 10.1091/mbc.3.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Hoekstra MF, DeMaggio AJ, Dhillon N, Vancura A, Kuret J, Johnston GC, Singer RA. Prenylated isoforms of yeast casein kinase I, including the novel Yck3p, suppress the gcs1 blockage of cell proliferation from stationary phase. Mol Cell Biol. 1996;16:5375–5385. doi: 10.1128/mcb.16.10.5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Ayscough KR, Drubin DG. A role for the actin cytoskeleton of Saccharomyces cerevisiae in bipolar bud-site selection. J Cell Biol. 1997;136:111–123. doi: 10.1083/jcb.136.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahner JE, Harkins HA, Pringle JR. Genetic analysis of the bipolar pattern of bud site selection in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:1857–1870. doi: 10.1128/mcb.16.4.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai L, Graves PR, Robinson LC, Italiano M, Culbertson MR, Rowles J, Cobb MH, DePaoli-Roach AA, Roach PJ. Casein kinase Iγ subfamily: molecular cloning, expression and characterization of three mammalian isoforms, and complementation of defects in the Saccharomyces cerevisiae YCK genes. J Biol Chem. 1995;270:12717–12724. doi: 10.1074/jbc.270.21.12717. [DOI] [PubMed] [Google Scholar]