Abstract
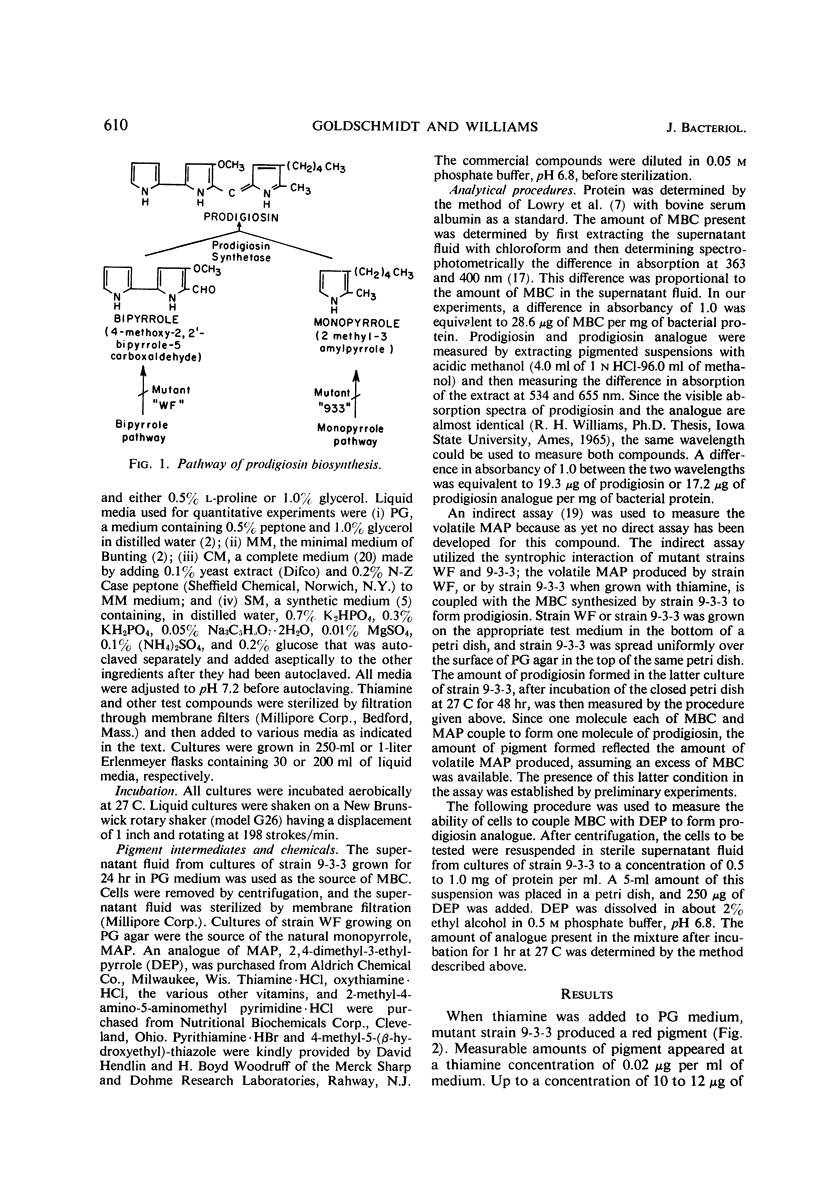
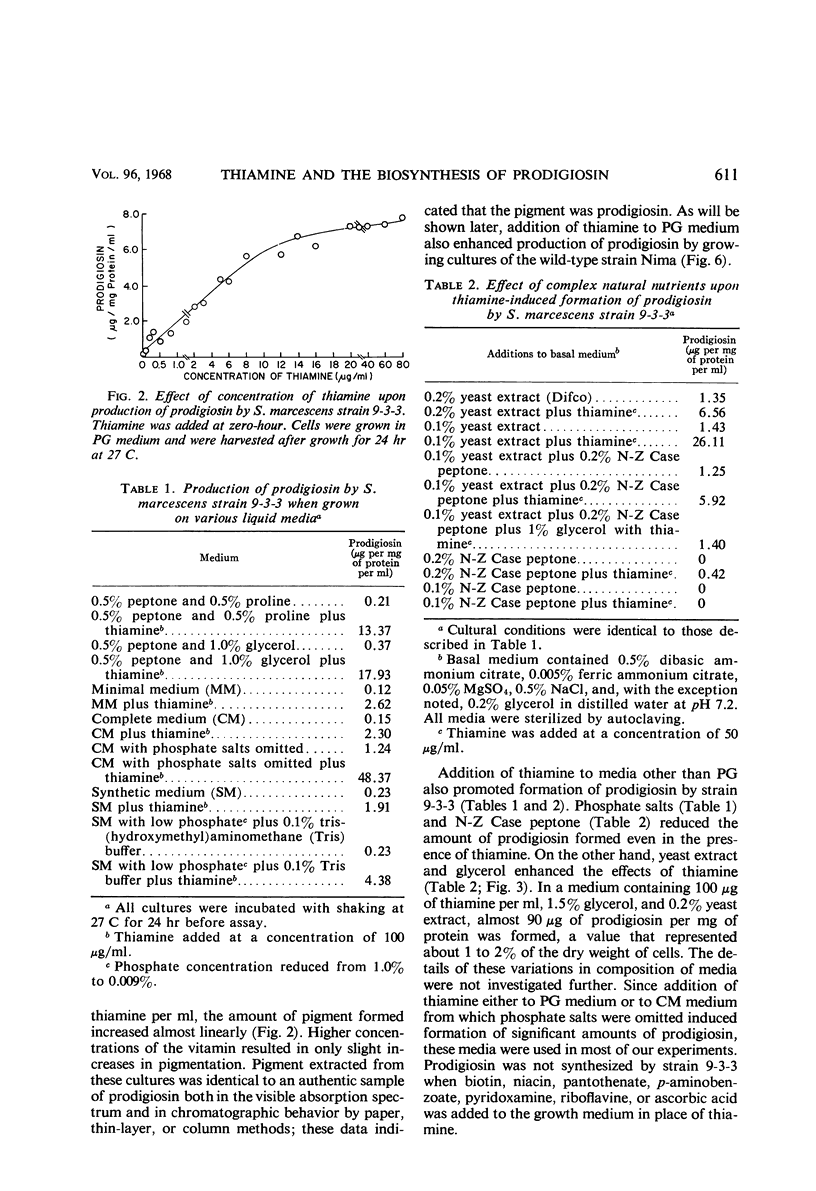
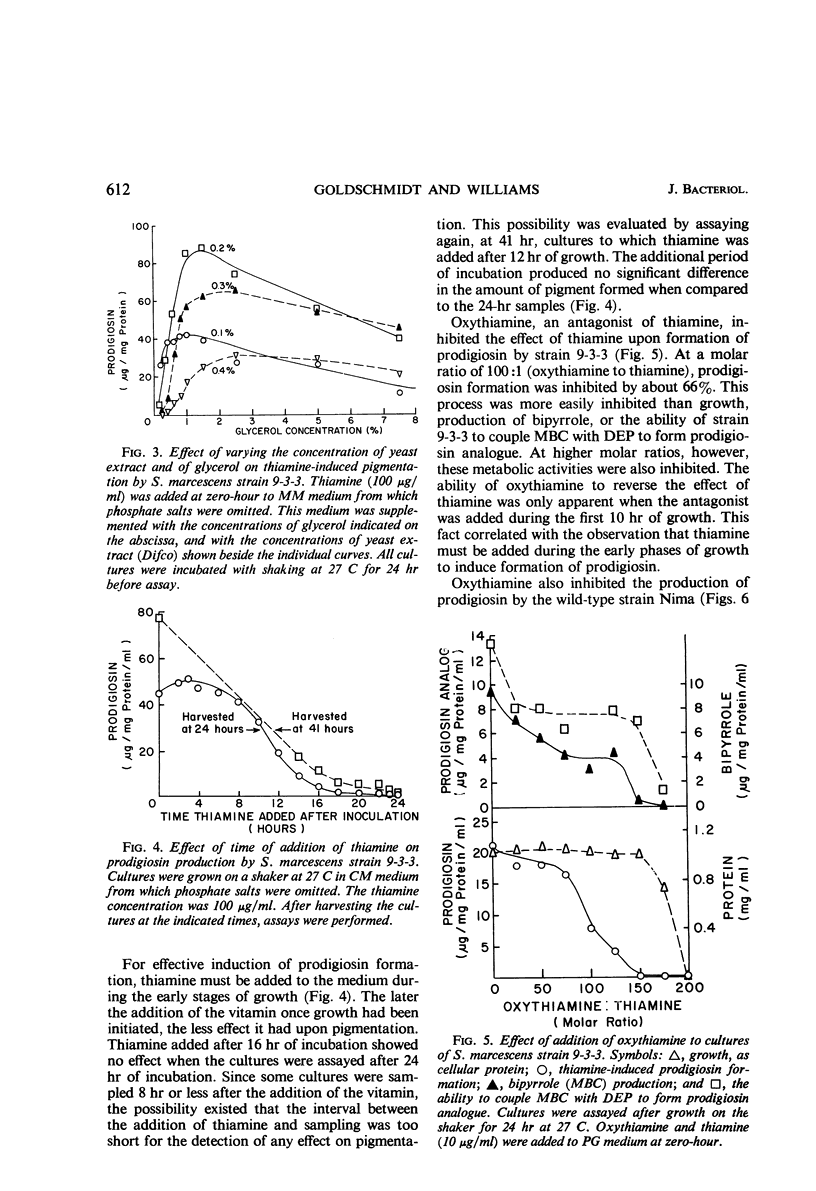
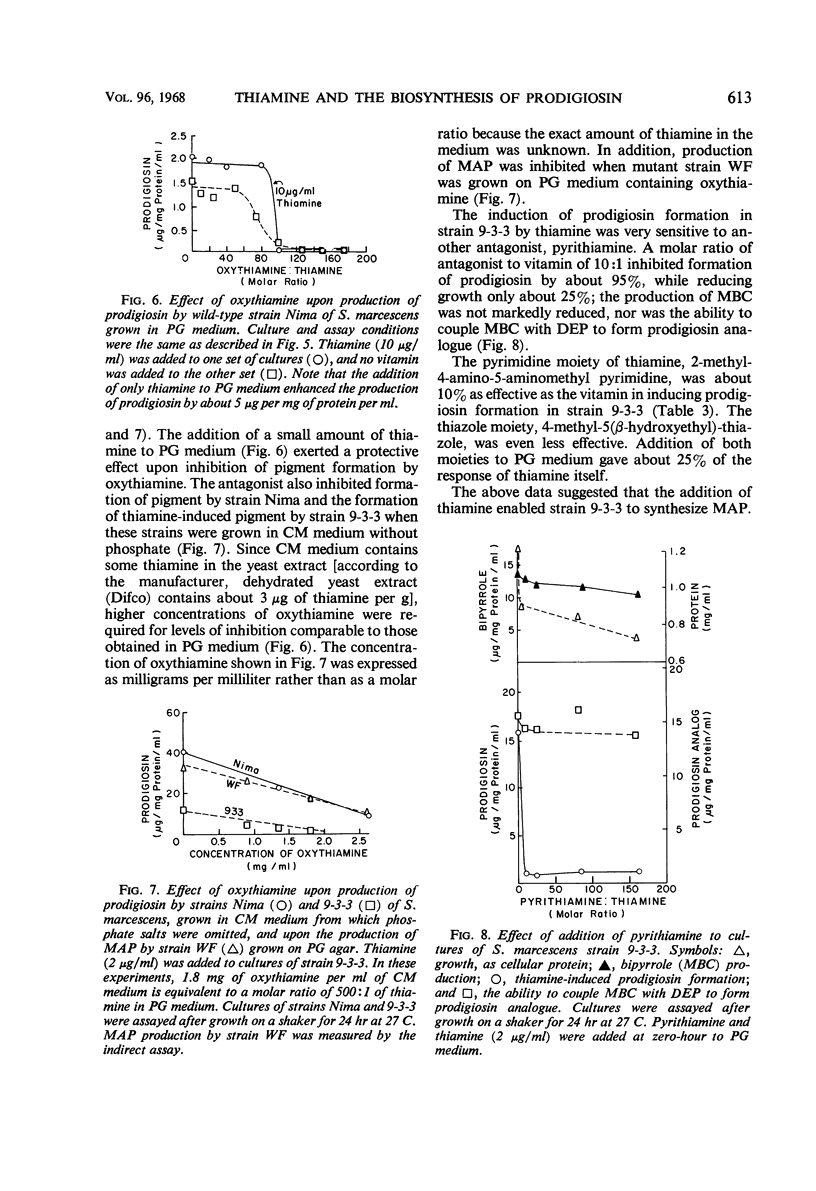
Thiamine stimulates the production of a red pigment, which is chromatographically and spectrophotometrically identical to prodigiosin, by growing cultures of Serratia marcescens mutant 9-3-3. This mutant is blocked in the formation of 2-methyl-3-amylpyrrole (MAP), the monopyrrole moiety of prodigiosin, but accumulates 4-methoxy-2,2,′-bipyrrole-5-carboxaldehyde (MBC) and can couple this compound with MAP to form prodigiosin. Addition of thiamine caused production of MAP, and as little as 0.02 mg of thiamine per ml in a peptone-glycerol medium stimulated production of measurable amounts of prodigiosin. Phosphate salts and another type of peptone decreased the thiamine-induced formation of prodigiosin; yeast extract and glycerol enhanced the formation of this substance. Thiamine also enhanced production of prodigiosin by wild-type strain Nima of S. marcescens. The thiamine antagonists, oxythiamine and pyrithiamine, inhibited thiamine-induced production of MAP and of prodigiosin by the mutant strain 9-3-3, formation of prodigiosin by the wild-type strain Nima, and production of MAP by another mutant, strain WF. The pyrimidine moiety of thiamine was only 10% as effective as the vitamin; the thiazole moiety, only 4%; and the two moieties together, 25%. Various other vitamins tested did not stimulate formation of prodigiosin by strain 9-3-3. Thiamine did not stimulate production of prodigiosin by a single-step mutant that showed the same phenotypic block in prodigiosin biosynthesis as strain 9-3-3. This is not surprising since strain 9-3-3 originated as a result of two mutational events. One event may involve thiamine directly, and the other may involve the biosynthesis of MAP. Thiamine is probably involved in the regulation of the biosynthesis of MAP, because the vitamin or inhibitory antagonists must be added during the early phases of growth in order to be effective.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bunting M. I. A Description of Some Color Variants Produced by Serratia marcescens, Strain 274. J Bacteriol. 1940 Jul;40(1):57–68. doi: 10.1128/jb.40.1.57-68.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunting M. I., Robinow C. F., Bunting H. FACTORS AFFECTING THE ELABORATION OF PIGMENT AND POLYSACCHARIDE BY SERRATIA MARCESCENS. J Bacteriol. 1949 Jul;58(1):114–115. [PMC free article] [PubMed] [Google Scholar]
- GREEN J. A., WILLIAMS R. P. Studies on pigmentation of Serratia marcescens. IV. Analysis of syntrophic pigment. J Bacteriol. 1957 Nov;74(5):633–636. doi: 10.1128/jb.74.5.633-636.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas F. L., Doudney C. O. A RELATION OF NUCLEIC ACID SYNTHESIS TO RADIATION-INDUCED MUTATION FREQUENCY IN BACTERIA. Proc Natl Acad Sci U S A. 1957 Oct 15;43(10):871–883. doi: 10.1073/pnas.43.10.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes D. W., Goldschmidt M. E., Cash H. P., Williams R. P. Production of purple pigment by a mutant of Serratia marcescens. Tex Rep Biol Med. 1966 Fall;24(3):489–493. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marks G. S., Bogorad L. STUDIES ON THE BIOSYNTHESIS OF PRODIGIOSIN IN SERRATIA MARCESCENS. Proc Natl Acad Sci U S A. 1960 Jan;46(1):25–28. doi: 10.1073/pnas.46.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. A. Prodigiosin synthesis in mutants of Serratia marcesens. J Bacteriol. 1966 Apr;91(4):1599–1604. doi: 10.1128/jb.91.4.1599-1604.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee P. P., Goldschmidt M. E., Williams R. P. Enzymic formation of prodigiosin analog by a cell-free preparation from Serratia marcescens. Biochim Biophys Acta. 1967 Feb 7;136(1):182–184. doi: 10.1016/0304-4165(67)90342-x. [DOI] [PubMed] [Google Scholar]
- SANTER U. V., VOGEL H. J. Prodigiosin synthesis in Serratia marcescens: isolation of a pyrrole-containing precursor. Biochim Biophys Acta. 1956 Mar;19(3):578–579. doi: 10.1016/0006-3002(56)90500-5. [DOI] [PubMed] [Google Scholar]
- WASSERMAN H. H., McKEON J. E., SANTER U. V. Studies related to the biosynthesis of prodigiosin in Serratia marcescens. Biochem Biophys Res Commun. 1960 Aug;3:146–149. doi: 10.1016/0006-291x(60)90211-4. [DOI] [PubMed] [Google Scholar]
- WILLIAMS R. P., GOLDSCHMIDT M. E., GOTT C. L. INHIBITION BY TEMPERATURE OF THE TERMINAL STEP IN BIOSYNTHESIS OF PRODIGIOSIN. Biochem Biophys Res Commun. 1965 Apr 9;19:177–181. doi: 10.1016/0006-291x(65)90500-0. [DOI] [PubMed] [Google Scholar]
- WILLIAMS R. P., GREEN J. A., RAPPO-PORT D. A. Studies on pigmentation of Serratia marcescens. I. Spectral and paper chromatographic properties of prodigiosin. J Bacteriol. 1956 Jan;71(1):115–120. doi: 10.1128/jb.71.1.115-120.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS R. P., SESSUMS J. H. Catalase activity and pigmentation in Serratia marcescens. Tex Rep Biol Med. 1959;17(2):259–266. [PubMed] [Google Scholar]
- Wasserman H. H., Friedland D. J., Morrison D. A. A novel dipyrrolyldipyrromethene prodigiosin analog from Serratia marcescens. Tetrahedron Lett. 1968 Feb;6:641–644. doi: 10.1016/s0040-4039(00)75602-4. [DOI] [PubMed] [Google Scholar]
- Williams R. P., Gott C. L. Inhibition by streptomycin of the biosynthesis of prodigiosin. Biochem Biophys Res Commun. 1964 May 22;16(1):47–52. doi: 10.1016/0006-291x(64)90209-8. [DOI] [PubMed] [Google Scholar]


