Abstract
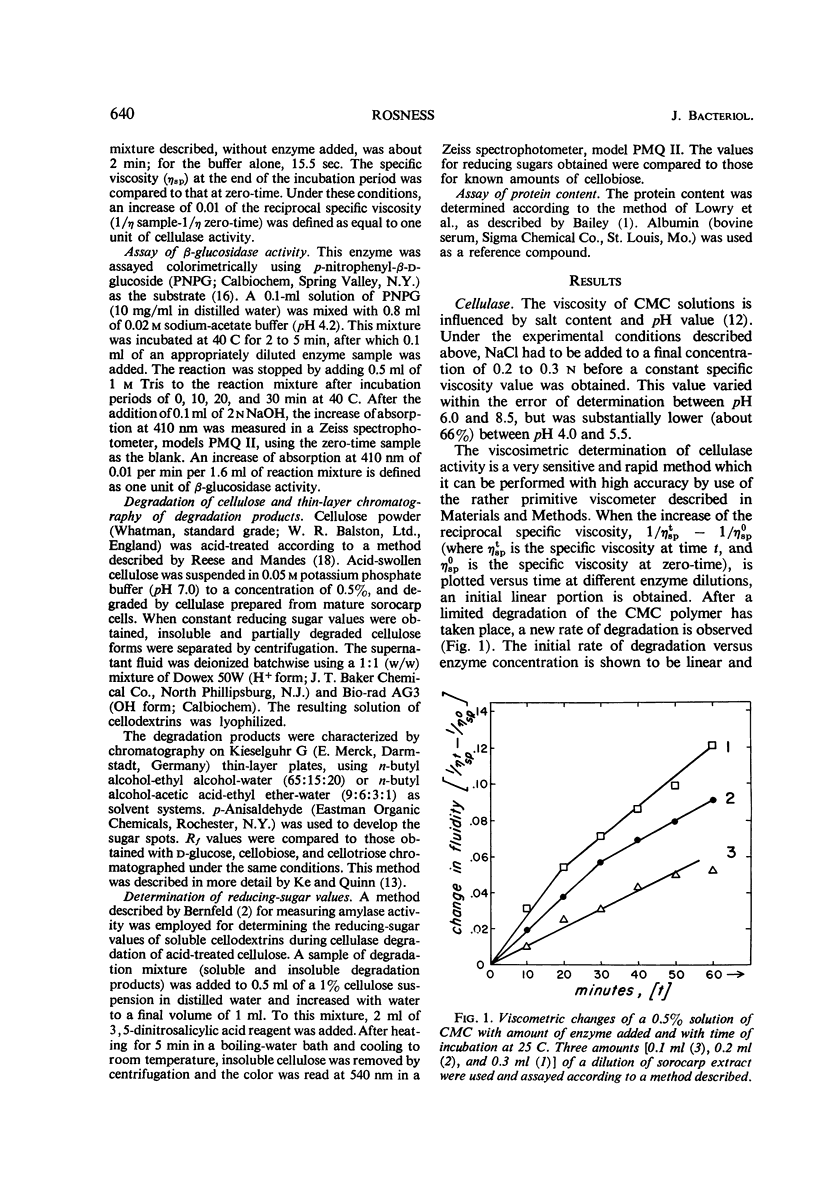
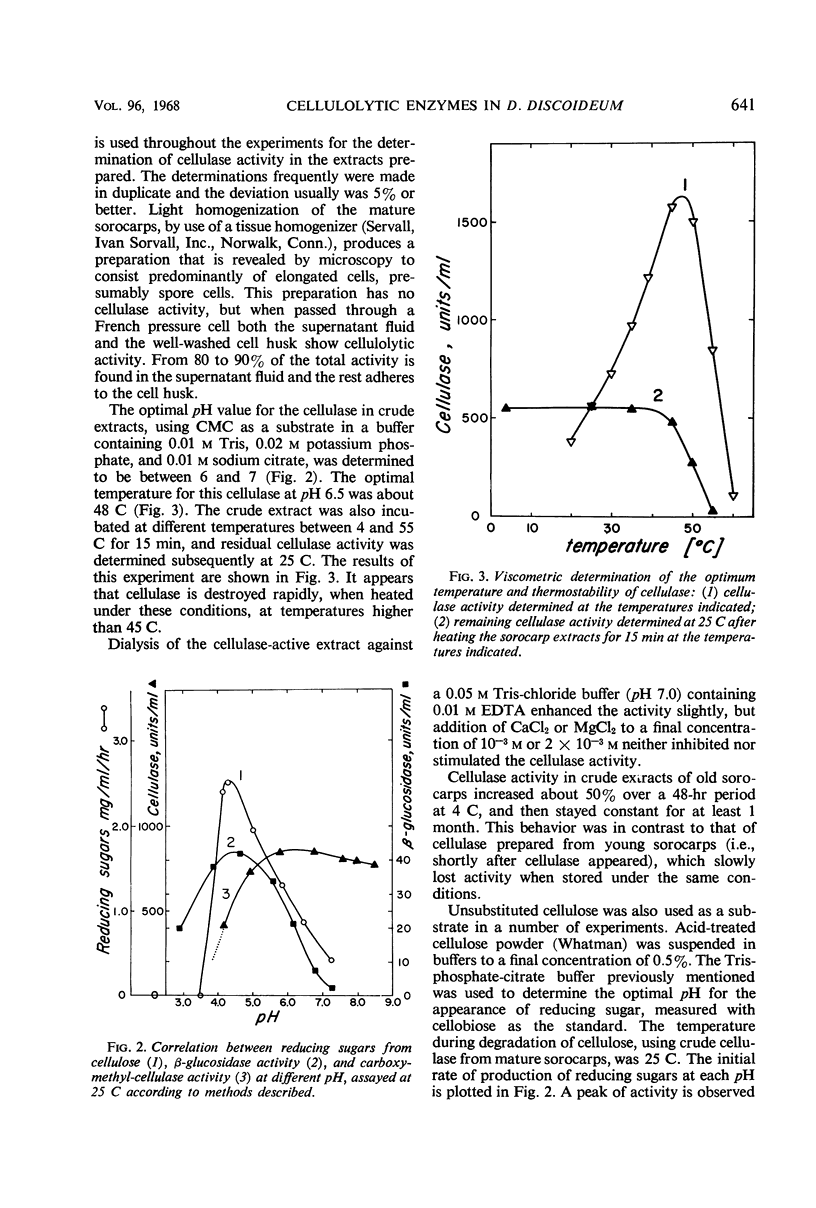
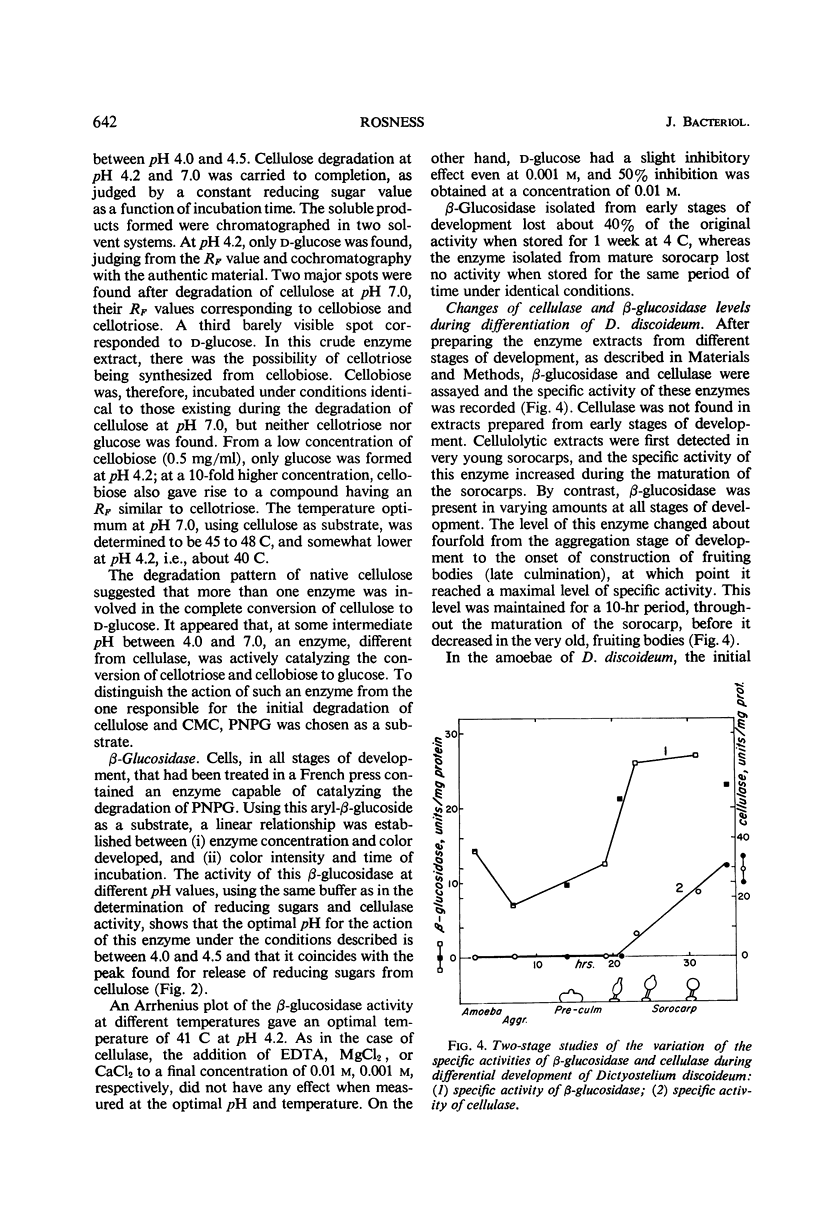
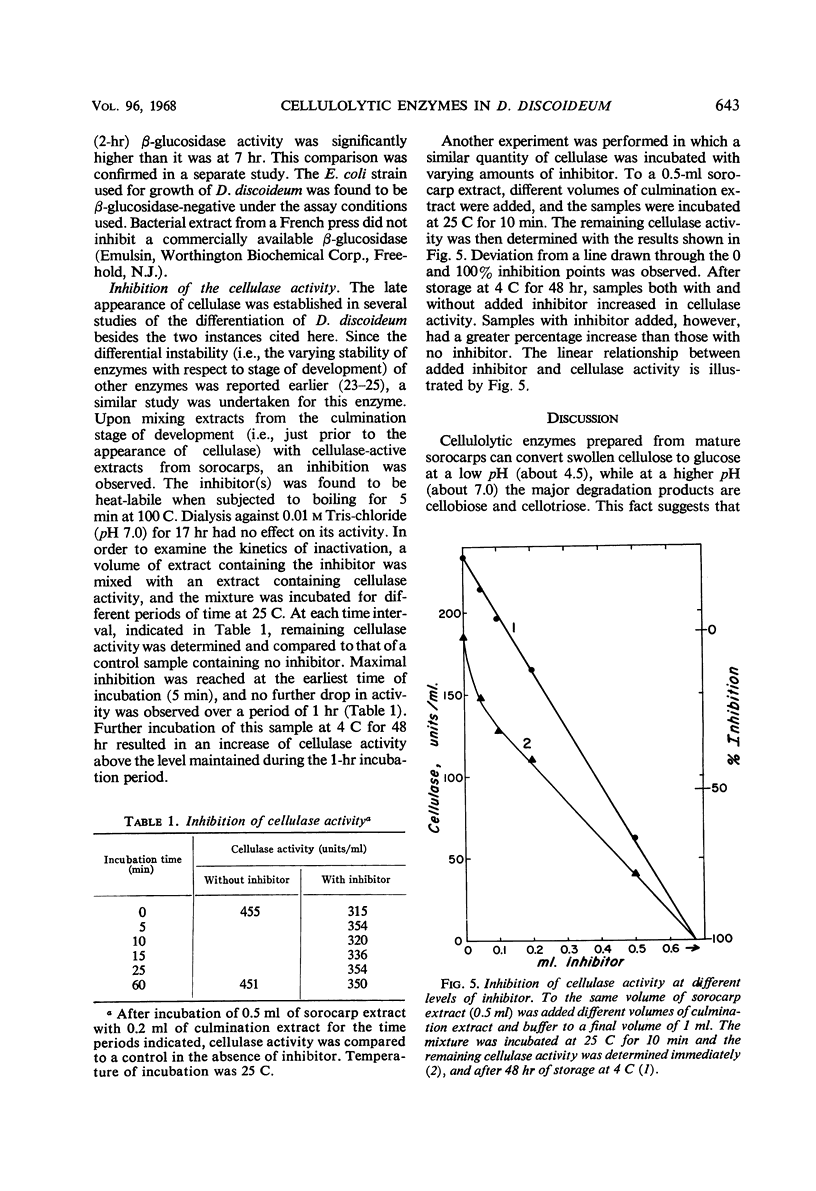
Extracts from Dictyostelium discoideum at the sorocarp stage of development catalyzed the degradation of acid-swollen cellulose to d-glucose at an optimal pH of about 4.5; cellobiose and cellotriose were the major products from this degradation at pH 7.0. The optimal temperature at pH 7.0, when swollen cellulose was used as substrate, was about 45 to 48 C, and was somewhat lower at pH 4.2 to 4.5, i.e., about 40 C. To account for this degradation pattern, two types of enzymes have been characterized, cellulase(s) and β-glucosidase. With carboxymethyl cellulose as substrate, cellulase activity was not found in extracts prior to the sorocarp stage of development, but increased rapidly during aging of the sorocarp. Evidence is presented that at earlier stages of development the cellulase(s) is present in an inactive form. An inhibitor found in these extracts is heat-labile and probably of protein nature. The β-glucosidase is present at all stages of development and the specific activity changes about fourfold, the highest activity occurring during the culmination and sorocarp stages of development.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CESKA M., FISHER J. R. Arginase activity. I. Partial purification and characterization of a stimulatory agent. Arch Biochem Biophys. 1960 Oct;90:288–293. doi: 10.1016/0003-9861(60)90581-6. [DOI] [PubMed] [Google Scholar]
- Ceccarini C. Trehalase from Dictyostelium discoideum: purification and properties. Science. 1966 Jan 28;151(3709):454–456. doi: 10.1126/science.151.3709.454. [DOI] [PubMed] [Google Scholar]
- Cotter D. A., Raper K. B. Spore germination in Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1966 Sep;56(3):880–887. doi: 10.1073/pnas.56.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEZELIUS K., RANBY B. G. Morphology and fine structure of the slime mold Dictyostelium discoideum. Exp Cell Res. 1957 Apr;12(2):265–289. doi: 10.1016/0014-4827(57)90141-6. [DOI] [PubMed] [Google Scholar]
- GEZELIUS K., WRIGHT B. E. ALKALINE PHOSPHATASE IN DICTYOSTELIUM DISCOIDEUM. J Gen Microbiol. 1965 Mar;38:309–327. doi: 10.1099/00221287-38-3-309. [DOI] [PubMed] [Google Scholar]
- GRAY J. L., PRIEST S. G., BLATT W. F., WESTPHAL U., JENSEN H. Isolation and characterization of a proteolytic inhibitor from bovine blood. J Biol Chem. 1960 Jan;235:56–59. [PubMed] [Google Scholar]
- IWASAKI T., TOKUYASU K., FUNATSU M. DETERMINATION OF CELLULASE ACTIVITY EMPLOYING GLYCOL CELLULOSE AS A SUBSTRATE. J Biochem. 1964 Jan;55:30–36. doi: 10.1093/oxfordjournals.jbchem.a127837. [DOI] [PubMed] [Google Scholar]
- LIDDEL G. U., WRIGHT B. E. The effect of glucose on respiration of the differentiating slime mold. Dev Biol. 1961 Jun;3:265–276. doi: 10.1016/0012-1606(61)90047-1. [DOI] [PubMed] [Google Scholar]
- Liu T. H., King K. W. Fragmentation during enzymic degradation of cellulose. Arch Biochem Biophys. 1967 May;120(2):462–463. doi: 10.1016/0003-9861(67)90265-2. [DOI] [PubMed] [Google Scholar]
- MAHADEVAN P. R., EBERHART B. THE BETA-GLUCOSIDASE SYSTEM OF NEUROSPORA CRASSA. II. PURIFICATION AND CHARACTERIZATION OF ARYL BETA-GLUCOSIDASE. Arch Biochem Biophys. 1964 Oct;108:22–29. doi: 10.1016/0003-9861(64)90350-9. [DOI] [PubMed] [Google Scholar]
- REESE E. T., SIU R. G. H., LEVINSON H. S. The biological degradation of soluble cellulose derivatives and its relationship to the mechanism of cellulose hydrolysis. J Bacteriol. 1950 Apr;59(4):485–497. doi: 10.1128/jb.59.4.485-497.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rackis J. J., Anderson R. L. Isolation of four soybean trypsin inhibitors by DEAE-cellulose chromatography. Biochem Biophys Res Commun. 1964 Mar 26;15(3):230–235. doi: 10.1016/0006-291x(64)90151-2. [DOI] [PubMed] [Google Scholar]
- SWARTZ M. N., KAPLAN N. O., FRECH M. E. Significance of heat-activated enzymes. Science. 1956 Jan 13;123(3185):50–53. doi: 10.1126/science.123.3185.50. [DOI] [PubMed] [Google Scholar]
- Wright B. E., Dahlberg D. Stability in vitro of uridine diphosphoglucose pyrophosphorylase in Dictyostelium discoideum. J Bacteriol. 1968 Mar;95(3):983–985. doi: 10.1128/jb.95.3.983-985.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright B. E. Multiple causes and controls in differentiation. Science. 1966 Aug 19;153(3738):830–837. doi: 10.1126/science.153.3738.830. [DOI] [PubMed] [Google Scholar]
- Wright B. E. ON ENZYME-SUBSTRATE RELATIONSHIPS DURING BIOCHEMICAL DIFFERENTIATION. Proc Natl Acad Sci U S A. 1960 Jun;46(6):798–803. doi: 10.1073/pnas.46.6.798. [DOI] [PMC free article] [PubMed] [Google Scholar]


