Abstract
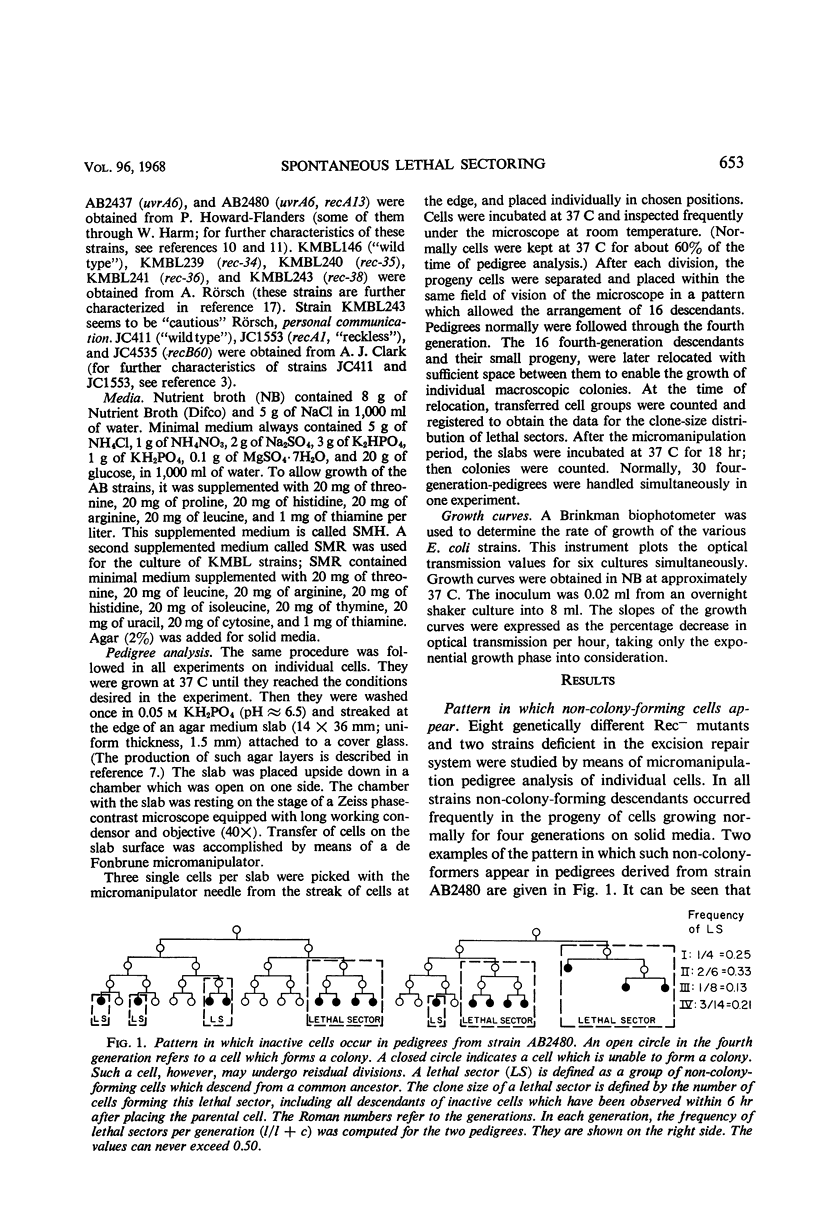
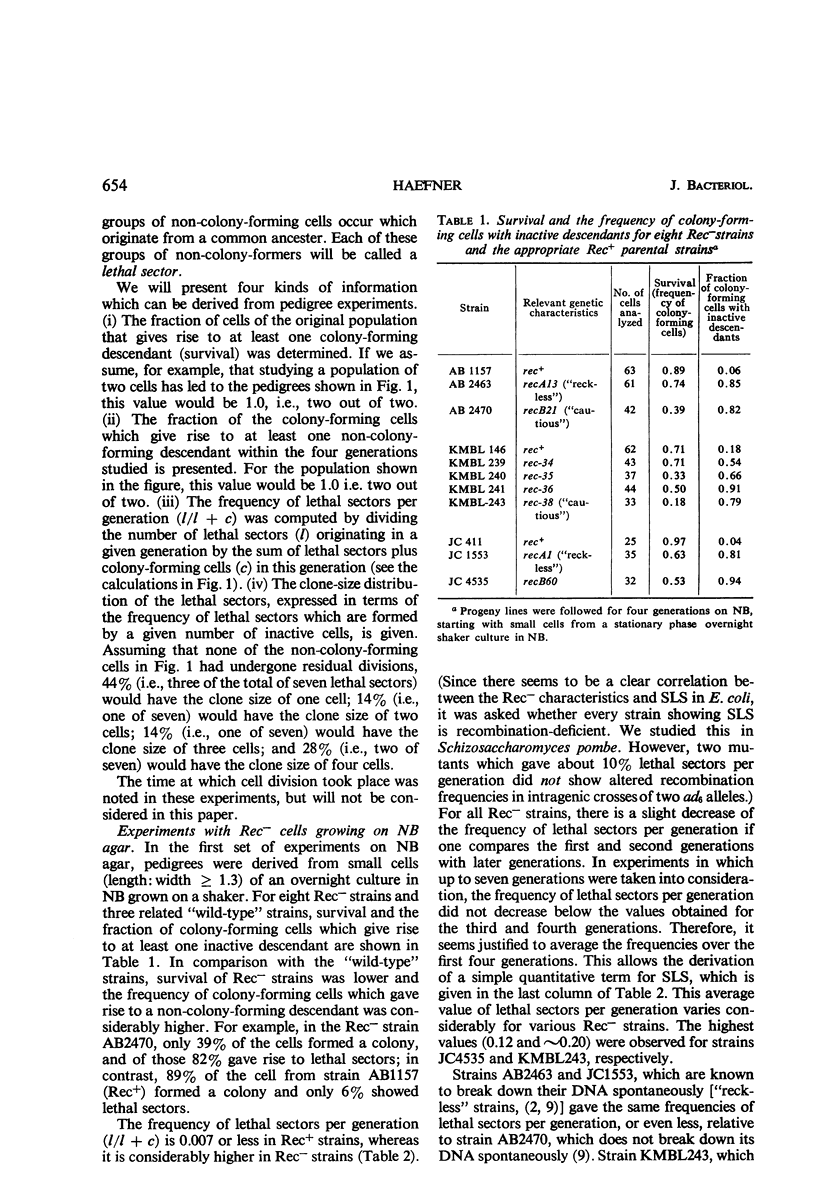
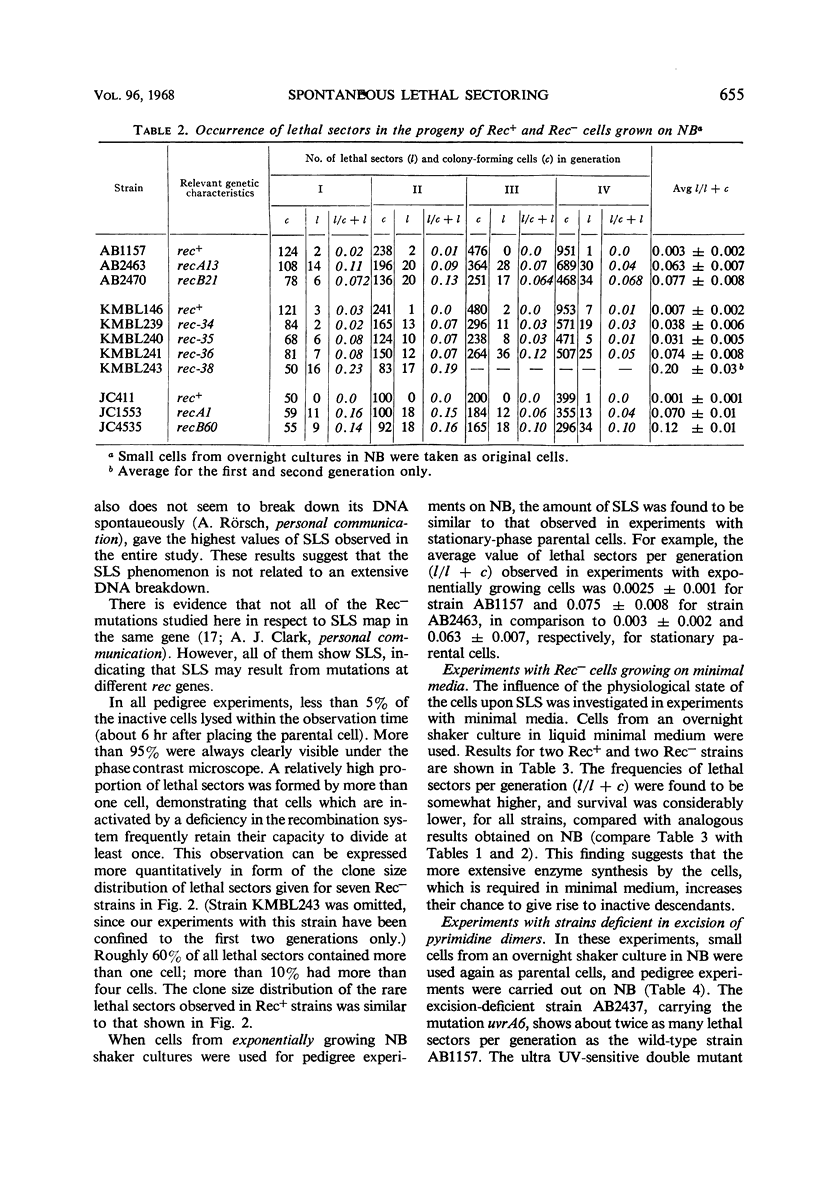
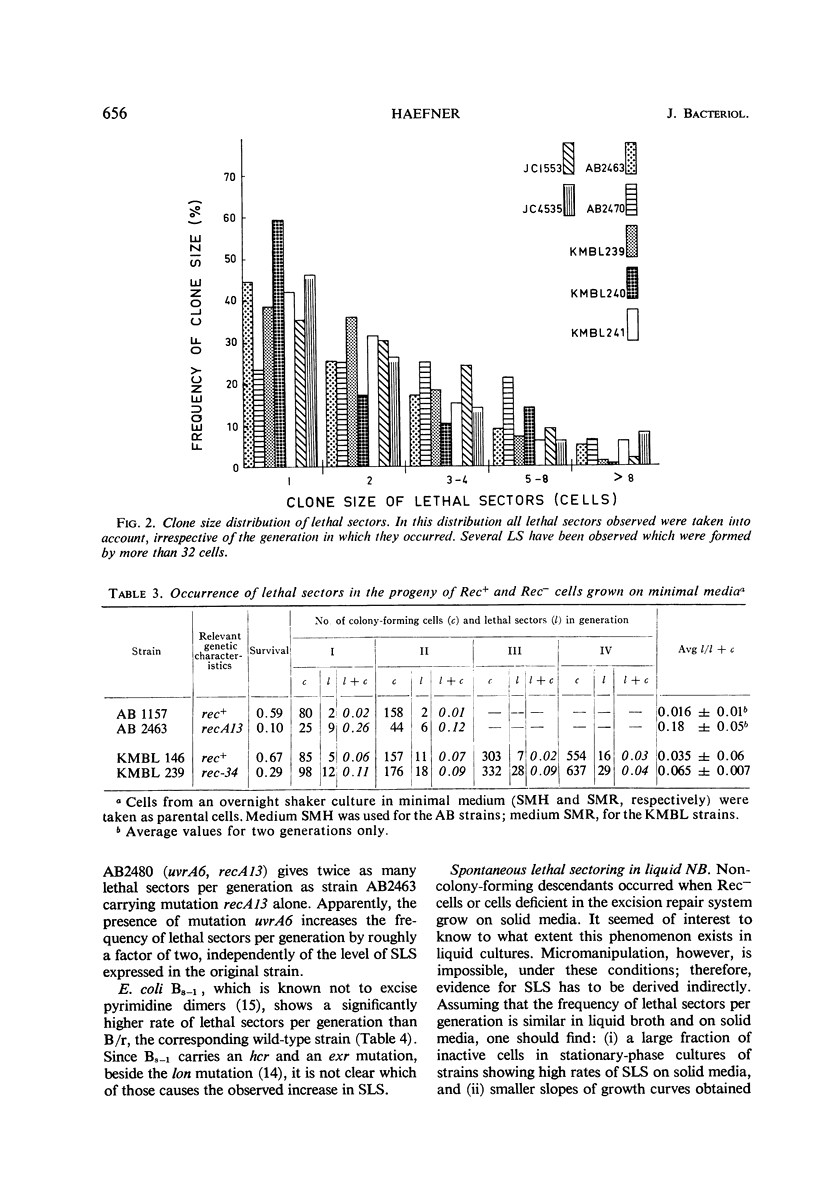
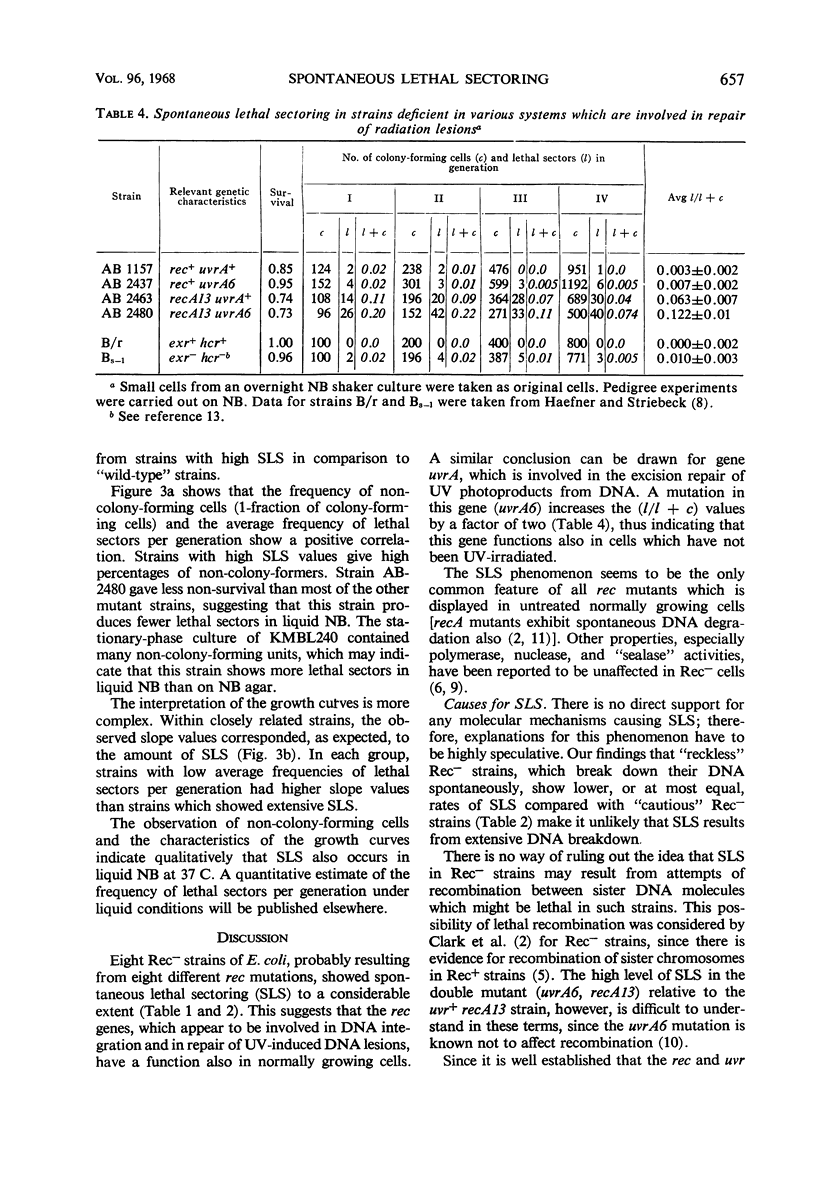
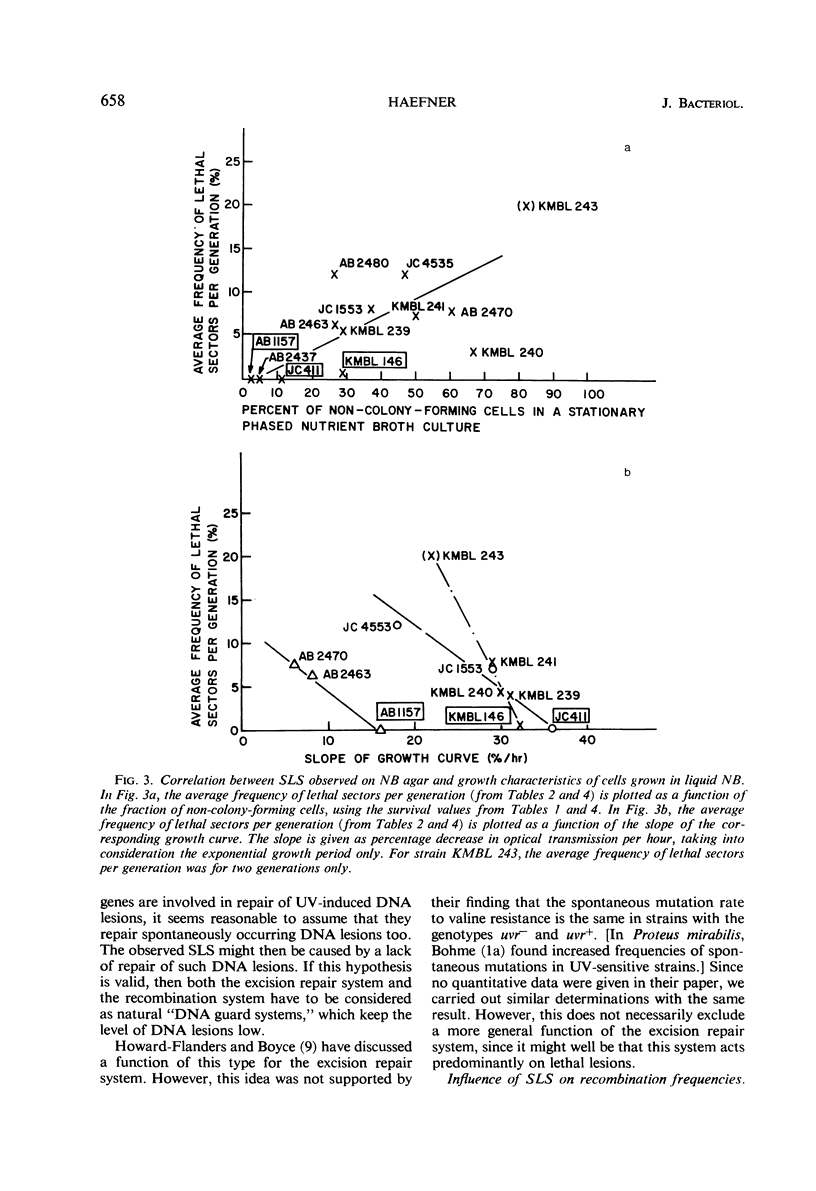
Eight recombination-deficient (Rec−) mutants of Escherichia coli were studied. Progeny lines were obtained on solid media, by means of micromanipulation, and the colony-forming ability of individual cells was analyzed. Cells of all eight strains gave rise to colony-forming as well as non-colony-forming descendants (“lethal sectoring”). Lethal sectors, i.e., groups of non-colony-forming cells which originate from a common ancestor, appeared with frequencies per generation ranging between 4 and 20% in Rec− strains, whereas lethal sectors were rare in Rec+ strains (less than 1%). A strain carrying a mutation (uvrA6) in one of the genes involved in pyrimidine dimer excision from deoxyribonucleic acid (DNA) showed twice as many lethal sectors per generation as a strain with the genotype uvrA+. Similarly, a double mutant (AB2480, uvrA6, recA13) showed twice as much spontaneous lethal sectoring as the corresponding Rec− strain (uvrA+, recA13). The kinetics of growth curves obtained in nutrient broth and the frequency of non-colony-forming units in stationary-phase broth cultures indicate clearly that lethal sectors occur in liquid cultures too. The causes for spontaneous lethal sectoring are unknown at present. It seems reasonable to assume that gene uvrA and the rec genes are somehow involved in the repair of spontaneously occurring DNA lesions, since a deficiency in this type of repair may cause lethal sectors. The extent to which spontaneous lethal sectoring (observed in all Rec− strains of E. coli studied) may contribute indirectly to the failure to form recombinants is discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON T. F. Recombination and segregation in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1958;23:47–58. doi: 10.1101/sqb.1958.023.01.007. [DOI] [PubMed] [Google Scholar]
- CLARK A. J., MARGULIES A. D. ISOLATION AND CHARACTERIZATION OF RECOMBINATION-DEFICIENT MUTANTS OF ESCHERICHIA COLI K12. Proc Natl Acad Sci U S A. 1965 Feb;53:451–459. doi: 10.1073/pnas.53.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. J., Chamberlin M., Boyce R. P., Howard-Flanders P. Abnormal metabolic response to ultraviolet light of a recombination deficient mutant of Escherichia coli K12. J Mol Biol. 1966 Aug;19(2):442–454. doi: 10.1016/s0022-2836(66)80015-3. [DOI] [PubMed] [Google Scholar]
- Clark A. J. The beginning of a genetic analysis of recombination proficiency. J Cell Physiol. 1967 Oct;70(2 Suppl):165–180. doi: 10.1002/jcp.1040700412. [DOI] [PubMed] [Google Scholar]
- Clowes R. C., Moody E. E. Chromosomal transfer from "recombination-deficient" strains of Escherichia coli K-12. Genetics. 1966 Apr;53(4):717–726. doi: 10.1093/genetics/53.4.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forro F., Jr Autoradiographic studies of bacterial chromosome replication in amino-acid deficient Escherichia coli 15T-. Biophys J. 1965 Sep;5(5):629–649. doi: 10.1016/S0006-3495(65)86741-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gefter M. L., Becker A., Hurwitz J. The enzymatic repair of DNA. I. Formation of circular lambda-DNA. Proc Natl Acad Sci U S A. 1967 Jul;58(1):240–247. doi: 10.1073/pnas.58.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haefner K. A simple apparatus for producing agar layers of uniform thickness for microbiological micromanipulator work. Z Allg Mikrobiol. 1967;7(3):229–231. [PubMed] [Google Scholar]
- Haefner K., Striebeck U. Radiation-induced lethal sectoring in Escherichia coli B/r and Bs-1. Mutat Res. 1967 Jul-Aug;4(4):399–407. doi: 10.1016/0027-5107(67)90002-4. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P. DNA repair and genetic recombination: studies on mutants of Escherichia coli defective in these processes. Radiat Res. 1966;(Suppl):156+–156+. [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P., Theriot L. Three loci in Escherichia coli K-12 that control the excision of pyrimidine dimers and certain other mutagen products from DNA. Genetics. 1966 Jun;53(6):1119–1136. doi: 10.1093/genetics/53.6.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P., Theriot L. Mutants of Escherichia coli K-12 defective in DNA repair and in genetic recombination. Genetics. 1966 Jun;53(6):1137–1150. doi: 10.1093/genetics/53.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederberg J. SIBLING RECOMBINANTS IN ZYGOTE PEDIGREES OF ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1957 Dec 15;43(12):1060–1065. doi: 10.1073/pnas.43.12.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattern I. E., Zwenk H., Rörsch A. The genetic constitution of the radiation-sensitive mutant Escherichia coli Bs-1. Mutat Res. 1966 Oct;3(5):374–380. doi: 10.1016/0027-5107(66)90047-9. [DOI] [PubMed] [Google Scholar]
- SETLOW R. B., CARRIER W. L. THE DISAPPEARANCE OF THYMINE DIMERS FROM DNA: AN ERROR-CORRECTING MECHANISM. Proc Natl Acad Sci U S A. 1964 Feb;51:226–231. doi: 10.1073/pnas.51.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa J. GENETIC STRUCTURE OF RECOMBINANT CHROMOSOMES FORMED AFTER MATING IN ESCHERICHIA COLI K12. Proc Natl Acad Sci U S A. 1960 Jan;46(1):91–101. doi: 10.1073/pnas.46.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood T. H. Genetic recombination in Escherichia coli: clone heterogeneity and the kinetics of segregation. Science. 1967 Jul 21;157(3786):319–321. doi: 10.1126/science.157.3786.319. [DOI] [PubMed] [Google Scholar]
- van de Putte P., Zwenk H., Rörsch A. Properties of four mutants of Escherichia coli defective in genetic recombination. Mutat Res. 1966 Oct;3(5):381–392. doi: 10.1016/0027-5107(66)90048-0. [DOI] [PubMed] [Google Scholar]



