Abstract
Diploid yeast develop pseudohyphae in response to nitrogen starvation, while haploid yeast produce invasive filaments which penetrate the agar in rich medium. We have identified a gene, FLO11, that encodes a cell wall protein which is critically required for both invasion and pseudohyphae formation in response to nitrogen starvation. FLO11 encodes a cell surface flocculin with a structure similar to the class of yeast serine/threonine-rich GPI-anchored cell wall proteins. Cells of the Saccharomyces cerevisiae strain Σ1278b with deletions of FLO11 do not form pseudohyphae as diploids nor invade agar as haploids. In rich media, FLO11 is regulated by mating type; it is expressed in haploid cells but not in diploids. Upon transfer to nitrogen starvation media, however, FLO11 transcripts accumulate in diploid cells, but not in haploids. Overexpression of FLO11 in diploid cells, which are otherwise not invasive, enables them to invade agar. Thus, the mating type repression of FLO11 in diploids grown in rich media suffices to explain the inability of these cells to invade. The promoter of FLO11 contains a consensus binding sequence for Ste12p and Tec1p, proteins known to cooperatively activate transcription of Ty1 elements and the TEC1 gene during development of pseudohyphae. Yeast with a deletion of STE12 does not express FLO11 transcripts, indicating that STE12 is required for FLO11 expression. These ste12-deletion strains also do not invade agar. However, the ability to invade can be restored by overexpressing FLO11. Activation of FLO11 may thus be the primary means by which Ste12p and Tec1p cause invasive growth.
INTRODUCTION
Diploid strains of Saccharomyces cerevisiae Σ1278b switch from a single-cell form of growth to develop pseudohyphae, long-branched chains of elongated cells, in response to nitrogen starvation (for review, see Gimeno and Fink, 1992; Kron and Gow, 1995). These invasive filaments have been interpreted to be a foraging adaptation to nitrogen starvation. Haploid yeast do not form pseudohyphae when starved for nitrogen, but they invade agar when cultured on rich medium (Roberts and Fink, 1994).
Several elements of the mitogen-activated protein kinase (MAPK) pathway that controls mating pheromone signal transduction in haploid cells are also required for pseudohyphae development and invasion: Ste20p, Ste11p, Ste7p, and Ste12p (Liu et al., 1993; Roberts and Fink, 1994). This may be the most well studied of all mitogen-activated protein (MAP) kinase systems (for review. see Herskowitz, 1995; Levin and Errede, 1995). The pheromone receptor and G protein which mediate the initial steps in the mating signal transduction pathway are not required for pseudohyphae formation (Liu et al., 1993). Instead, the G proteins encoded by RAS2 and CDC42 appear to control activation of the MAPK pathway leading to pseudohyphae development (Mosch et al., 1996). The kinases Ste20p, Ste11p (MEKK), and Ste7p (MEK) are required for phosphorylation of the transcription factor Ste12p in response to mating pheromone in haploid cells (Elion et al., 1993); these same proteins are required for invasion (Roberts and Fink, 1994) and pseudohyphae formation (Liu et al., 1993). This pathway thus controls signal transduction in response to mating pheromones in haploid cells and pseudohyphae development in response to nitrogen starvation in diploid cells. Since pheromone-responsive genes are not detectably induced during haploid pseudohyphae formation (Roberts and Fink, 1994), Ste12p must have at least two functional states in the haploid cell. These two activities of Ste12p appear to be determined by association with an accessory transcription factor, Tec1p. A transcriptional reporter which is induced during filamentous growth and invasion but not during mating has been developed using regulatory sequences from the transposable element Ty1 fused to lacZ (Mosch et al., 1996). Transcription of this reporter is dependent on the cooperative binding of Ste12p and Tec1p to a filamentation and invasion response element (FRE) in the Ty1 sequences (Madhani and Fink, 1997). To date, the only other gene reported to be regulated by a FRE is the TEC1 gene itself (Madhani and Fink, 1997).
Surprisingly, although haploid cells invade rich agar, diploids do not (Roberts and Fink, 1994). Haploid invasiveness requires the same components of the MAPK pathway that are required for diploid pseudohyphal development, including the transcriptional activator Ste12p (Roberts and Fink, 1994). Invasiveness is distinct from formation of the pseudohyphal filaments in both haploids and diploids (Mosch and Fink, 1997; Roberts and Fink, 1994). Invasion does not require BUD gene functions, which regulate the budding patterns of haploid and diploid cells. Strains with mutations in BUD1 and BUD2 penetrate agar as efficiently as wild-type strains, even though these strains do not form ordered filaments (Roberts and Fink, 1994).
Investigations of pseudohyphae development to date have largely focused on regulators of the pseudohyphal pathway. These studies have revealed the involvement of transcription factors and the kinases and phosphatases characteristic of signal transduction pathways (Blacketer et al., 1993, 1994; Liu et al., 1993, 1996; Gimeno and Fink, 1994; Gavrias et al., 1996; Ward et al., 1995; Mosch and Fink, 1997; Roberts et al., 1997). There must also be downstream genes that are regulated during pseudohyphae development that result in alterations of the cell surface, changes in cell shape and cell cycle control, and the ability to invade. We have recently characterized a gene (open reading frame YIR019c) that we have named FLO11 which encodes a cell surface flocculin (Lo and Dranginis, 1996). In single-cell yeasts that are not developing pseudohyphae, FLO11 expression causes cell–cell adhesion of the calcium-dependent type called flocculation. FLO11 is identical to MUC1, a gene shown to be required for pseudohyphae formation by amylase-secreting strains of S. cerevisiae var. diastaticus in which both haploids and diploids switch to a pseudohyphal form of growth in response to carbon limitation (Lambrechts et al., 1996). We report here that FLO11 is essential for both invasion and filament formation by the Σ1278b strain of S. cerevisiae that form pseudohyphae efficiently in response to nitrogen starvation and on which most of the studies of filamentous growth have been performed (Gimeno et al., 1992). We place FLO11 in the signal transduction pathway downstream of STE12 and show that it is likely to be the only target of STE12 required for invasion. Flo11p represents the first specific cell surface requirement for the formation of pseudohyphae and invasion, and connects the processes of cell adhesion, invasion, and pseudohyphae formation in yeast.
MATERIALS AND METHODS
Strains and Growth Conditions
Yeast strains used in this study are described in Table 1. They were derived from strains of the Σ1278b genetic background (Gimeno et al., 1992) generously provided by members of the laboratory of Gerald Fink. Strain YSL1015 contains the plasmids pRS423 and pRS424 and strain YSL1012 contains the plasmid pRS426 (Christianson et al., 1992) solely to facilitate the construction of these strains as diploids. Standard yeast culture medium was prepared as described (Kaiser et al., 1994). YEPD plates [2% yeast extract (Difco, Wilmington, DE), 4% Bacto peptone (Difco), 2% glucose, and 2% Bacto-agar (Difco)] were allowed to dry at room temperature before use. Low ammonia medium (SLAD) for pseudohyphal growth in diploids was prepared as described [6.7 g/l yeast nitrogen base without amino acids and ammonium sulfate (Difco), 2% glucose, 2% washed Bacto-agar (Difco), and appropriate auxotrophic requirements] (Gimeno et al., 1992). Strains scored for pseudohyphal filament formation were streaked on SLAD plates and observed and photographed after 1 wk of growth at 30°C. For the invasive growth assay, strains were patched onto YEPD plates with a toothpick, with care being taken to avoid scatching the surface of agar. Patches were allowed to grow at 30°C for 3 d and incubated at room temperature for an additional 2 d. Cells that penetrated the agar were examined after washing the cells off the agar surface with deionized water (Roberts and Fink, 1994). Samples were photographed with Polaroid 65 film (Sigma, St. Louis, MO).
Table 1.
Strains and plasmid list
| Strain or Plasmid | Genotype or Description | Reference Source Derivation |
|---|---|---|
| S. cerevisiae strain | ||
| L5486 | MATa, ura3-52, leu2::hisG | Roberts and Fink (1994) |
| L5487 | MATα, ura3-52, leu2::hisG | Roberts and Fink (1994) |
| 10560-4A | MATa, ura3-52, leu2::hisG, | Laboratory of G. Fink |
| trp1::hisG, his3::hisG | ||
| 10560-6B | MATα, ura3-52, leu2::hisG, | Laboratory of G. Fink |
| trp1::hisG, his3::hisG | ||
| YSL1012 | MATa/MATα, ura3-52/ura3-52, | This work. L5487 × YIY318 |
| LUE2/leu2::hisG [URA3] | (Yamashita et al., 1985) | |
| + plasmid pRS426 | ||
| (Christianson et al., 1992) | ||
| YSL1015 | MATa/MATα, ura3/ura3-52, | This work. 10560-4A × |
| leu2::hisG/leu2::hisG | 10560-6B + plasmid pRS423 | |
| trp1::hisG,/trp1::hisG | + plasmid pRS424 | |
| his3::hisG/his3::hisG [TRP1][HIS3] | Christianson et al. (1992) | |
| L5487flo11-4 | MATα, ura3-52, leu2::hisG, | This work |
| flo11::URA3 | Derived from L5487 | |
| YSL12 | MATα, ura3-52, leu2::hisG, | This work |
| ste12::LEU2 | Derived from L5487 | |
| Plasmids | ||
| pRS315-a1 | CEN-LEU2 plasmid carrying MATa1 | This work |
| pRS415-α2 | CEN-LEU2 plasmid carrying MATα2 | Sharon Y. Roth |
| pYSL12 | 2μ-LEU2 plasmid carrying FLO11 | This work |
| pYSL13 | 2μ-URA3 plasmid carrying FLO11 | This work |
| pSL35 | pGEM3Z(f+) containing a 746-bp | This work |
| HindIII–PvuII FL011 fragment | ||
| pGEM-ACT300 | pGEM3Z containing a 300-bp BalII | K. Wang; Ng and Abelson, 1980 |
| fragment from pYACT1 | ||
| pSUL16 | ste12::LEU2-containing plasmid | Fields and Herskowitz, 1987 |
| pSY2 | 2μ-LEU2 plasmid carrying STE12 | Dolan and fields, 1990 |
Plasmid Constructions
Plasmids used in this work are listed in Table 1. pRS315-a1 contains the 1.8-kb XhoI–HindIII fragment containing MATa1 isolated from the XhoI linker insertion mutation matax 12 (Tatchell et al., 1981) cloned into the polylinker of pRS315 (Sikorski and Heiter, 1989). pRS415-α2 contains the MATα2 gene with its own promoter, amplified by polymerase chain reaction from base 1 to base 969 (Tatchell et al., 1981) using primers with SalI and SpeI restriction sites at the 5′ ends, cloned into the CEN-LEU2 vector pRS415 (Christianson et al., 1992); it was a generous gift from Sharon Y. Roth. pSY2 is a 2 μ-LEU2 plasmid containing the STE12 gene (Dolan and Fields, 1990) in the vector YEp13.
pYSL12 contains the entire FLO11 gene (sequence number relative to initiating AUG: −870 to 4107) which was amplified by long polymerase chain reaction (Lo and Dranginis, 1996) as a SalI–BglII fragment; the FLO11 fragment was cloned into the LEU2–2 μ yeast shuttle vector pRS425 (Christianson et al., 1992). pYSL13 is the same FLO11 fragment cloned into the URA3–2 μ yeast shuttle vector pRS426 (Christianson et al., 1992).
Clones used for the synthesis of 32P-labeled riboprobes for Northern blots were as follows. The FLO11 probe was made from pSL35, which contains a HindIII–PvuII fragment of FLO11 (sequence number relative to initiating AUG of coding sequence of FLO11: 2669–3415) cloned into pGEM3Z(f+) vector (Promega, Madison, WI). The actin probe was made from pGEM-Act300, a gift from Kevin Wang, which contains a 300-bp BalII fragment from pYACT1 (Ng and Abelson, 1980).
Gene Disruptions
Disruption of FLO11 was performed as previously described (Lo and Dranginis, 1996). STE12 was disrupted using the ste12::LEU2-containing plasmid pSUL16 (Fields and Herskowitz, 1987). All gene replacements and disruptions were confirmed by Southern blotting.
Northern Blot Analysis
Total RNA was prepared by a modification of published methods (Carlson and Botstein, 1982; Dranginis, 1989) as follows. Haploid and diploid yeast cells were grown in different media and harvested at different growth stages (time points: 0.5, 2, 4, 6, 8, and 24 h of growth). Cell density was estimated for each sample by measuring A600 (1OD600 unit equals approximately 2 × 107 cells). The cells were collected by centrifugation, transferred in 1 ml of water to microfuge tubes, and collected again. To the cell pellet was added 0.4 g of washed glass beads (0.45–0.5 mm, Thomas Scientific, NJ), followed by 0.3 ml of cracking buffer (0.5 M NaCl, 0.2 M Tris-HCl, pH 7.4, 10 mM EDTA, and 1% SDS) and 0.3 ml of 25:24:1 phenol:chloroform:isoamyl alcohol. The samples were vortexed at top speed for 1 min. Another 0.3 ml of cracking buffer and 0.3 ml of phenol:chloroform:isoamyl alcohol was added, and the mixture was vortexed for 30 s. The samples were centrifuged 5 min and the aqueous phase was extracted with 0.3 ml of phenol:chloroform:isoamyl alcohol. The sample was precipitated with 1 ml of ethanol at −20°C and then centrifuged for 1 min. The pellet was washed with 70% ethanol and suspended in RNase-free water.
Ten micrograms of RNA per sample was run in 3-(N-morpholino)propanesulfonic acid-formaldehyde gels as described (Sambrook et al., 1989). Gels were washed in the following way: 15 min with distilled water, 30 min with 0.01 N NaOH, 15 min with 0.1 M Tris-Hcl (pH 7.5), and 30 min with 0.1× SSC. Transfer to GeneScreen membrane (DuPont-New England Nuclear Research Products, Boston, MA) was performed by capillary blot using 10× SSC. RNA was cross-linked to the membrane with a Stratalinker (Stratagene, La Jolla, CA) at 120,000 μJ/cm2.
Membranes were hybridized with 32P-labeled riboprobes for FLO11 and ACT1, produced from the plasmids described above by transcription with T7 RNA polymerase (New England Biolabs, Beverly, MA). The quantitation of the mRNA was performed using a PhosphorImager (Molecular Dynamics, Sunnyvale, CA). FLO11 RNA was normalized to the hybridization signal from the actin RNA (ACT1) which was used as a control for RNA loading. The FLO11:ACT1 RNA ratio was plotted against OD600 of the cell sample.
Photomicroscopy
Colonies were viewed through the bottom of the Petri dish with a Zeiss standard 16 microscope at 100× total magnification and photographed using a 35-mm camera using TMAX 400 (Kodak) film.
RESULTS
FLO11 Is Required for Invasive Growth and Pseudohyphae Formation
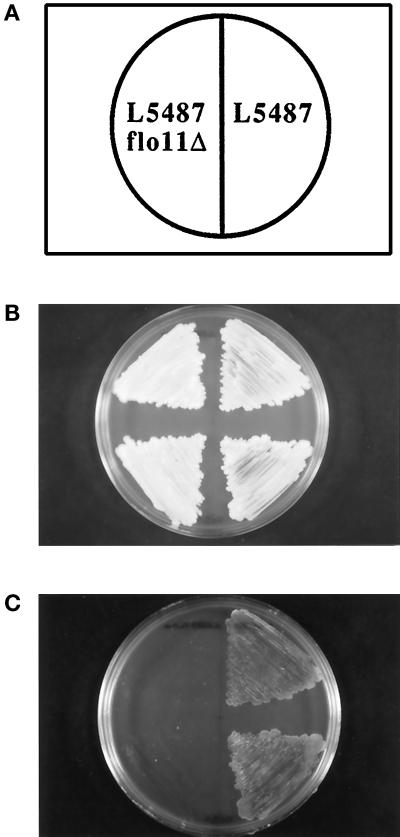
We have recently described FLO11, a gene that encodes a cell surface flocculin (Lo and Dranginis, 1996). Expression of FLO11 was examined by RNA hybridization analysis in several different yeast strain backgrounds. The 4.2-kb FLO11 RNA was detected in the pseudohyphae-forming strain Σ1278b (L5486 and L5487; see Table 1) and in S. cerevisiae var. diastaticus, whose flocculation properties were previously studied (Lo and Dranginis, 1996), but FLO11 transcripts were not detected in the laboratory strain S288C, which does not form pseudohyphae (Gimeno et al., 1992; Liu et al., 1996; our unpublished results). To test the role of this cell surface molecule in invasion and formation of pseudohyphae, we constructed a gene deletion of FLO11 in yeast strain Σ1278b, on which many studies of filamentous growth have been performed. Figure 1 shows that FLO11 is required for invasiveness of haploid cells. Cells were patched on rich medium (YEPD) and incubated at 30°C for 3 d and at room temperature for an additional 2 d (Figure 1B). Cells that penetrated the agar were examined after washing the cells off the agar surface with deionized water (Roberts and Fink, 1994). The wild-type cells on the right were able to invade the agar, but the isogeneic strain with FLO11 deleted was no longer able to penetrate the agar (Figure 1C).
Figure 1.
FLO11 is required for invasiveness of haploid cells. Yeast were streaked on YEPD medium and grown for 3 d at 30°C followed by 2 d of growth at room temperature. As shown in the diagram in A, haploid α cells of strain L5487 are on the right half of the Petri dish while isogeneic cells with a deletion of FLO11 (L5487-flo11–4) are on the left half of the dish. (B) Total yeast growth on the surface of the agar, shown before the plate was rinsed with water. (C) The same plate after rinsing cells off the surface of the agar with a gentle stream of water. Invasive growth is demonstrated by cells that remain in the agar.
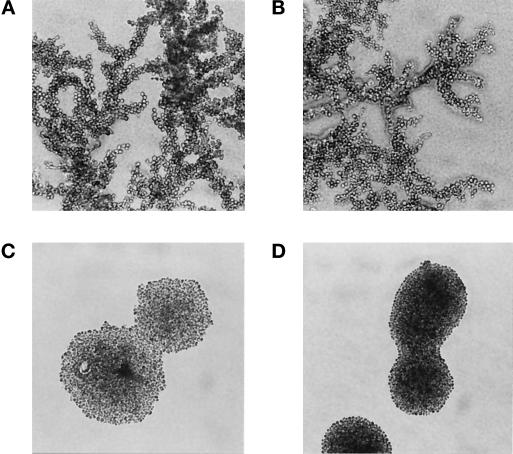
We then tested the ability of diploids with a deletion of FLO11 to form pseudohyphae in response to nitrogen starvation. Figure 2A shows that diploid cells form highly branched pseudohyphae when starved for nitrogen, but haploid colonies exhibit a smooth circular outline (Figure 2D). It has previously been shown that pseudohyphae formation in diploids is dependent on the a1/α2 repressor (Gimeno et al., 1992; Lo et al., 1998). Like the natural diploid, a pseudodiploid which is made by transforming α haploids with a single copy plasmid containing MATa1 forms filaments efficiently (Figure 2B). The same pseudodiploid with a deletion of FLO11 fails to form any filaments (Figure 2C). We have recently confirmed this result using true diploid cells homozygous for the FLO11 deletion (our unpublished observations). FLO11 is thus required for diploid cells to form pseudohyphae in response to nitrogen starvation.
Figure 2.
FLO11 is required for pseudohyphae formation by diploids in response to nitrogen starvation. The morphology of yeast colonies grown on nitrogen starvation medium (SLAD) for 1 wk is shown. (A) Diploid strain YSL1012 (MATa/MATα). (B) “Pseudodiploid” constructed by transforming α haploid strain L5487 with a single-copy plasmid containing the MATa1 gene (pRS315-a1). (C) FLO11 genomic deletion in the pseudodiploid strain shown in B (strain L5487flo11–4 containing pRS315-a1). (D) Haploid α strain L5487.
FLO11 mRNA Is Induced in Diploids, But Not in Haploids by Nitrogen Starvation
The requirement for FLO11 in the diploid to form pseudohyphae was puzzling, since we have previously shown that FLO11 expression is repressed by the action of the a1/α2 repressor in diploids grown in rich media (Lo and Dranginis, 1996). One hypothesis is that FLO11 expression, while repressed in rich media, is induced when diploids are transferred to nitrogen starvation media. Experiments with STA1, a related gene whose promoter has extensive sequence homology to the promoter of FLO11, support this hypothesis. STA1 is repressed in a manner dependent on the a1/α2 repressor in diploids grown in rich media, but the RNA is induced when diploids are transferred to sporulation medium, which starves cells for nitrogen in the presence of acetate as a carbon source (Dranginis, 1989).
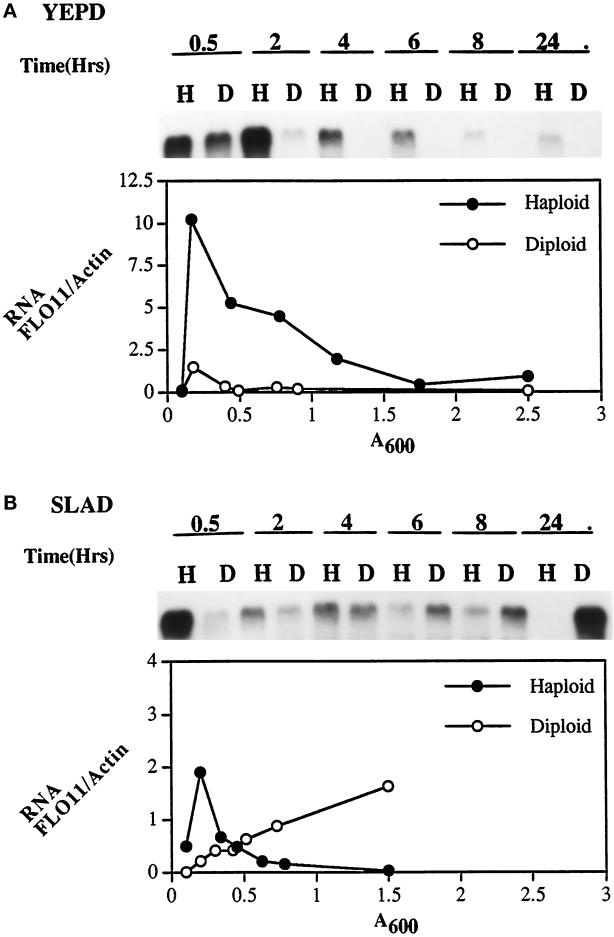
To test this hypothesis, we measured the expression of FLO11 in both haploids and diploids in response to growth in rich medium and to nitrogen starvation by Northern blot analysis (Figure 3). The isogeneic α haploid strain (L5487) and a/α diploid strain (YSL1015) were grown in YEPD liquid medium overnight, then diluted to an initial OD600 of 0.10–0.125 into YEPD (rich) or SLAD (nitrogen-deficient) media. After incubation at 30°C, cells were harvested at different time points (0.5, 2, 4, 6, 8, and 24 h). The cell density of each sample was measured, and RNA was purified. RNA from each time point was run in formaldehyde gels and transferred to nylon membrane, and the FLO11 and actin mRNA were detected by hybridization with riboprobes. Figure 3A shows the FLO11 RNA from haploids (H) and diploids (D) which were cultured in YEPD media. The FLO11 signal reaches its peak intensity after a 30-min incubation in both strains. However, the levels of FLO11 RNA rapidly declined in the diploids, beginning with the 2-h sample and disappearing entirely after a 4-h culture (OD600 ∼0.4) whereas the expression of FLO11 in haploids was still high (Figure 3A).
Figure 3.
FLO11 mRNA is induced in diploids, but not in haploids by nitrogen starvation. The isogeneic haploid α strain (L5487) and diploid strain (YSL1015) were inoculated into YEPD and SLAD media, and RNA samples were prepared at the indicated time points (0.5, 2, 4, 6, 8, and 24 h). The Northern blots were hybridized with a 32P-labeled FLO11 riboprobe and then subsequently stripped and rehybridized with a 32P-labeled actin riboprobe control as described. Lanes H indicate haploid samples; D indicates diploid. (A) RNA from cells grown in YEPD, hybridized with FLO11. (B) RNA from cells grown in low nitrogen medium (SLAD), hybridized with FLO11. Ratio of FLO11 RNA to actin RNA at various growth stages (OD600) of cells grown in YEPD and SLAD is plotted beneath the Northern blots. The RNA band intensities were determined with the PhosphorImager. Closed circles, haploid (L5487, Matα); open circles, diploid (YSL1015, Mata/α).
The blots were stripped and rehybridized with an actin probe to control for amount of RNA. To quantitate the expression, the band intensities were measured using a PhosphorImager and the FLO11:actin ratio was calculated and plotted as a function of cell growth (Figure 3). In rich media (YEPD), the ratio of FLO11 to actin is maximum at early log phase for both haploid (FLO11/actin = 10) and diploid (FLO11/actin = 1.5). The FLO11 signal in the haploid was initially higher than that in the diploid. The ratio of FLO11 to actin RNA declined rapidly in the diploid after a 4-h culture (FLO11/actin = 0.08) but in the haploid the ratio was still 0.9 after a 24-h culture (Figure 3A).
In contrast, when cells were transferred to nitrogen starvation media (SLAD) the FLO11 signal declined very rapidly in haploids after 0.5 h and disappeared at 24 h (OD600 ∼1.5), but the expression of FLO11 in diploids continued to increase in an overnight culture (Figure 3B). The FLO11:actin ratio climbed steadily in the diploids, reaching 1.7 at 24 h (Figure 3B). Similar curves were obtained when 28S rRNA was used as control for loading instead of actin (our unpublished observations).
These data indicate that FLO11 expression is regulated by both mating type and nitrogen starvation. FLO11 transcripts are induced in diploid cells upon starvation for nitrogen, but not in haploid cells. The pattern of FLO11 expression may explain why diploids but not haploids form pseudohyphae when starved for nitrogen, whereas haploids but not diploids are invasive in rich medium.
Overexpression of FLO11 Induces Invasive Growth in Diploids
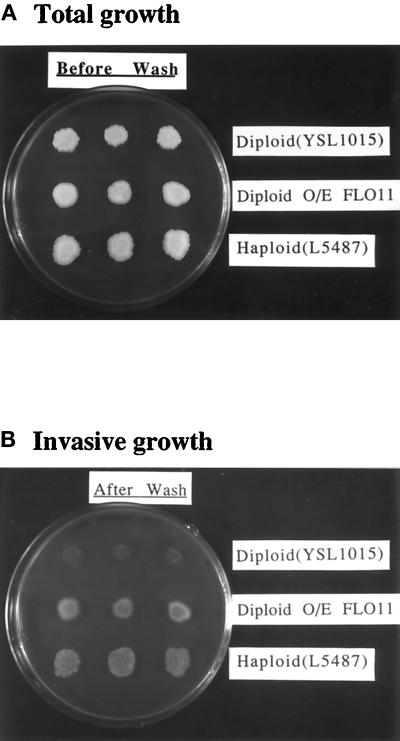
Could the inability of diploid cells to invade rich media be accounted for by the repression of FLO11 in these circumstances? We tested this hypothesis by overexpressing cloned FLO11 in a diploid and assaying for invasive growth on rich medium. A diploid strain (YSL1015) was transformed with a high-copy number vector containing the 2 μ origin of replication and the cloned FLO11 gene (pYSL12) (Figure 4, middle row of colonies). The same strain was transformed with the vector only (pRS425), without the cloned FLO11 gene, as a control (Figure 4, top row of colonies). The lower row of colonies is the isogeneic haploid (L5487), which is known to be invasive on rich agar. The plate was incubated for 3 d at 30°C followed by 2 d of growth at room temperature. Figure 4A shows the total growth on the surface of the agar before the plate was rinsed with water, and Figure 4B shows the same plate after rinsing cells off the surface of the agar with a gentle stream of deionized water. Invasive growth is indicated by cells that remain in the agar after washing. The diploid transformed with vector alone shows very weak invasive growth, but when the FLO11 gene was present on a high-copy plasmid the diploid strain is as strongly invasive as the haploid. Therefore, expression of FLO11 from a high-copy plasmid in diploids induces invasive growth on rich medium. Mating-type repression of FLO11 suffices to explain the inability of diploid cells to invade.
Figure 4.
Overexpression of FLO11 in diploids growing on rich media enables them to invade agar. A diploid strain (YSL1015) was transformed with either the vector control pRS425 (first row of colonies) or with a multicopy plasmid carrying FLO11, pYSL12 (middle row of colonies). Invasive growth is compared with that of a wild-type isogeneic haploid strain, L5487 (bottom row of colonies). Cells were patched onto YEPD medium and grown for 3 d at 30°C followed by 2 d of growth at room temperature. (A) The total growth on the surface of the agar, shown before the plate was rinsed with water. (B) The same plate after rinsing cells off the surface of the agar with a gentle stream of deionized water. Invasive growth is demonstrated by cells that remain in the agar.
Overexpression of FLO11 Restores Invasiveness to Yeast with a Deletion of STE12
Ste12p is a critical transcription activator in the filamentous pathway in yeast (for review, see Kron and Gow, 1995). Yeast with a mutation of STE12 are defective for filamentous growth (Mosch and Fink, 1997). Using RNA blots, we have shown that yeast with a deletion of STE12 do not express FLO11 transcripts (our unpublished results), indicating that STE12 is required for FLO11 expression. To determine the role of FLO11 in this signal transduction pathway, we performed epistasis studies to understand the relation between FLO11 and STE12. A wild-type α strain and an isogeneic ste12-deletion strain were transformed with a vector not containing FLO11 (pRS426) and grown on the top and middle rows, respectively, of the agar dish shown in Figure 5A. The ste12-deletion strain transformed with FLO11 cloned into the high-copy (2 μ) vector is shown as the bottom row of colonies in Figure 5A. The plate was incubated as described above before invasive growth was assayed by washing the plate (Figure 5B). Figure 5 shows that strains with a deletion of STE12 cannot penetrate agar. The ability to invade can be restored by the presence of FLO11 on a high-copy plasmid (pYSL13) in these ste12-deletion strains.
Figure 5.
Overexpression of FLO11 restores invasiveness to yeast with a deletion of STE12. Invasiveness of the following strains is compared: Haploid strain L5487 (first row of colonies); strain YSL12 (L5487 with a deletion of STE12) transformed with the vector control pRS426 (middle row of colonies); and strain YSL12 (STE12 deletion) transformed with pYSL13, a 2μ-URA3 plasmid containing the FLO11 gene (bottom row of colonies). Cells were patched onto YEPD medium and grown for 3 d at 30°C followed by 2 d of growth at room temperature. (A) The total growth on the surface of the agar, shown before the plate was rinsed with water. (B) The same plate after rinsing cells off the surface of the agar with a gentle stream of deionized water. Invasive growth is demonstrated by cells that remain in the agar.
However, the inability to invade caused by a deletion of FLO11 could not be overcome by overexpression of STE12 (Figure 6). Ste12p is known to activate many genes in the mating pheromone signal transduction pathway (see for review, Sprague and Thorner, 1992) and to be required along with Tec1p for activation of a filamentation-specific reporter gene during the filamentous growth response (Madhani and Fink, 1997). Overexpression of STE12 by expressing the gene from a 2 μ plasmid in this way has been shown to enhance filamentation and invasion (Mosch and Fink, 1997). The data presented here place FLO11 downstream of STE12 in the invasive growth pathway and furthermore suggest that FLO11 may be the only target of STE12 which is responsible for invasive growth.
Figure 6.
Overexpression of STE12 does not restore invasiveness to yeast with a deletion of FLO11. Invasive growth of the following strains were compared: wild-type strain L5487 (first row of colonies); L5487flo11–4 (L5487 with a deletion of FLO11) transformed with the 2μ vector control pRS426 (middle row of colonies); and L5487flo11–4 transformed with a multiple copy (2μ) plasmid carrying STE12 (pSY2). Cells were patched onto YEPD medium and grown for 3 d at 30°C followed by 2 d of growth at room temperature. (A) The total growth on the surface of the agar, shown before the plate was rinsed with water. (B) The same plate after rinsing cells off the surface of the agar with a gentle stream of deionized water. Invasive growth is demonstrated by cells that remain in the agar.
The Upstream Region of the FLO11 Promoter Contains a Filamentation and Invasion Response Element (FRE)
Although Ste12p can act alone to activate genes in the mating pathway, it has recently been reported that activation of the filamentous response requires the cooperative binding of Ste12p and Tec1p to gene promoters (Madhani and Fink, 1997). This combinatorial binding site has been called the filamentation and invasion response element (FRE) and it is comprised of a Ste12p-binding site (consensus: TGAAACA) and a Tec1p-binding site (consensus: CATTCC or CATTCT) in close proximity (Madhani and Fink, 1997). Examination of the sequence of the upstream region of FLO11 revealed that it contains a sequence which is an almost exact match to the FRE between bases −725 and −699 with respect to the start codon (Figure 7). There is only one base divergent from the FRE consensus sequence; this is at position −723 in the Ste12p-binding site where C is found instead of A. There are 14 bp separating the Ste12p and Tec1p sites in the FLO11 sequence, which is identical to the spacing of these sites in the Ty1 FRE (Madhani and Fink, 1997).
Figure 7.
Models for FLO11 action in the filamentous growth pathway. (A) In the filamentous signaling pathway Flo11p functions downstream of Ste12p and is the primary target of Ste12p to effect invasion. (B) The FLO11 promoter region contains a filamentous response element (FRE), a site for the cooperative binding of the proteins Ste12p and Tec1p which transcriptionally activate genes during the filamentous response (Madhani and Fink, 1997).
The only genes previously reported to have FRE sequences in their promoters are the Ty1 transposable element and TEC1 itself (Madhani and Fink, 1997). FLO11 is the first downstream effector of the filamentous pathway reported to contain a FRE.
DISCUSSION
Studies of regulation of pseudohyphae development and invasiveness in yeast have resulted in the identification of genes encoding kinases, phosphatases, and transcription factors (Blacketer et al., 1993, 1994; Liu et al., 1993, 1996; Gimeno and Fink, 1994; Ward et al., 1995; Gavrias et al., 1996; Roberts et al., 1997). Ultimately, changes must occur at the cell surface to enable cells to invade substrate or to adhere to form a filamentous chain of cells. Flo11p has been previously identified as a flocculin, a cell surface molecule responsible for the calcium-dependent nonsexual aggregation known as flocculation (Lo and Dranginis, 1996). The work described here demonstrates that this flocculin is required for invasion and pseudohyphae development. Flo11p is the first cell wall protein shown to be involved in these processes. Recently, a screen for mutants defective in filamentous growth led to the identification of genes encoding structural proteins that interact with the cytoskeleton and genes responsible for bud site selection as well as previously identified components of the signal transduction pathway, but did not recover FLO11 (Mosch and Fink, 1997). However, this screen also did not lead to the identification of several other genes known to be required for filamentous growth, suggesting that the screen was not saturated.
The transcriptional activator Ste12p is a downstream target of the pheromone-responsive MAPK cascade, elements of which are also required for the filamentous response (Liu et al., 1993). Ste12p activates two different sets of genes, the mating genes and the filamentation genes, in response to different signals. A transcriptional reporter containing promoter sequences from the Ty1 element, FG(TyA)::lacZ, has been shown to be activated by Ste12p during filamentous growth, but not during the mating response (Mosch et al., 1996). This filamentation-specific transcriptional induction was recently shown to be a consequence of the cooperative binding of Ste12p and Tec1p to a filamentation and invasion response element (FRE), which is comprised of a binding site for Ste12p adjacent to a binding site for Tec1p (Madhani and Fink, 1997). Two genes have thus far been described which have FRE elements: Ty1 and the transcriptional factor TEC1 itself, which seems to participate in a positive feedback loop (Madhani and Fink, 1997). FLO11, however, represents the first reported functionally important downstream gene in the filamentous response pathway which contains a FRE in its promoter.
We present evidence that FLO11 is regulated by the transcriptional activator Ste12p. First, strains with a deletion of STE12 do not express FLO11 RNA. Second, the upstream region of FLO11 contains a consensus FRE sequence. Finally, expression of FLO11 on a high-copy plasmid overcomes the inability of the ste12-deletion strain to invade agar, indicating that activation of FLO11 is the primary mechanism by which the transcriptional activator Ste12p promotes invasion.
It is possible that the adhesion, invasion, and filamentation functions of FLO11 are related. One hypothesis is that FLO11 is responsible for the adhesion of mother and daughter cells in the filamentous chain of cells of pseudohyphae. The adhesion properties of Flo11p might also account for its critical role in invasiveness. Adhesion to substrate may play a role in the mechanism of invasion or cell–cell adhesion may provide a stable filament of cells, allowing the force of cell division to drive invasion. A relationship between flocculation and pseudohyphae formation was recently suggested by the discovery that PHD10, an activator of pseudohyphae development, is the same gene as FLO8 (Liu et al., 1996), a putative transcriptional activator of the flocculin FLO1 (Kobayashi et al., 1996). A nonsense mutation in FLO8 accounts for the inability of the common laboratory yeast strain S288C to form pseudohyphae (Liu et al., 1996). It is possible that Flo8p also activates transcription of FLO11, and that this could account for the role of FLO8 in pseudohyphae formation. Other flocculins such as Flo1p must not play the same role as Flo11p in Σ1278b strains of yeast, since deletion of FLO11 abolishes the ability to invade or to form pseudohyphae. Flocculation per se does not cause invasiveness or filamentation, since we and others (Gimeno et al., 1992) have observed flocculent strains that do not invade or form filaments.
We show that FLO11 transcripts are induced in diploid cells upon starvation for nitrogen, but not in haploids. Conversely, in rich media FLO11 transcripts persist in haploids but are not expressed in diploids. It may be that the diploid-specific repression of FLO11 in rich media is accomplished through the activity of the FRE, since a lacZ reporter construct regulated by a FRE was shown to be expressed in diploids grown on rich media at less than one-tenth the level in haploids grown in rich media (Madhani and Fink, 1997). Interestingly, overexpression of FLO11 in diploids on rich media enables them to invade, suggesting that the differential expression of FLO11 accounts for the differences in the abilities of haploid and diploid cells to invade. The expression of the FLO11 flocculin on the cell surface may represent a final common pathway of the transcription activators and other regulators of invasiveness and pseudohyphae development.
ACKNOWLEDGMENTS
This work was supported by an award from the Clare Boothe Luce Fund of the Henry Luce Foundation and by grant GM-51059 from the National Institutes of Health to A.M.D. The authors thank Diana Bartelt for the use of equipment and for critical reading of the manuscript, Kevin Wang for plasmids, Gerald Fink and members of his laboratory for generously sharing yeast strains, and two anonymous reviewers for thoughtful critiques.
REFERENCES
- Blacketer MJ, Koehler CM, Coats SG, Myers AM, Madaule P. Regulation of dimorphism in Saccharomyces cerevisiae: involvement of the novel protein kinase homolog elm1p and protein phosphatase 2A. Mol Cell Biol. 1993;13:5567–5581. doi: 10.1128/mcb.13.9.5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacketer MJ, Madaule P, Myers AM. The Saccharomyces cerevisiae mutation elm4–1 facilitates pseudohyphal differentiation and interacts with a deficiency in phosphoribosylpyrophosphate synthase activity to cause constitutive pseudohyphal growth. Mol Cell Biol. 1994;14:4671–4681. doi: 10.1128/mcb.14.7.4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson M, Botstein D. Two differentially regulated mRNAs with different 5′ ends encode secreted and intracellular forms of yeast invertase. Cell. 1982;28:145–154. doi: 10.1016/0092-8674(82)90384-1. [DOI] [PubMed] [Google Scholar]
- Christianson TW, Sikorski RS, Dante M, Shero JH, Heiter P. Multifunctional yeast high-copy-number shuttle vectors. Gene. 1992;110:119–122. doi: 10.1016/0378-1119(92)90454-w. [DOI] [PubMed] [Google Scholar]
- Dolan JW, Fields S. Overproduction of the yeast STE12 protein leads to constitutive transcriptional induction. Genes Dev. 1990;4:492–502. doi: 10.1101/gad.4.4.492. [DOI] [PubMed] [Google Scholar]
- Dranginis AM. Regulation of STA1 gene expression by MAT during the life cycle of Saccharomyces cerevisiae. Mol Cell Biol. 1989;9:3992–3998. doi: 10.1128/mcb.9.9.3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elion EA, Satterberg B, Kranz JE. FUS3 phosphorylates multiple components of the mating signal transduction cascade; evidence for STE12 and FAR1. Mol Biol Cell. 1993;4:495–510. doi: 10.1091/mbc.4.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields S, Herskowitz I. Regulation by the yeast mating-type locus of STE12, a gene required for cell-type-specific expression. Mol Cell Biol. 1987;7:3818–3821. doi: 10.1128/mcb.7.10.3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrias V, Andrianopoulos A, Gimeno CJ, Timberlake WE. Saccharomyces cerevisiae TEC1 is required for pseudohyphal growth. Mol Microbiol. 1996;19:1255–1263. doi: 10.1111/j.1365-2958.1996.tb02470.x. [DOI] [PubMed] [Google Scholar]
- Gimeno CJ, Fink GR. The logic of cell division in the life cycle of yeast. Science. 1992;257:626–626. doi: 10.1126/science.1496375. [DOI] [PubMed] [Google Scholar]
- Gimeno CJ, Fink GR. Induction of pseudohyphal growth by overexpression of PHD1, a Saccharomyces cerevisiae gene related to transcriptional regulators of fungal development. Mol Cell Biol. 1994;14:2100–2112. doi: 10.1128/mcb.14.3.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno CJ, Ljungdahl PO, Styles CA, Fink GR. Unipolar cell divisions in the yeast Saccharomyces cerevisiae lead to filamentous growth: regulation by starvation and RAS. Cell. 1992;68:1077–1090. doi: 10.1016/0092-8674(92)90079-r. [DOI] [PubMed] [Google Scholar]
- Herskowitz I. MAP kinase pathways in yeast: for mating and more. Cell. 1995;80:187–197. doi: 10.1016/0092-8674(95)90402-6. [DOI] [PubMed] [Google Scholar]
- Kaiser C, Michaelis S, Mitchell A. Methods in Yeast Genetics. Plainview, NY: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- Kobayashi O, Suda H, Ohtani T, Sone H. Molecular cloning and analysis of the dominant flocculation gene FLO8 from Saccharomyces cerevisiae. Mol Gen Genet. 1996;251:707–715. doi: 10.1007/BF02174120. [DOI] [PubMed] [Google Scholar]
- Kron SJ, Gow NA R. Budding yeast morphogenesis: signalling, cytoskeleton and cell cycle. Curr Opin Cell Biol. 1995;7:845–855. doi: 10.1016/0955-0674(95)80069-7. [DOI] [PubMed] [Google Scholar]
- Lambrechts MG, Bauer FF, Marmur J, Pretorius IS. Muc1, a mucin-like protein that is regulated by Mss10, is critical for pseudohyphal differentiation in yeast. Proc Natl Acad Sci USA. 1996;93:8419–8424. doi: 10.1073/pnas.93.16.8419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin DE, Errede B. The proliferation of MAP kinase signaling pathways in yeast. Curr Opin Cell Biol. 1995;7:197–202. doi: 10.1016/0955-0674(95)80028-x. [DOI] [PubMed] [Google Scholar]
- Liu H, Styles CA, Fink GR. Elements of the yeast pheromone response pathway required for filamentous growth of diploids. Science. 1993;262:1741–1744. doi: 10.1126/science.8259520. [DOI] [PubMed] [Google Scholar]
- Liu H, Styles CA, Fink GR. Saccharomyces cerevisiae S288C has a mutation in FLO8, a gene required for filamentous growth. Genetics. 1996;144:967–978. doi: 10.1093/genetics/144.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo WS, Dranginis AM. FLO11, a yeast gene related to the STA genes, encodes a novel cell surface flocculin. J Bacteriol. 1996;178:7144–7151. doi: 10.1128/jb.178.24.7144-7151.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo, W.S., Raitses, E.I., and Dranginis, A.M. (1998). Development of pseudohyphae by haploid and diploid yeast; effect of mating type and budding pattern. Curr. Genet. (in press). [DOI] [PubMed]
- Madhani HD, Fink GR. Combinatorial control required for the specificity of yeast MAPK signaling. Science. 1997;275:1314–1317. doi: 10.1126/science.275.5304.1314. [DOI] [PubMed] [Google Scholar]
- Mosch H-U, Fink GR. Dissection of filamentous growth by transposon mutagenesis in Saccharomyces cerevisiae. Genetics. 1997;145:671–684. doi: 10.1093/genetics/145.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosch H-U, Roberts RL, Fink GR. Ras2 signals via the Cdc42/Ste20/mitogen-activated protein kinase module to induce filamentous growth in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1996;93:5352–5356. doi: 10.1073/pnas.93.11.5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng R, Abelson J. Isolation and sequence of the gene for actin in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1980;77:3912–3916. doi: 10.1073/pnas.77.7.3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RL, Fink GR. Elements of a single MAP kinase cascade in Saccharomyces cerevisiae mediate two developmental programs in the same cell type: mating and invasive growth. Genes Dev. 1994;8:2974–2985. doi: 10.1101/gad.8.24.2974. [DOI] [PubMed] [Google Scholar]
- Roberts RL, Mosch H-U, Fink GR. 14, 3–3 proteins are essential for RAS/MAPK cascade signaling during pseudohyphal development in S. cerevisiae. Cell. 1997;89:1055–1065. doi: 10.1016/s0092-8674(00)80293-7. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning, A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sikorski RS, Heiter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague GF, Jr, Thorner JW. Pheromone response and signal transduction during the mating process of Saccharomyces cerevisiae. In: Jones EW, Pringle JR, Broach JR, editors. The Molecular and Cellular Biology of the Yeast Saccharomyces: Gene Expression. Plainview, NY: Cold Spring Harbor Laboratory Press; 1992. pp. 657–744. [Google Scholar]
- Tatchell K, Nasmyth KA, Hall BD, Astell C, Smith M. In vitro mutation analysis of the mating-type locus in yeast. Cell. 1981;27:25–35. doi: 10.1016/0092-8674(81)90357-3. [DOI] [PubMed] [Google Scholar]
- Ward MP, Gimeno CJ, Fink GR, Garrett S. SOK2 may regulate cyclic AMP-dependent protein kinase-stimulated growth and pseudohyphal development by repressing transcription. Mol Cell Biol. 1995;15:6854–6863. doi: 10.1128/mcb.15.12.6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita I, Takano Y, Fukui S. Control of STA1 gene expression by the mating-type locus in yeasts. J Bacteriol. 1985;164:769–773. doi: 10.1128/jb.164.2.769-773.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]