Abstract
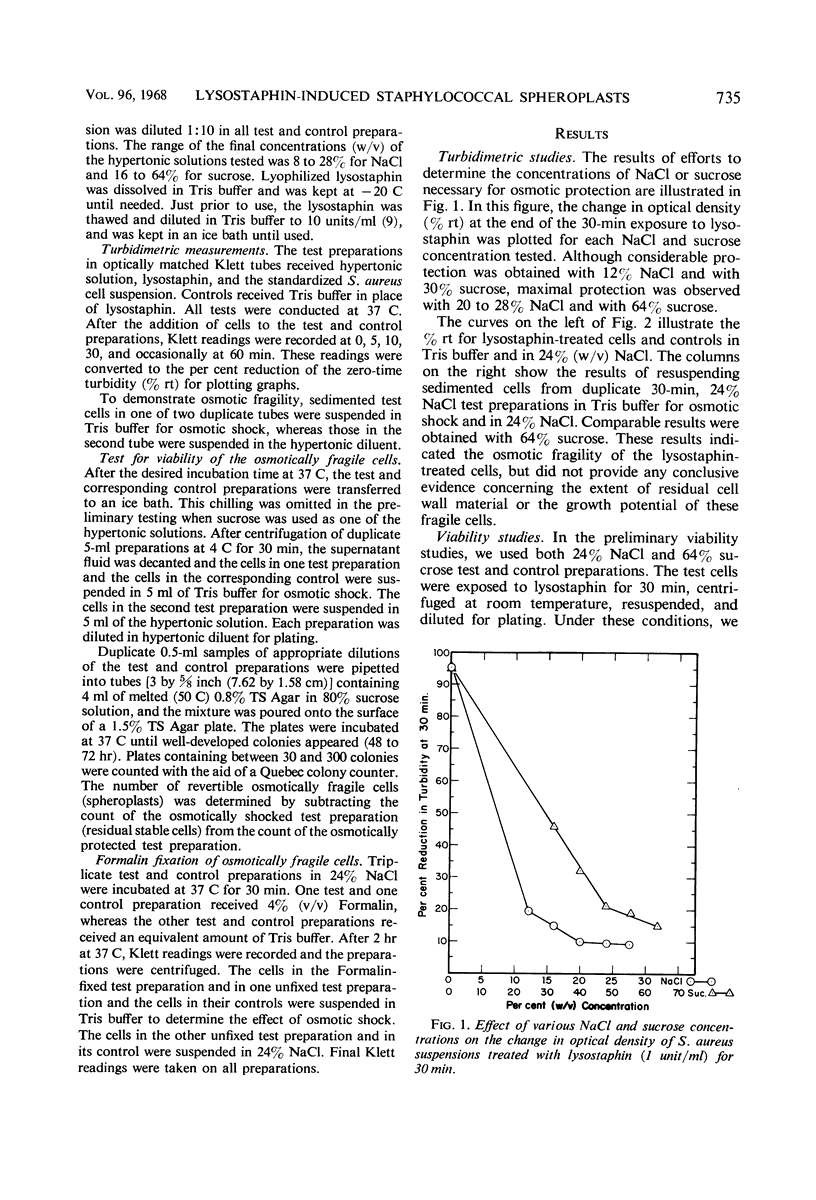
When Staphylococcus aureus FDA 209P cells were treated with lysostaphin (1 unit/ml) in hypertonic sodium chloride or sucrose environments, viable, osmotically fragile spheroplasts were produced. Turbidimetric studies indicated that 64% (w/v) sucrose or 20 to 28% (w/v) sodium chloride gives maximal protection against lysis of the lysostaphin-treated cells. The NaCl appeared to give greater protection than the sucrose and proved to be much more suitable for viability and related studies. Viability of both shocked and nonshocked treated cells was determined by S. aureus colony counts on agar plates overlayered with the test dilution of the cells suspended in 4 ml of semisolid agar containing 72% sucrose. The difference in the counts represented the number of revertible spheroplasts. Under these conditions, 30 to 50% of the test cells were recovered as osmotically fragile, but revertible, spheroplasts after 5 to 10 min of exposure to lysostaphin in 24% NaCl. This rewere obtained after 5 to 10 min of exposure to lysostaphin in 24% NaCl. This recovery rate fell off rapidly with prolonged exposure. In view of residual turbidity of 30- and even 60-min exposure preparations, it appeared probable that most of the osmotically fragile cells were eventually converted to protoplasts by the prolonged lysostaphin treatment. Osmotically fragile cells were converted to osmotic stability by fixation with 4% (v/v) Formalin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWDER H. P., ZYGMUNT W. A., YOUNG J. R., TAVORMINA P. A. LYSOSTAPHIN: ENZYMATIC MODE OF ACTION. Biochem Biophys Res Commun. 1965 Apr 23;19:383–389. doi: 10.1016/0006-291x(65)90473-0. [DOI] [PubMed] [Google Scholar]
- Burke M. E., Pattee P. A. Purification and characterization of a staphylolytic enzyme from Pseudomonas aeruginosa. J Bacteriol. 1967 Mar;93(3):860–865. doi: 10.1128/jb.93.3.860-865.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASH J. H., WISHNICK M., MILLER P. A. FORMATION OF "PROTOPLASTS" OF STAPHYLOCOCCUS AUREUS WITH A FUNGAL N-ACETYLHEXOSAMINIDASE. J Bacteriol. 1964 Feb;87:432–437. doi: 10.1128/jb.87.2.432-437.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHELL P., MOYLE J. Autolytic release and osmotic properties of protoplasts from Staphylococcus aureus. J Gen Microbiol. 1957 Feb;16(1):184–194. doi: 10.1099/00221287-16-1-184. [DOI] [PubMed] [Google Scholar]
- Martin H. H. Bacterial protoplasts--a review. J Theor Biol. 1963 Jul;5(1):1–34. doi: 10.1016/0022-5193(63)90034-1. [DOI] [PubMed] [Google Scholar]
- SCHINDLER C. A., SCHUHARDT V. T. LYSOSTAPHIN: A NEW BACTERIOLYTIC AGENT FOR THE STAPHYLOCOCCUS. Proc Natl Acad Sci U S A. 1964 Mar;51:414–421. doi: 10.1073/pnas.51.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler C. A. The role of NaC1 in the lysis of Staphylococcus aureus by lysostaphin. J Gen Microbiol. 1965 Aug;40(2):199–205. doi: 10.1099/00221287-40-2-199. [DOI] [PubMed] [Google Scholar]
- WEIBULL C. Bacterial protoplasts. Annu Rev Microbiol. 1958;12:1–26. doi: 10.1146/annurev.mi.12.100158.000245. [DOI] [PubMed] [Google Scholar]


