Abstract
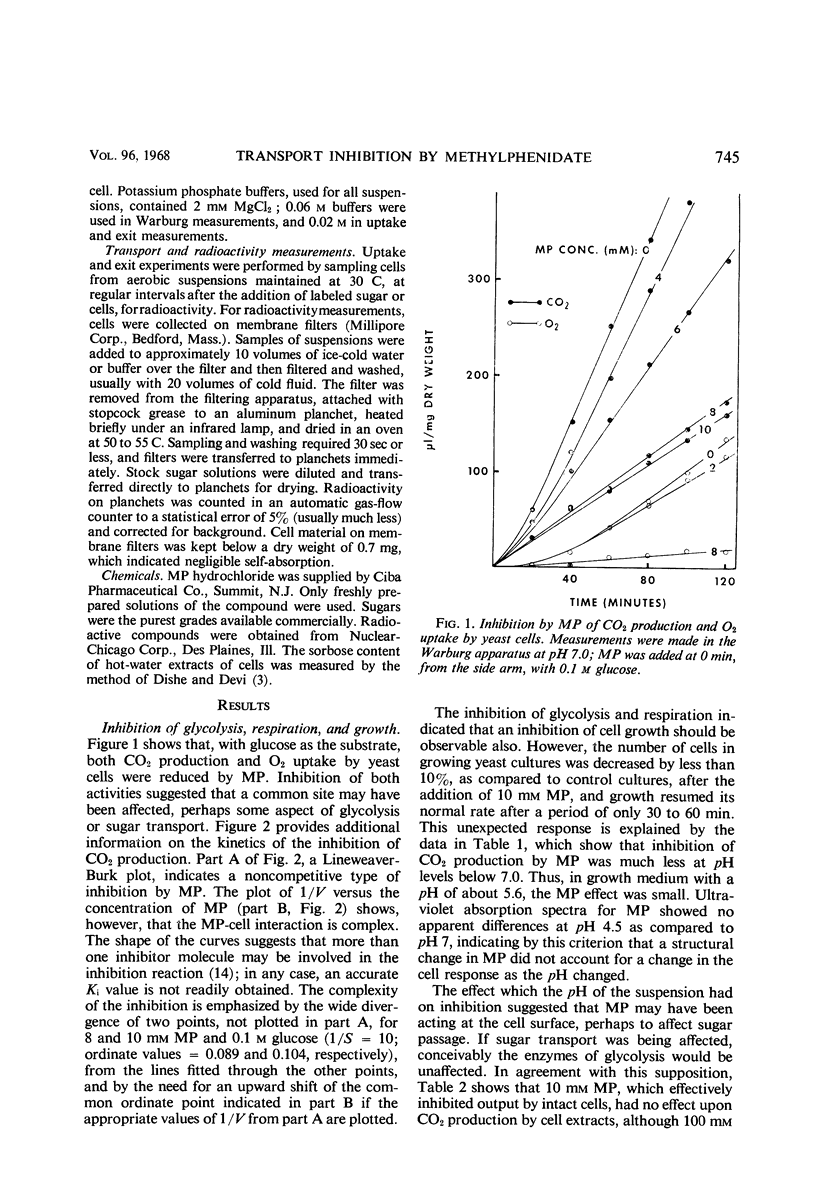
The influence of methylphenidate on glycolysis in yeast cells was studied to describe more fully the nature of the reactions in which this drug participates. CO2 production and O2 uptake of yeast cells was inhibited 75% by a 10 mm concentration of the compound. This effect, with glucose as a substrate, occurred at pH 7.0, but not at pH 4.5. Kinetic data indicated that the reaction was noncompetitive and complex; the methylphenidate effect on CO2 production could not readily be reversed. Glycolysis by cell-free extracts was not inhibited at the 10-mm concentration, but was affected at 100 mm. Utilization of O2 with maltose and ethyl alcohol as substrates also was reduced. Entry into the cells of a number of different carbohydrates and of glycine was inhibited to different degrees. The loss from suspended cells of materials absorbing at 280 nm was reduced, and the efflux of sorbose, arabinose, and lactose was decreased. Thus, transport into and out of the cells was inhibited and leakage, or permeability, was reduced. It is hypothesized that methylphenidate reacts with a cell membrane constituent, or constituents, and inhibits glycolysis by blocking sugar passage.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarado F. Hypothesis for the interaction of phlorizin and phloretin with membrane carriers for sugars. Biochim Biophys Acta. 1967 Jul 3;135(3):483–495. doi: 10.1016/0005-2736(67)90038-7. [DOI] [PubMed] [Google Scholar]
- DISCHE Z., DEVI A. A new colorimetric method for the determination of ketohexoses in presence of aldoses, ketoheptoses and ketopentoses. Biochim Biophys Acta. 1960 Mar 25;39:140–144. doi: 10.1016/0006-3002(60)90129-3. [DOI] [PubMed] [Google Scholar]
- GERHARDT P., JUDGE J. A. POROSITY OF ISOLATED CELL WALLS OF SACCHAROMYCES CEREVISIAE AND BACILLUS MEGATERIUM. J Bacteriol. 1964 Apr;87:945–951. doi: 10.1128/jb.87.4.945-951.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILES C. H., McKAY R. B. Studies in hydrogen bond formation. XI. Reactions between a variety of carbohydrates and proteins in aqueous solutions. J Biol Chem. 1962 Nov;237:3388–3392. [PubMed] [Google Scholar]
- HOAGLAND R. J., McCARTY R. J. Treatment of drug-induced coma: effectiveness of methylphenidate. Am J Med Sci. 1963 Feb;245:189–197. [PubMed] [Google Scholar]
- Kabara J. J., Alvares A. P., Riegel C., McLaughlin J. T. Brain cholesterol x: effect of scheduling on the sterol lowering capability of methylphenidate (Ritalin). Proc Soc Exp Biol Med. 1965 Oct;120(1):37–41. doi: 10.3181/00379727-120-30437. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- SPOERL E., CARLETON R. Studies on cell division; nitrogen compound changes in yeast accompanying an inhibition of cell division. J Biol Chem. 1954 Oct;210(2):521–529. [PubMed] [Google Scholar]
- Spoerl E., Doyle R. J., Thompson M. E. Limiting factors involved in CO2 production by starved and x-irradiated starved yeast cells. J Cell Physiol. 1965 Apr;65(2):271–276. doi: 10.1002/jcp.1030650215. [DOI] [PubMed] [Google Scholar]
- VANSTEVENINCK J., WEED R. I., ROTHSTEIN A. LOCALIZATION OF ERYTHROCYTE MEMBRANE SULFHYDRYL GROUPS ESSENTIAL FOR GLUCOSE TRANSPORT. J Gen Physiol. 1965 Mar;48:617–632. doi: 10.1085/jgp.48.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]


