Abstract
The nuclear accumulation of β-catenin plays an important role in the Wingless/Wnt signaling pathway. This study describes an examination of the nuclear import of β-catenin in living mammalian cells and in vitro semi-intact cells. When injected into the cell cytoplasm, β-catenin rapidly migrated into the nucleus in a temperature-dependent and wheat germ agglutinin–sensitive manner. In the cell-free import assay, β-catenin rapidly migrates into the nucleus without the exogenous addition of cytosol, Ran, or ATP/GTP. Cytoplasmic injection of mutant Ran defective in its GTP hydrolysis did not prevent β-catenin import. Studies using tsBN2, a temperature-sensitive mutant cell line that possesses a point mutation in the RCC1 gene, showed that the import of β-catenin is insensitive to nuclear Ran-GTP depletion. These results show that β-catenin possesses the ability to constitutively translocate through the nuclear pores in a manner similar to importin β in a Ran-unassisted manner. We further showed that β-catenin also rapidly exits the nucleus in homokaryons, suggesting that the regulation of nuclear levels of β-catenin involves both nuclear import and export of this molecule.
INTRODUCTION
The trafficking of macromolecules across the nuclear envelope plays a key role in the coordination of cytoplasmic and nuclear events. The exchange of macromolecules occurs at the nuclear pore complex (NPC), which spans the double lipid bilayer of the nuclear envelope. The NPC is a large proteinaceous structure of ∼125 MDa in size and mediates bidirectional transport via several different mechanisms (for reviews, see Davis, 1995; Fabre and Hurt, 1997). Small molecules, such as ions, low-molecular-weight metabolites, and proteins smaller than 20–40 kDa cross 10-nm-diameter aqueous channels of the NPC by passive diffusion, whereas larger molecules are generally transported through the gated channels of the NPC via an active, receptor-mediated mechanism.
A number of recent discoveries have led to the development of a model for receptor-mediated active nuclear import and export (for reviews, see Corbett and Silver, 1997; Görlich, 1997; Nakielny et al., 1997; Nigg, 1997; Ullman et al., 1997; Imamoto et al., 1998; Mattaj and Englmeier, 1998; Ohno et al., 1998). The model involves two essential elements, which are required for both the import and export pathways: 1) soluble transport factors, which recognize respective signals present in each protein, which is either imported into or exported out of the nucleus; and 2) a small GTPase Ran that affects the affinity between the transport factors and signals by binding directly to the transport factors. Import substrates form a complex with import factors in the cytoplasm, are transported through the NPC, and are then released from the import factors on the nucleoplasmic side of NPC when the GTP-bound form of Ran binds to the import factors. Export substrates form a complex with export factors and Ran-GTP inside the nucleus, are transported through the NPC, and are then released from the export factors when Ran-GTP is converted into Ran-GDP in the cytoplasm or on the cytoplasmic side of NPC.
Proteins that contain a basic-type nuclear localization signal (NLS) are recognized by importin α/β and form a nuclear pore-targeting complex in the cytoplasm to target nuclear pores (Imamoto et al., 1995c). Importin α specifically recognizes the NLS, whereas importin β docks the NLS-containing proteins to NPC by binding directly to importin α and NPC (Adam and Gerace, 1991; Adam and Adam, 1994; Görlich et al., 1994, 1995; Chi et al., 1995; Enenkel et al., 1995; Imamoto et al., 1995a,b; Moroianu et al., 1995; Radu et al., 1995; Weis et al., 1995). Several other importin β–related proteins have been identified as import factors for proteins that contain different types of NLS. For example, transportin binds directly to the M9 sequence of heterogeneous nuclear ribonucleoprotein A1 and has been shown to mediate the import of the M9 sequence–containing protein (Pollard et al., 1996). Importin β requires importin α family proteins (Miyamoto et al., 1997; Tsuji et al., 1997) to bind to its import cargos, whereas the other importin β–related proteins bind directly to their import cargos with no additional adaptor proteins such as importin α being required. Aside from these differences, importin β and its related import factors possess two common properties: their abilities of binding directly to NPC components as well as to Ran-GTP. The binding of Ran-GTP causes the dissociation of import cargos from the import factors (Rexach and Blobel, 1995; Chi et al., 1996; Görlich et al., 1996; Izaurralde et al., 1997).
Other importin β–related proteins have also been identified as export factors. These include CRM1, an export factor for leucine-rich nuclear export signals (Fornerod et al., 1997a; Fukuda et al., 1997; Stade et al., 1997), CAS for importin α (Kutay et al., 1997), and exportin-t for tRNA (Arts et al., 1998; Kutay et al., 1998). These export factors possess homologous N-terminal regions, which are required for Ran binding (Fornerod et al., 1997b; Görlich et al., 1997) and share two common properties: their abilities of interacting with NPC components as well as to Ran-GTP. In contrast to import factors, the binding of Ran-GTP to export factors stabilizes the complex with their export cargos (Fornerod et al., 1997a; Kutay et al., 1997, 1998).
As a result, the GTPase cycle of Ran plays a key role in nuclear import and export, as mediated by importin β–related proteins (for reviews, see Koepp and Silver, 1996; Goldfarb, 1997; Cole and Hammell, 1998; Melchior and Gerace, 1998). Because the only known Ran GTPase-activating protein, Ran GAP1, is localized in the cytoplasm and the cytoplasmic face of NPC (Bischoff et al., 1995; Matunis et al., 1996; Mahajan et al., 1997), and the only known GDP/GTP exchange factor for Ran, RCC1, is localized in the nucleus (Ohtsubo et al., 1989; Bischoff and Ponstingl, 1991), nuclear Ran is thought to be predominantly in its GTP form, whereas cytoplasmic Ran is in its GDP form. Because Ran-GTP affects the binding of cargos with their import and export factors in an opposite manner, a steep gradient of Ran-GTP and Ran-GDP across the nuclear envelope assures the directional transport of cargos through the NPC (Izaurralde et al., 1997). Disruption of the Ran-GTP and Ran-GDP gradient across the nuclear envelope has been shown to severely impair both import and export pathways mediated by importin β–related soluble transport factors. In addition to its role in affecting transport complex formation, GTP hydrolysis of Ran is thought to provide an energy source for the energy-dependent NPC translocation of import substrates (Melchior et al., 1995a), but the precise translocation mechanism remains obscure. On the other hand, in the previous study, we showed that importin β itself does not require Ran or its GTP hydrolysis for translocating through the NPC when not carrying import cargos (Kose et al., 1997). Similar observations have also been reported for other importin β–related proteins, indicating Ran-unassisted import could be a common feature for importin β–related proteins (Kutay et al., 1998; Nakielny and Dreyfuss, 1998).
In addition to the constitutive nuclear import of proteins such as SV40 T-antigen NLS substrates, the extracellular signal-dependent nuclear protein import has also been studied. We have recently shown that the interferon-γ–dependent nuclear import of Stat1 (signal transducer and activator of transcription 1) is mediated by the nuclear pore-targeting complex via the influenza virus nucleoprotein interacter 1 family, but not the Rch1 family, of importin α in conjunction with importin β, and Ran (Sekimoto et al., 1996, 1997). In this study, we focused on and examined the nuclear import of β-catenin. It is well known that β-catenin functions as a key signaling molecule involved in the Wingless/Wnt signal transduction pathway. β-Catenin was first identified as a component of the cell–cell adhesion complex that binds directly to the cadherin adhesion molecule (McCrea et al., 1991), but the participation of β-catenin in signal transduction has been shown to be dependent on its nuclear function (Funayama et al., 1995; Behrens et al., 1996; Molenaar et al., 1996; Orsulic and Peifer, 1996; Schneider et al., 1996). The Wingless/Wnt signal triggers an increase in the cytoplasmic pool of β-catenin, and free cytoplasmic β-catenin then migrates into the nucleus, where it regulates the transcription of Wingless responsible genes with DNA-binding proteins called the LEF/TCF (lymphocyte enhancer factor/T-cell factor) family (for reviews, see Cavallo et al., 1997; Gumbiner, 1997; Kuhl and Wedlich, 1997; Shapiro, 1997; Brown and Moon, 1998). It has been proposed that the cytoplasmic level of β-catenin is largely regulated by degradation, which is initiated by phosphorylation through the action of glycogen synthase kinase 3β and interaction with adenomatous polyposis coli (APC) protein (Munemitsu et al., 1995; Rubinfeld et al., 1996). A number of recent reports showing the frequent genetic alteration of APC or β-catenin gene in colorectal cancer (Korinek et al., 1997; Morin et al., 1997; Iwao et al., 1998), melanoma (Rubinfeld et al., 1997) or hepatocellular carcinomas (Miyoshi et al., 1998), which results in the stabilization of β-catenin, suggest a close correlation between the nuclear accumulation of β-catenin and the formation of several types of cancer.
It was previously proposed that β-catenin accumulates in the nucleus in the form of a complex with the LEF/TCF family possessing typical basic-type NLS, based on the observation that β-catenin strongly accumulated in the nucleus in TCF/LEF-overexpressing cells (Behrens et al., 1996; Huber et al., 1996; Molenaar et al., 1996). However, in living cells, we found that the nuclear import of β-catenin is insensitive to cytoplasmically injected mutant Ran defective in its GTP hydrolysis (G19V Ran), which is a potent inhibitor of importin β- and transportin-dependent import pathways. Studies using tsBN2 cells, temperature-sensitive baby hamster kidney cells that possess a point mutation in the RCC1 gene, further showed that the nuclear import of β-catenin is insensitive to disruption of the Ran-GTP/Ran-GDP gradient across the nuclear envelope. In the in vitro transport assay, β-catenin rapidly migrates into the nucleus without the addition of cytosolic extract or Ran, and its import was not inhibited by the addition of G19V Ran-GTP. These results show that β-catenin migrates into the nucleus without the aid of soluble transport factors, such as importin α/β or transportin, and such an import pathway requires neither Ran nor its GTP hydrolysis. The nuclear migration of β-catenin was saturable and competitively inhibited by importin β, showing that specific molecular interactions at NPC are involved in β-catenin import. The further observation that the β-catenin also rapidly exits the nucleus suggests that the regulation of the nuclear level of β-catenin involves the balance between nuclear import and export of this protein.
MATERIALS AND METHODS
Cell Culture
Madin–Darby bovine kidney (MDBK) cells were cultured in DMEM supplemented with 5% FBS (Dainippon Pharmaceutical, Osaka, Japan). Baby hamster kidney 21 (BHK21) and tsBN2 cells were cultured in DMEM supplemented with 10% FBS at 37 and 33.5°C, respectively. MDBK cells were plated on coverslips for microinjection experiments or on eight-well multitest slides (ICN Biomedicals, Costa Mesa, CA) for in vitro transport assay 24 h-36 h before each experiment. BHK21 cells were grown on coverslips for 48 h at 37°C, and tsBN2 cells were grown on coverslips for 24 h at 33.5°C (permissive temperature) or 39.5°C (nonpermissive temperature) before use in microinjection experiments. BHK21 cells were fused by the hemagglutinating virus of Japan (Sendai virus) as described previously (Tachibana, et al., 1994). Homokaryons were incubated for 3 h at 37°C before microinjection experiments.
Recombinant Expression and Purification
The recombinant mouse β-catenin proteins were generated as follows. The pBluescript SK(−) carrying the entire coding region of mouse β-catenin with BamHI and KpnI sites at the ends was kindly provided by Drs. A. Nagafuchi and Sh. Tsukita (Faculty of Medicine, Kyoto University, Kyoto, Japan). The insert of full-length β-catenin was subcloned into a modified pGEX-6P2 expression vector at the BamHI and KpnI sites, and oligonucleotides encoding the FLAG epitope (DYKDDDDK) were ligated into the N terminus of the β-catenin gene at the BamHI site. To construct the expression vector of the green fluorescent protein (GFP) chimera of β-catenin, the full-length mouse β-catenin ORF was amplified by PCR using the synthetic oligonucleotides (5′-GATCGGATCCATGGCTATCCAAGTCGACC-3′ and 5′-GATCGCGGCCGCGGACGATTTACAGGTCAG-3′), and this PCR product was inserted into the BamHI and NotI sites of pGEX6P2 carrying the S65A/Y145F humanized GFP gene at the N terminus of multicloning sites (kindly provided by Dr. S. Kuroda, Osaka University, Institute of Scientific and Industrial Research).
The expression vectors described above were transformed into Escherichia coli strain BL21(DE3), and the expression was induced by addition of 0.2 mM isopropyl-β-d-thiogalactopyranoside and incubation for 12 h at 20°C. Bacteria were lysed in buffer A (50 mM Tris-HCl, pH 8.0, 50 mM NaCl, 2 mM DTT, which contained 1 μg/ml aprotinin, leupeptin, and pepstatin) with freeze–thaw and sonication and clarified by centrifugation (45,000 rpm, 30 min). The resultant supernatant was incubated with glutathione-Sepharose at 4°C for 30 min. After extensive washing, the Sepharose beads were incubated with PreScission Protease (Pharmacia, Piscataway, NJ) for 5 h at 4°C in buffer B (50 mM phosphate, pH 7.2, 50 mM NaCl, 2 mM DTT, containing 1 μg/ml aprotinin, leupeptin, and pepstatin) to recover GST cleaved FLAG- or GFP-β-catenin in the supernatant. The FLAG- or GFP-β-catenin was further purified on a MonoQ column (Pharmacia) with a linear gradient of buffer B containing 50 mM–1.0 M NaCl at a flow rate of 0.5 ml/min. The first peak fractions containing the β-catenin proteins were collected, desalted with a PD10 column (Pharmacia) equilibrated with buffer B, and concentrated by ultrafiltration using Microcon 50 (Amicon, Danvers, MA).
E. coli strains expressing wild-type and G19V Ran were obtained as described previously (Sekimoto et al., 1996). Recombinant wild-type and G19V Ran were expressed, purified, and charged with GTP and GDP according to the methods of Bischoff and Ponstingl (1995) and Melchior et al. (1995b) with slight modifications. Briefly, expression was induced by addition of 1 mM isopropyl-β-d-thiogalactopyranoside and incubation for 14 h at 20°C. The E. coli cells were lysed in buffer C (50 mM Tris-HCl, pH 8.0, 75 mM NaCl, 1 mM MgCl2, 0.1 mM PMSF, 1 mM DTT, 1 μg/ml aprotinin, leupeptin, and pepstatin) by freeze–thaw and the addition of lysozyme. The clarified lysates were applied to a diethylaminoethyl-Sepharose FF column (Pharmacia), and flow-through fractions were collected. After precipitation in 60% saturated ammonium sulfate solution, 2 mM GTP or GDP was added, and the solution was incubated for at least 1 h in buffer D (50 mM phosphate buffer, pH 7.0, 1 mM 2-mercaptoethanol, 10% glycerol, 2 μM GTP or GDP) on ice. The samples were applied to a HiPrep Sephacryl S-200 HR fast-performance liquid chromatography column (Pharmacia) equilibrated with buffer D, and peak fractions containing Ran proteins were pooled. GTP and GDP forms of Ran were further separated on a Fractogel EMDSO3-650 (s) column (Merck, Darmstadt, Germany) with a linear gradient of buffer D containing 50 mM phosphate to 500 mM phosphate. The purified GTP or GDP form of Ran was desalted with a PD10 column equilibrated with transport buffer (see below) and concentrated. Ninety-five percent of purified wild-type and G19V Ran-GTP were charged with GTP, and 100% of purified wild-type Ran-GDP was charged with GDP by this procedure.
The human transportin/karyopherin β2 gene (Pollard et al., 1996) was amplified from a Hela cell cDNA library by PCR using the synthetic oligonucleotide primers (5′-CTCAGCGGATCCATGGAGTATGAGTGGAAACCTGAC-3′ and 5′-CTCAGCGGTACCTTAAACACCATAAAAAGCTGCAAG-3′). The PCR product was inserted into the BamHI and KpnI sites of a modified pGEX-6P-3 (Pharmacia) and verified by DNA sequencing. Recombinant GST-transportin was expressed as described previously (Imamoto et al., 1995b) and purified to homogeneity using glutathione-Sepharose (Pharmacia) following the manufacturer’s recommended protocol.
A nuclear localization domain (M9) in the heterogeneous nuclear ribonucleoprotein A1 protein (Siomi and Dreyfuss, 1995) was amplified from a Hela cell cDNA library by the PCR using the synthetic oligonucleotide primers (5′-CTCAGCGGATCCGGAGGTGGTGGAAGCTACAATG-3′ and 5′-ATAGCCACCTTGGTTTCGTGG-3′). The PCR product was inserted into the BamHI and SmaI sites of pGST-GFP (Tachibana et al., 1996) and verified by DNA sequencing. The oligonucleotide encoding SV40 large T-antigen NLS (PKKKRKVEDP) was ligated into the BamHI and SmaI sites of pGST-GFP to obtain the GST-NLS-GFP expression plasmid. The GST-M9-GFP and GST-NLS-GFP fusion proteins were expressed as described previously (Imamoto et al., 1995b) and purified to homogeneity using glutathione-Sepharose (Pharmacia) following the manufacturer’s recommendations.
Recombinant mouse importin β and GFP-importin β proteins were expressed and purified as described previously (Kose et al., 1997).
In Vitro Transport Assay
Digitonin-permeabilized MDBK cells were prepared as described previously (Kose et al., 1997). Unless described differently in figure legends, 10 μl of testing solution usually contained 8 pmol of FLAG-β-catenin in transport buffer (20 mM HEPES, pH 7.3, 110 mM potassium acetate, 2 mM magnesium acetate, 5 mM sodium acetate, 0.5 mM EGTA, 2 mM DTT, 1 μg/ml aprotinin, leupeptin, and pepstatin) containing 2% BSA. Where indicated, cytosol prepared from mouse Ehrlich ascites tumor cells, recombinant wild-type or mutant Ran proteins, or recombinant importin β or transportin and energy source (ATP and GTP) were included in the above 10-μl testing solution. Reactions involving the addition of ATP and GTP were performed in the presence of 1 mM ATP (Sigma, St. Louis, MO; A-6410), 5 mM phosphocreatine (Sigma, P-6502), 20 U of creatine kinase (Sigma, C-3755), and 0.5 mM GTP (Boehringer Mannheim, Indianapolis, IN), whereas reactions without nucleotides were performed in the absence of nucleotides and ATP-regenerating systems. Pretreatment of permeabilized cells with apyrase was performed as follows. Digitonin-permeabilized cells were incubated with transport buffer containing 0.1 U/ml apyrase (Sigma, A-6410) and 2% BSA for 5 min at 30°C. After rinsing the cells with transport buffer, they were incubated with import mixtures containing β-catenin or NLS substrate. For wheat germ agglutinin (WGA) treatment, permeabilized cells were incubated with 0.5 mg/ml WGA (EY Laboratories, San Mateo, CA) for 5 min on ice before the import reaction. The import reaction was performed for 20 min at 30°C or on ice, and the cells were then washed twice with ice-cold transport buffer and fixed with 3.7% formaldehyde in transport buffer (minus DTT) for 10 min at room temperature.
Microinjection
Recombinant β-catenin protein was injected through a glass capillary into the cytoplasm or nucleus of cells grown on coverslips. Where indicated, WGA, G19V Ran-GTP, or NLS substrate was coinjected with β-catenin protein. After incubation for 30 min at 37°C or on ice, the cells were washed twice with PBS and fixed with 3.7% formaldehyde in PBS for 10 min at room temperature.
Indirect Immunofluorescence
To examine the localization of FLAG-β-catenin, the fixed cells were permeabilized with 0.5% Triton X-100 in PBS for 5 min at room temperature, incubated with 3% skim milk in PBS for 20 min, and then incubated with 30 μg/ml murine immunoglobulin G1 (IgG1) monoclonal anti-FLAG M2 antibody (Eastman Kodak, Rochester, NY) for 1 h at room temperature. The mouse antibody was detected with CY3-labeled goat antibodies to mouse IgG (Tago, Burlingame, CA). The samples were examined using an Axiophoto microscope (Carl Zeiss, Thornwood, NY).
Conjugation of Texas Red with BSA
BSA was dissolved at 5 mg/ml in 0.1 M carbonate buffer, pH 9.5, and mixed with 0.5 mg of Texas Red. After incubation for 2 h at room temperature, free fluorophore was removed by gel filtration on a PD10 (Pharmacia) equilibrated with 20 mM HEPES, pH 7.3, 110 mM potassium acetate. Peak fractions containing Texas Red–labeled BSA were collected and dialyzed against 20 mM HEPES, pH 7.3, 110 mM potassium acetate.
Recombinant FLAG-β-Catenin Injection and Treatment of Xenopus Embryos
Egg collection and fertilization were performed as described previously (Guger and Gumbiner, 1995). The equatorial region of a single prospective ventral blastomere of the four-cell stage embryo was injected with purified recombinant FLAG-β-catenin in volumes of ∼25 nl (∼25 ng of protein). Embryos were then allowed to develop at room temperature in 0.1× modified Barth’s solution [88 mM NaCl, 1 mM KCl, 0.82 mM MgSO4, 0.41 mM CaCl2, 0.33 mM Ca(NO3)2, 2.4 mM NaHCO3, 10 mM HEPES, pH 7.4].
Others
Allophycocyanin (Calbiochem, La Jolla, CA) was chemically conjugated to a synthetic peptide containing the amino acid sequence of SV40 large T-antigen NLS (CYGGPKKKRKVEDP; purchased from the Peptide Institute, Mino, Osaka, Japan ) as described previously (Imamoto et al., 1995c). Ehrlich ascites tumor cell cytosol was prepared as described previously (Imamoto et al., 1995c).
RESULTS
β-Catenin Rapidly Migrates into the Nucleus in a Temperature-dependent and WGA-sensitive Manner in Living Cells
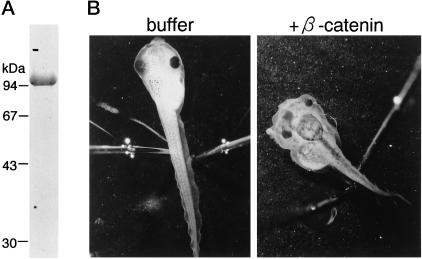
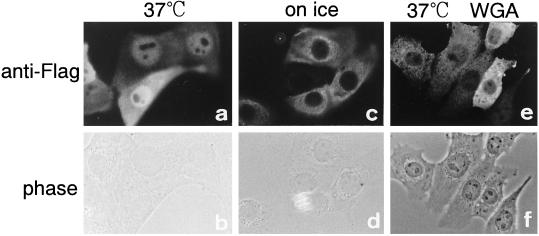
To examine the nuclear import of β-catenin, recombinant mouse β-catenin, tagged with a FLAG epitope at its N terminus, was bacterially expressed, purified to homogeneity, and used as an import substrate. The purified FLAG-β-catenin was confirmed to possess signaling activity by examining its ability to induce axis duplication by directly injecting the recombinant protein into a Xenopus embryo (Figure 1). As shown in Figure 2, the FLAG-β-catenin, when injected into the cytoplasm of living mammalian cells, rapidly migrated into the nucleus within 5–10 min. A portion of the injected β-catenin was also observed at the plasma membrane, probably because of interaction with its known binding proteins. The nuclear migration of β-catenin in living cells was temperature dependent and was potently inhibited by WGA. The inhibition of import by WGA shows that the observed nuclear migration of β-catenin does not result from the passive diffusion of degradated products but that this protein migrates into the nucleus through gated channels of NPC (Finlay et al., 1987; Yoneda et al., 1987). Such import of β-catenin has been observed in Hela cells, BHK21 cells, and MDBK cells.
Figure 1.
Double axis formation induced by purified recombinant β-catenin. (A) SDS-PAGE profile of purified β-catenin. The purified FLAG-β-catenin was subjected to 10% SDS-PAGE and stained with Coomassie Brilliant Blue. (B) Right panel, a Xenopus embryo was injected at the four-cell stage with purified recombinant FLAG-β-catenin (∼25 ng protein/25 nl) in 50 mM potassium phosphate buffer, pH 7.2, and 50 mM NaCl. Tadpoles exhibit axis duplication, including two eye pairs and two cement glands. Left panel, control experiment demonstrating that the injection of BSA in the same buffer had no effect.
Figure 2.
β-Catenin migrates into the nucleus of living cells in a temperature-dependent and WGA-sensitive manner. Purified recombinant mouse FLAG-β-catenin (1 mg/ml) was injected into the cytoplasm of MDBK cells with (e and f) or without (a–d) 2 mg/ml WGA. After incubation for 30 min at 37°C (a, b, e, and f) or on ice (c and d), cells were fixed with 3.7% formaldehyde in PBS. Injected FLAG-β-catenin was visualized by indirect immunofluorescence with anti-FLAG mouse mAb and CY3-labeled anti-mouse goat IgG. The localization of β-catenin was examined by Axiophoto microscopy (Zeiss).
β-Catenin Rapidly Migrates into the Nucleus of Permeabilized Cells without the Addition of Soluble Factors and ATP/GTP
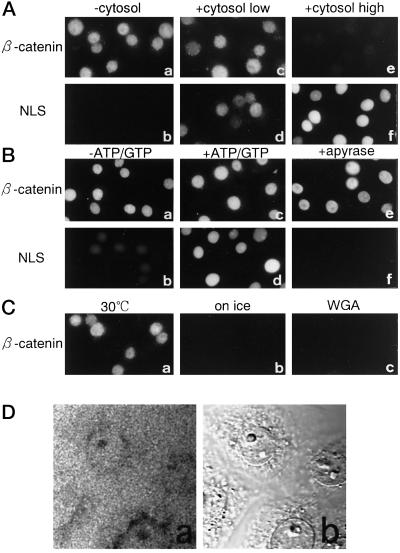
We further examined the import of β-catenin using a digitonin-permeabilized cell-free transport assay (Adam et al., 1990). We found that β-catenin rapidly migrated into the nucleus in the absence of exogenously added cytosol or nucleotides (Figure 3, A and B). The nuclear migration of β-catenin was sensitive to temperature and was inhibited by WGA as in living cells (Figure 3C). Furthermore, it was found that the addition of cytosol did not stimulate but rather inhibited import at high concentration. We also examined whether β-catenin associates with importin α and β, either directly or indirectly, through the pull-down assay using GST-β-catenin, and the immunoprecipitation of FLAG-β-catenin using an anti-FLAG antibody. β-Catenin, when incubated in the crude cytosol prepared from Ehrlich ascites tumor cells or alone with purified recombinant importin α or β or both proteins, did not associate with either importin α or β (our unpublished results).
Figure 3.
β-Catenin migrates into the nucleus in the absence of exogenous soluble factors or ATP in the digitonin-permeabilized cell-free import assay. (A) Digitonin-permeabilized MDBK cells were incubated with 10 μl of testing solution containing 8 pmol of FLAG-β-catenin (a, c, and e) or 1 μg of allophycocyanin-NLS (b, d, and f) in the presence of 10 mg/ml cytosol (e and f) or 1 mg/ml cytosol (c and d) or in the absence of cytosol (a and b). All reaction mixtures contained ATP and GTP. After incubation for 20 min at 30°C, cells were fixed, and FLAG-β-catenin was visualized by indirect immunofluorescence with anti-FLAG mouse mAb and CY3-labeled anti-mouse goat IgG. Allophycocyanin-NLS was directly examined by Axiophoto microscopy (Zeiss) after fixation. (B) Digitonin-permeabilized MDBK cells were incubated with 10 μl of testing solution containing 8 pmol of FLAG-β-catenin (a, c, and e) without cytosol or 1 μg of allophycocyanin-NLS (b, d, and f) supplemented with 10 mg/ml cytosol in the absence (a, b, e, and f) or presence (c and d) of ATP and GTP. e and f show import reactions performed in the permeabilized cells, which were pretreated with apyrase (see MATERIALS AND METHODS). After incubation for 20 min at 30°C, cells were fixed, and the localization of β-catenin and allophycocyanin-NLS was examined as in A. (C) Digitonin-permeabilized MDBK cells were incubated with 8 pmol of FLAG-β-catenin for 20 min at 30°C (a and c) or on ice (b). c shows the import reaction performed in the permeabilized cells, which were pretreated with WGA (see MATERIALS AND METHODS). After incubation, cells were fixed, and localization of β-catenin was examined as in A. (D) Digitonin-permeabilized MDBK cells were incubated with 8 pmol of GFP-β-catenin in the absence of energy source as in B. After 20 min of incubation, distribution of GFP-β-catenin (a) was examined by confocal laser scanning microscope (Zeiss, LSM410) using a 40× objective with oil immersion, without washing and fixation of the cells. Cells were recorded simultaneously by interference contrast (b). A 1-μm section, corresponding to the equator of the nucleus, is shown. The final figure was produced using Adobe Photoshop (Adobe Systems, Mountain View, CA).
Furthermore, the addition of either ATP or GTP or both failed to stimulate the import, and preincubation of cells with apyrase also did not inhibit import (Figure 3B), whereas the import of allophycocyanin conjugated to SV40 T-antigen NLS peptide (allophycocyanin-NLS) was completely abolished in the apyrase-pretreated permeabilized cells. It should be noted that the incubation of permeabilized cells with apyrase can readily diminish free ATP from permeabilized cells but does not completely deplete the nucleotides retained in the cells, even after prolonged incubation (our unpublished results). Therefore, these data do not necessarily show that the nuclear import of β-catenin is completely independent of ATP/GTP as well as their hydrolysis, but differences in sensitivity to nucleotide depletion between import of β-catenin and SV40 T-antigen NLS substrate show that the requirement for ATP and/or GTP is much less for the β-catenin import pathway compared with the importin α/β–mediated import pathway. Moreover, as shown in Figure 3D, N-terminally tagged GFP-β-catenin, which showed the same import behavior as FLAG-β-catenin both in living cells and the in vitro transport assay, did not accumulate into the nucleus of permeabilized cells against a concentration gradient when the cells were examined directly without fixation, indicating that the β-catenin import is not an energy-dependent active process.
Nuclear Migration of β-Catenin Is Insensitive to Excess Cytoplasmic Ran-GTP
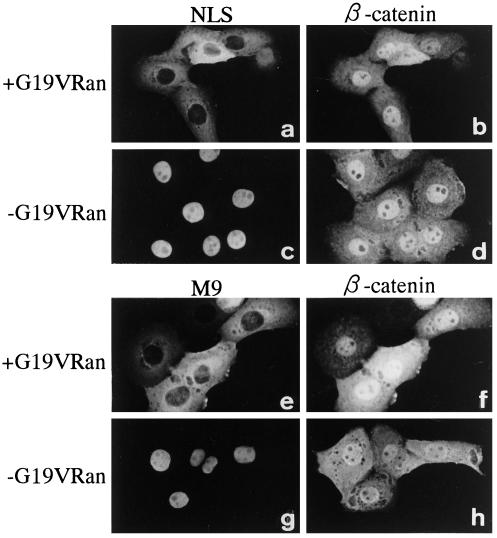
To understand the role of Ran in the nuclear import of β-catenin, we first examined the effect of mutant Ran defective in its GTP hydrolysis (G19V Ran) on the nuclear accumulation of β-catenin in living cells. The G19V Ran-GTP, when coinjected into the cytoplasm, has been shown to potently inhibit nuclear import mediated by importin α/β (Sekimoto et al., 1996; Kose et al., 1997). G19V Ran-GTP also inhibited transport mediated by transportin (Figure 4). However, in the case of β-catenin, we observed no effect of coinjection of G19V Ran-GTP on its nuclear accumulation (Figure 4, b and f). It is noteworthy that raising the concentration of G19V Ran-GTP up to fivefold, which completely inhibits the nuclear import of the SV40 T-antigen NLS-containing substrate, still had no effect on the β-catenin import (import of the NLS substrate was completely inhibited by the coinjection of 1 mg/ml G19V Ran-GTP). These results show that the import of β-catenin is not affected by excess cytoplasmic Ran-GTP.
Figure 4.
Nuclear accumulation of β-catenin is not inhibited by mutant Ran (G19V Ran)-GTP in living cells. A mixture of FLAG-β-catenin (0.8 mg/ml) and GST-NLS-GFP (0.5 mg/ml) (a–d) or GST-M9-GFP (1 mg/ml) (e–h) was coinjected with 5 mg/ml G19V Ran-GTP (a, b, e, and f) or alone (c, d, g, and h) into the cytoplasm of MDBK cells. After incubation for 30 min at 37°C, cells were fixed, and the localization of FLAG-β-catenin (b, d, f, and h) was examined as in Figure 2. The localization of GST-NLS-GFP (a and c) or GST-M9-GFP (e and g) shows that G19V Ran-GTP strongly inhibited the nuclear accumulation of these two substrates, whereas the nuclear accumulation of FLAG-β-catenin was not affected in the same cells.
Nuclear Migration of β-Catenin Is Insensitive to Nuclear Ran-GTP Depletion
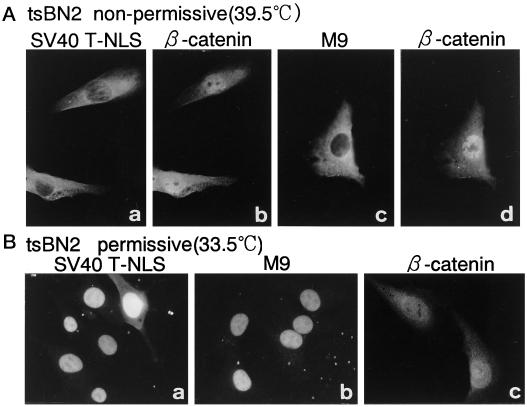
We next asked whether the depletion of Ran-GTP from the nucleus affects β-catenin import. For this, we examined β-catenin import in tsBN2 cells, a temperature-sensitive BHK21 cell line that possesses a point mutation in the RCC1 gene (Nishimoto et al., 1978; Uchida et al., 1990). In tsBN2 cells, when cultured at nonpermissive temperature, RCC1 rapidly loses its activity, and as a result, nuclear Ran-GTP declines (Nishitani et al., 1991; Ren et al., 1993). When nuclear import was examined in tsBN2 cells cultured at nonpermissive temperature for 24 h, the import of SV40 T-antigen NLS substrate was inhibited in 50–60% of the cells examined 30 min after cytoplasmic injection, which is consistent with our previous report (Tachibana et al., 1994). The import of M9 sequence-containing substrates was found to be even more inhibitory (80–90% inhibition) under the same conditions. In contrast, no import inhibition or decline of β-catenin in these cells was observed (Figure 5). β-Catenin migrated into the nucleus of tsBN2 cells to a similar extent whether nuclear migration of coinjected SV40 T-antigen NLS substrate was impaired. The import inhibition of β-catenin by WGA was confirmed in these cells (our unpublished results). These results indicate that the import of β-catenin is the most insensitive to nuclear Ran-GTP depletion among the import substrates examined.
Figure 5.
Nuclear accumulation of β-catenin is insensitive to nuclear Ran-GTP depletion in living cells. A mixture of 0.8 mg/ml FLAG-β-catenin and 0.5 mg/ml GST-NLS-GFP (a and b) or 1 mg/ml GST-M9-GFP (c and d) was injected into the cytoplasm of tsBN2 cells cultured at nonpermissive temperature (upper panels). After further incubation for 30 min at nonpermissive temperature, cells were fixed, and localization of FLAG-β-catenin (b and d) was examined as in Figure 2. Localization of GST-NLS-GFP (a) or GST-M9-GFP (c) shows that nuclear accumulation of these two substrates declined in the tsBN2 cells incubated at nonpermissive temperature, whereas nuclear accumulation of FLAG-β-catenin was not affected in the same cells. Lower panels, control experiments showing that GST-NLS-GFP (a), GST-M9-GFP (b), and FLAG-β-catenin (c) all migrate normally in the nucleus of tsBN2 cells, which were incubated at permissive temperature within 30 min after cytoplasmic injection.
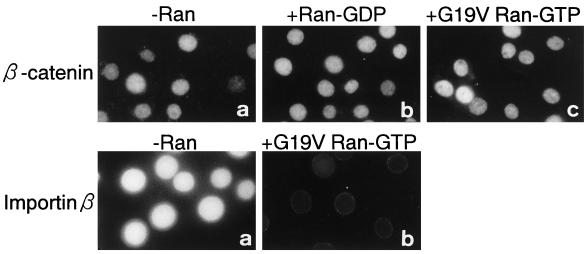
Last, we examined the requirement of Ran for β-catenin import using the in vitro transport assay. The addition of Ran, at various concentrations, completely failed to stimulate the import (Figure 6). Moreover, addition of G19V Ran-GTP, at a concentration that completely inhibits the nuclear accumulation of importin β (Figure 6, lower panels) and the SV40 T-antigen NLS-containing substrate (our unpublished results), failed to inhibit the nuclear accumulation of β-catenin. These results provide further supporting evidence that Ran, as well as its GTP hydrolysis, is not required for the import of β-catenin.
Figure 6.
Nuclear accumulation of β-catenin does not require Ran in vitro. Upper panel, digitonin-permeabilized MDBK cells were incubated with a 10-μl testing solution containing 8 pmol of FLAG-β-catenin in the absence (a) or presence of 80 pmol of Ran-GDP (b) or 80 pmol of G19V Ran-GTP (c). After incubation for 30 min at 30°C, the cells were fixed, and the localization of FLAG-β-catenin was examined as in Figure 3. Lower panel, digitonin-permeabilized MDBK cells were incubated with 10 μl of testing solution containing 3 pmol GFP-importin β in the absence (a) or presence of 80 pmol of G19V Ran-GTP (b). After incubation for 20 min at 30°C, the cells were fixed, and the localization of GFP-importin β was examined by Axiophoto microscopy (Zeiss).
Nuclear Migration of β-Catenin Is Saturable
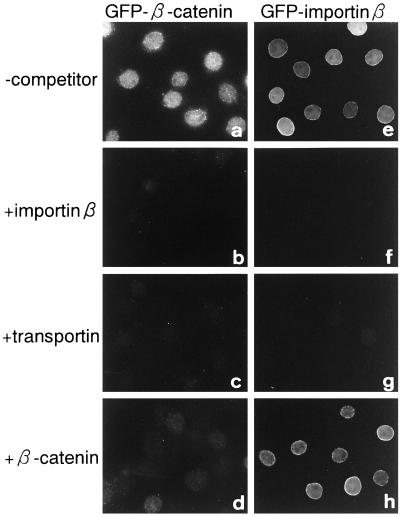
WGA potently inhibited β-catenin import, showing that this protein migrates into the nucleus of the gated channels of the NPC but not the diffusive channel. To further confirm whether nuclear accumulation of β-catenin actually involves a facilitated process, we examined the saturability of import. As shown in Figure 7, the nuclear accumulation of GFP-β-catenin was competitively inhibited by FLAG-β-catenin. The nuclear accumulation of β-catenin was also strongly inhibited by both importin β and transportin. These results show that the import of β-catenin is saturable, and that the migration occurs via an interaction with specific NPC components, which importin β and transportin interact with. However, excess FLAG-β-catenin did not inhibit the nuclear migration of GFP-importin β, while transportin did inhibit the nuclear migration of GFP-importin β, which will be discussed below.
Figure 7.
Nuclear accumulation of β-catenin is saturable and competitively inhibited by importin β family proteins. Digitonin-permeabilized MDBK cells were incubated with 10 μl of testing solution containing 4 pmol of GFP-β-catenin (a–d) or 4 pmol of GFP-importin β (e–h) in the absence (a and e) or presence of 80 pmol of importin β (b and f), 80 pmol of GST-transportin (c and g), or 80 pmol of FLAG-β-catenin (d and h). After incubation for 10 min at 30°C, cells were fixed, and localization of the GFP fusion proteins was examined by Axiophoto microscopy (Zeiss).
β-Catenin Is Able to Rapidly Exit the Nucleus
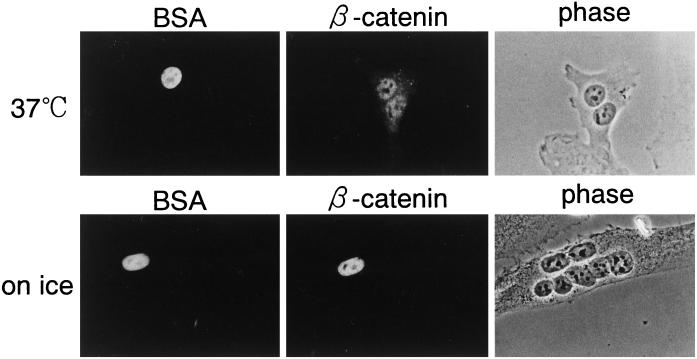
The above data show that β-catenin possesses the ability to migrate rapidly into the nucleus constitutively, without forming a complex with LEF and TCF families. However, β-catenin is not constitutively and exclusively localized in the nuclei of all cells that express β-catenin. The intracellular level of β-catenin is largely regulated by its degradation, which is thought to occur in the cytoplasm. Such physiological evidence prompted us to investigate whether β-catenin exits the nucleus. To determine whether β-catenin is exported from the nucleus, microinjection studies were performed in homokaryons of mammalian cells. As shown in Figure 8, β-catenin, when injected into one of the nuclei in multinucleated cells, rapidly exited the nucleus and migrated into all the nuclei within 30 min after injection, whereas coinjected Texas Red–labeled BSA stayed only in the injected nucleus. This reaction was temperature dependent. These results show that β-catenin has the capability of exiting the nucleus of a living cell.
Figure 8.
β-Catenin rapidly exits the nucleus in a temperature-dependent manner. A mixture of 1 mg/ml GFP-β-catenin and 0.5 mg/ml Texas Red–labeled BSA was injected into the nuclei of homokaryon of BHK21 cells. After incubation for 30 min at 37°C (upper panels) or on ice (lower panels), cells were fixed, and localization of GFP-β-catenin (middle panels) and Texas Red–labeled BSA (left panels) was examined by Axiophoto microscopy (Zeiss). The localization of Texas Red–labeled BSA shows an injection site.
DISCUSSION
The NLS-mediated import process involves multiple sequential steps. NLS-containing karyophiles target the NPC after the formation of a complex with the import factors in an energy-independent manner. The subsequent energy-dependent translocation through the NPC requires Ran (Moore and Blobel, 1993; Melchior et al., 1993). Ran has been proposed to play at least two roles in the NPC translocation step. One is to release import substrates from their import factors at the nucleoplasmic side of NPC, which results in the completion of transport and the clearance of import factors from the NPC (Görlich et al., 1996). The other is to provide an energy source for the energy-dependent NPC translocation via its GTP hydrolysis at the cytoplasmic side of NPC (Melchior et al., 1995a; Mahajan et al., 1997).
Although cytoplasmic injection of dominant-negative G19V Ran-GTP potently inhibits the nuclear import of the SV40 T-antigen NLS substrate and the M9 sequence-containing substrate, it has no effect on β-catenin import (Figure 4). It has been shown that both importin-β and transportin release their cargos upon binding to the GTP form of Ran in vitro (Rexach and Blobel, 1995; Chi et al., 1996; Görlich et al., 1996; Izaurralde et al., 1997). The most likely explanation for the inhibitory effects of G19V Ran-GTP, when injected into the cytoplasm, is that it prevents import complex formation in the cytoplasm. The lack of inhibition of β-catenin import, therefore, suggests that β-catenin is not imported by soluble transport factors, such as importin β or transportin, the import complex formation of which is sensitive to G19V Ran-GTP. Moreover, the results obtained from the in vitro transport assay showed that β-catenin migrated into the nucleus without the addition of cytosol and that its import was not inhibited by the addition of G19V Ran-GTP. From these findings, we conclude that β-catenin can be imported into the nucleus without the aid of soluble transport factors such as importin α/β or transportin, which are largely extracted during permeabilization with digitonin. The lack of inhibition by G19V Ran-GTP also shows that GTP hydrolysis of Ran is not required for the nuclear import of β-catenin.
Fagotto et al. (1998), using the digitonin-permeabilized cell-free import assay, examined the import of β-catenin. Their results are consistent with ours, in that the β-catenin accumulates in the nucleus in an importin α/β-independent manner without requiring the addition of exogenous cytosol, although they also reported the inhibition of import by a mutant Ran, which was defective in its GTP hydrolysis (Q69L Ran), the effect of which was observed only after preincubation of the permeabilized cells. To precisely interpret the effects of preincubation, the ongoing events in the permeabilized cells, which are preincubated with Q69L Ran-GTP, need to be examined.
To understand whether the import of β-catenin requires nuclear Ran-GTP, we examined the effect of nuclear Ran-GTP depletion on the import of β-catenin. Previous studies (Tachibana et al., 1994; Dickmanns et al., 1996) and this study as well (Figure 5) demonstrated that the efficiency of the importin α/β- and transportin-dependent import pathways clearly declines in tsBN2 cells, which had been cultured at nonpermissive temperature. In contrast, it was found that the import of β-catenin did not decline in these cells, showing that the import of β-catenin is most insensitive to the depletion of nuclear Ran-GTP in living cells. In vitro results further showed that the addition of Ran completely failed to stimulate the import of β-catenin (Figure 6), which are consistent with the results reported by Fagotto et al. (1998). Collectively, these results indicate that Ran is not involved in all aspects of the nuclear import of β-catenin, although differences in sensitivity to nuclear Ran-GTP depletion between importin α/β- and transportin-dependent nuclear import pathways remain to be elucidated.
We did not detect a clear requirement for nucleotides for β-catenin import as reported by Fagotto et al. (1998). We included apyrase only during the preincubation period but not during the incubation of permeabilized cells with β-catenin, because both apyrase and hexokinase were found to destabilize FLAG-β-catenin and to induce its degradation for unknown reasons. Although pretreatment with apyrase did not completely deplete the nucleotides from the permeabilized cells, the treatment was sufficient to completely abolish the import of SV40 T-antigen NLS substrate (Figure 3B). Our present data clearly show differences in the requirement of nucleotides between Ran-unassisted β-catenin import and the Ran-assisted conventional import pathways. Moreover, β-catenin did not accumulate in the nucleus of permeabilized cells against a concentration gradient, indicating that the β-catenin import does not involve active mechanisms. However, as has also been reported by Fagotto et al. (1998), we observed the partial inhibition of import of β-catenin on preincubation of the cells with a nonhydrolyzable GTP analogue, guanylyl-imido diphosphate, only in the presence of an ATP source (our unpublished results), although the extent of inhibition of import differed from cell to cell. These results leave the possibility open that other GTPases might affect the β-catenin import either directly or indirectly.
In a previous study, we demonstrated that importin β migrates into the nucleus without Ran as well as its GTP hydrolysis when it does not carry importin α and the NLS substrate (Kose et al., 1997). Ran-unassisted import also has been observed with other importin β–related proteins (Kutay et al., 1998; Nakielny and Dreyfuss, 1998). In addition to transport factors such as importin β and its related proteins, β-catenin represents a novel example of a compound that can be translocated through NPC without the aid of Ran.
β-Catenin possesses 12 tandem repeating motifs called armadillo (Peifer et al., 1994; Huber et al., 1997), and the armadillo repeats of β-catenin have been reported to be necessary and sufficient for its nuclear accumulation (Funayama et al., 1995). On the other hand, importin β and its related proteins possess tandem repeating motifs called HEAT motifs (Aitchison et al., 1996). Recently, Malik et al. (1997) showed that the armadillo motifs and HEAT motifs are fundamentally similar in their structure and proposed that these repeating motifs may share a functional similarity. Moreover, Fagotto et al. (1998) showed that β-catenin binds directly to the FXFG repeat-containing C-terminal fragment of yeast nucleoporin NUP1. We showed that the nuclear import of both importin β and β-catenin occurs in a Ran-unassisted manner, and that importin β competitively inhibits the import of β-catenin. These results indicate that β-catenin migrates into the nucleus via an interaction with the same site of the NPC as importin β (Figure 7). However, excess β-catenin failed to competitively inhibit the import of importin β, although it effectively inhibited its own import, leaving the possibility that β-catenin may require a specific carrier protein, which is tightly retained in the permeabilized cells and competes with importin β for binding to NPC. Alternatively, β-catenin may interact with NPC components with much lower affinity than importin β to translocate through the NPC. Further studies will be required to distinguish between these possibilities.
The nuclear accumulation of β-catenin plays a key role in the Wingless/Wnt signal transduction pathway. In this study, we showed that β-catenin possesses the ability to migrate rapidly and constitutively into the nucleus without forming a complex with the LEF/TCF family. β-Catenin is not localized constitutively in the nucleus, but its nuclear accumulation is regulated by upstream events, which include Wingless/Wnt signal transduction. At present, the nuclear concentration of β-catenin is generally thought to be regulated mainly by cytoplasmic degradation. Because our present study shows that β-catenin possesses the ability to migrate rapidly into the nucleus through NPC by a facilitated mechanism without requiring importin β-related transport factors and Ran, and also rapidly exits the nucleus, the nuclear level of β-catenin could be regulated by several alternative mechanisms. The prevention of nuclear accumulation of β-catenin observed in the presence of cytosol in vitro (Figure 3A) raises the possibility that cytoplasmic factors exist that prevent β-catenin import and/or stimulate its nuclear export. In addition, because β-catenin apparently does not accumulate in the nucleus against a concentration gradient by active mechanisms (Figure 3D), the nuclear retention of β-catenin should be considered for its nuclear accumulation. In summary, we propose that regulation of the nuclear levels of β-catenin involves a balance between nuclear import and export as well as cytoplasmic and nuclear retention of this protein.
ACKNOWLEDGMENTS
We thank Drs. A. Nagafuchi and Sh. Tsukita (Kyoto University, Faculty of Medicine) for the generous gift of β-catenin plasmid. We also thank Dr. S. Kuroda (Osaka University, Institute of Scientific and Industrial Research) for the gift of pGEX-6P-2-hGFP, Dr. M. Nakanishi (Osaka University, Research Institute for Microbial Diseases) for the hemagglutinating virus of Japan, and Dr. T. Shimamoto (Osaka University Medical School) for HeLa cell cDNA library. We are grateful to Drs. N. Masuyama and E. Nishida (Kyoto University, Graduate School of Science) for their advice on the Xenopus embryo injection experiments. This work was supported by a grant-in-aid for scientific research on priority areas 07282103, grant-in-aid for scientific research (B) 08458229, grant-in-aid for scientific research (C) 08680764, and grant-in-aid for Center-of-Excellence research 07CE2006 from the Japanese Ministry of Education, Science, Sports and Culture.
REFERENCES
- Adam EJ, Adam SA. Identification of cytosolic factors required for nuclear location sequence-mediated binding to the nuclear envelope. J Cell Biol. 1994;125:547–555. doi: 10.1083/jcb.125.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam SA, Gerace L. Cytosolic proteins that specifically bind nuclear location signals are receptors for nuclear import. Cell. 1991;66:837–847. doi: 10.1016/0092-8674(91)90431-w. [DOI] [PubMed] [Google Scholar]
- Adam SA, Marr RS, Gerace L. Nuclear protein import in permeabilized mammalian cells requires soluble cytoplasmic factors. J Cell Biol. 1990;111:807–816. doi: 10.1083/jcb.111.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitchison JD, Blobel G, Rout MP. Kap104p: a karyopherin involved in the nuclear transport of mRNA binding proteins. Science. 1996;274:624–627. doi: 10.1126/science.274.5287.624. [DOI] [PubMed] [Google Scholar]
- Arts G-J, Fornerod M, Mattaj IW. Identification of a nuclear export receptor for tRNA. Curr Biol. 1998;8:305–314. doi: 10.1016/s0960-9822(98)70130-7. [DOI] [PubMed] [Google Scholar]
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of β-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- Bischoff FR, Krebber H, Kempf T, Hermes I, Ponstingl H. Human RanGTPase-activating protein RanGAP1 is a homologue of yeast Rna1p involved in mRNA processing and transport. Proc Natl Acad Sci USA. 1995;92:1749–1753. doi: 10.1073/pnas.92.5.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff FR, Ponstingl H. Catalysis of guanine nucleotide exchange on Ran by the mitotic regulator RCC1. Nature. 1991;354:80–82. doi: 10.1038/354080a0. [DOI] [PubMed] [Google Scholar]
- Bischoff FR, Ponstingl H. Catalysis of guanine nucleotide exchange of Ran by RCC1 and stimulation of hydrolysis of Ran-bound GTP by Ran-GAP1. Methods Enzymol. 1995;257:135–144. doi: 10.1016/s0076-6879(95)57019-5. [DOI] [PubMed] [Google Scholar]
- Brown JD, Moon RT. Wnt signaling: why is everything so negative? Curr Biol. 1998;10:182–187. doi: 10.1016/s0955-0674(98)80140-3. [DOI] [PubMed] [Google Scholar]
- Cavallo R, Rubenstein D, Peifer M. Armadillo and dTCF: a marriage made in the nucleus. Curr Opin Genet Dev. 1997;7:459–466. doi: 10.1016/s0959-437x(97)80071-8. [DOI] [PubMed] [Google Scholar]
- Chi NC, Adam EJ, Adam SA. Sequence and characterization of cytoplasmic nuclear protein import factor p97. J Cell Biol. 1995;130:265–274. doi: 10.1083/jcb.130.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi NC, Adam EJ, Visser GD, Adam SA. RanBP1 stabilizes the interaction of Ran with p97 nuclear protein import. J Cell Biol. 1996;135:559–569. doi: 10.1083/jcb.135.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole CN, Hammell CM. Nucleocytoplasmic transport: driving and directing transport. Curr Biol. 1998;8:368–372. doi: 10.1016/s0960-9822(98)70239-8. [DOI] [PubMed] [Google Scholar]
- Corbett AH, Silver PA. Nucleocytoplasmic transport of macromolecules. Microbiol Mol Biol Rev. 1997;61:193–211. doi: 10.1128/mmbr.61.2.193-211.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis LI. The nuclear pore complex. Annu Rev Biochem. 1995;64:865–896. doi: 10.1146/annurev.bi.64.070195.004245. [DOI] [PubMed] [Google Scholar]
- Dickmanns A, Bischoff FR, Marshallsay C, Lührmann R, Ponstingl H, Fanning E. The thermolability of nuclear protein import in tsBN2 cells is suppressed by microinjected Ran-GTP or Ran-GDP, but not by RanQ69L or RanT24N. J Cell Sci. 1996;109:1449–1457. doi: 10.1242/jcs.109.6.1449. [DOI] [PubMed] [Google Scholar]
- Enenkel C, Blobel G, Rexach M. Identification of a yeast karyopherin heterodimer that targets import substrate to mammalian nuclear pore complexes. J Biol Chem. 1995;270:16499–16502. doi: 10.1074/jbc.270.28.16499. [DOI] [PubMed] [Google Scholar]
- Fabre E, Hurt E. Yeast genetics to dissect the nuclear pore complex and nucleocytoplasmic trafficking. Annu Rev Genet. 1997;31:277–313. doi: 10.1146/annurev.genet.31.1.277. [DOI] [PubMed] [Google Scholar]
- Fagotto F, Gluck U, Gumbiner BM. Nuclear localization signal-independent and importin/karyopherin-independent nuclear import of β-catenin. Curr Biol. 1998;8:181–190. doi: 10.1016/s0960-9822(98)70082-x. [DOI] [PubMed] [Google Scholar]
- Finlay DR, Newmeyer DD, Price TM, Forbes DJ. Inhibition of in vitro nuclear transport by a lectin that binds to nuclear pores. J Cell Biol. 1987;104:189–200. doi: 10.1083/jcb.104.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornerod M, Ohno M, Yoshida M, Mattaj IW. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997a;90:1051–1060. doi: 10.1016/s0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- Fornerod M, van Deursen J, van Baal S, Reynolds A, Davis D, Murti KG, Fransen J, Grosveld G. The human homologue of yeast CRM1 is in a dynamic subcomplex with CAN/Nup214 and a novel nuclear pore component Nup88. EMBO J. 1997b;16:807–816. doi: 10.1093/emboj/16.4.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Asano S, Nakamura T, Adachi M, Yoshida M, Yanagida M, Nishida E. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature. 1997;390:308–311. doi: 10.1038/36894. [DOI] [PubMed] [Google Scholar]
- Funayama N, Fagotto F, McCrea P, Gumbiner BM. Embryonic axis induction by the armadillo repeat domain of β-catenin: evidence for intracellular signaling. J Cell Biol. 1995;128:959–968. doi: 10.1083/jcb.128.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb DS. Whose finger is on the switch? Science. 1997;276:1814–1816. doi: 10.1126/science.276.5320.1814. [DOI] [PubMed] [Google Scholar]
- Görlich D. Nuclear protein import. Curr Opin Cell Biol. 1997;9:412–419. doi: 10.1016/s0955-0674(97)80015-4. [DOI] [PubMed] [Google Scholar]
- Görlich D, Dabrowski M, Bischoff FR, Kutay U, Bork P, Hartmann E, Prehn S, Izaurralde E. A novel class of RanGTP binding proteins. J Cell Biol. 1997;138:65–80. doi: 10.1083/jcb.138.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görlich D, Kostka S, Kraft R, Dingwall C, Laskey RA, Hartmann E, Prehn S. Two different subunits of importin cooperate to recognize nuclear localization signals and bind them to the nuclear envelope. Curr Biol. 1995;5:383–392. doi: 10.1016/s0960-9822(95)00079-0. [DOI] [PubMed] [Google Scholar]
- Görlich D, Pante N, Kutay U, Aebi U, Bischoff FR. Identification of different roles for RanGDP and RanGTP in nuclear protein import. EMBO J. 1996;15:5584–5594. [PMC free article] [PubMed] [Google Scholar]
- Görlich D, Prehn S, Laskey RA, Hartmann E. Isolation of a protein that is essential for the first step of nuclear protein import. Cell. 1994;79:767–778. doi: 10.1016/0092-8674(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Carcinogenesis: a balance between β-catenin and APC. Curr Biol. 1997;7:443–446. doi: 10.1016/s0960-9822(06)00214-4. [DOI] [PubMed] [Google Scholar]
- Guger KA, Gumbiner BM. β-Catenin has Wnt-like activity and mimics the nieuwkoop signaling center in Xenopus dorsal-ventral patterning. Dev Biol. 1995;172:115–125. doi: 10.1006/dbio.1995.0009. [DOI] [PubMed] [Google Scholar]
- Huber AH, Nelson WJ, Weis WI. Three-dimentional structure of the armadillo repeat region of β-catenin. Cell. 1997;90:871–882. doi: 10.1016/s0092-8674(00)80352-9. [DOI] [PubMed] [Google Scholar]
- Huber O, Korn R, McLaughlin J, Ohsugi M, Herrmann BG, Kemler R. Nuclear localization of β-catenin by interaction with transcription factor LEF-1. Mech Dev. 1996;59:3–10. doi: 10.1016/0925-4773(96)00597-7. [DOI] [PubMed] [Google Scholar]
- Imamoto N, Kamei Y, Yoneda Y. Nuclear transport factors: function, behavior and interaction. Eur J Histochem. 1998;42:9–20. [PubMed] [Google Scholar]
- Imamoto N, Shimamoto T, Kose S, Takao T, Tachibana T, Matsubae M, Sekimoto T, Shimonishi Y, Yoneda Y. The nuclear pore-targeting complex binds to nuclear pores after association with a karyophile. FEBS Lett. 1995a;368:415–419. doi: 10.1016/0014-5793(95)00699-a. [DOI] [PubMed] [Google Scholar]
- Imamoto N, Shimamoto T, Takao T, Tachibana T, Kose S, Matsubae M, Sekimoto T, Shimonishi Y, Yoneda Y. In vivo evidence for involvement of a 58 kDa component of nuclear pore-targeting complex in nuclear protein import. EMBO J. 1995b;14:3617–3626. doi: 10.1002/j.1460-2075.1995.tb00031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamoto N, Tachibana T, Matsubae M, Yoneda Y. A karyophilic protein forms a stable complex with cytoplasmic components prior to nuclear pore binding. J Biol Chem. 1995c;270:8559–8565. doi: 10.1074/jbc.270.15.8559. [DOI] [PubMed] [Google Scholar]
- Iwao K, Nakamori S, Kameyama M, Imaoka S, Kinoshita M, Fukui T, Ishiguro S, Nakamura Y, Miyoshi Y. Activation of the β-catenin gene by interstitial deletions involving exon 3 in primary colorectal carcinomas without adenomatous polyposis coli mutations. Cancer Res. 1998;58:1021–1026. [PubMed] [Google Scholar]
- Izaurralde E, Kutay U, von Kobbe C, Mattaj IW, Görlich D. The asymmetric distribution of the constituents of the Ran system is essential for transport into and out of the nucleus. EMBO J. 1997;16:6535–6547. doi: 10.1093/emboj/16.21.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepp DM, Silver PA. A GTPase controlling nuclear trafficking: running the right way or walking RANdomly? Cell. 1996;87:1–4. doi: 10.1016/s0092-8674(00)81315-x. [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a β-catenin-Tcf complex in APC−/− colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- Kose S, Imamoto N, Tachibana T, Shimamoto T, Yoneda Y. Ran-unassisted nuclear migration of a 97-kDa component of nuclear pore-targeting complex. J Cell Biol. 1997;139:841–849. doi: 10.1083/jcb.139.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl M, Wedlich D. Wnt signaling goes nuclear. Bioessays. 1997;19:101–104. doi: 10.1002/bies.950190204. [DOI] [PubMed] [Google Scholar]
- Kutay U, Bischoff FR, Kostka S, Kraft R, Görlich D. Export of importin α from the nucleus is mediated by a specific nuclear transport factor. Cell. 1997;90:1061–1071. doi: 10.1016/s0092-8674(00)80372-4. [DOI] [PubMed] [Google Scholar]
- Kutay U, Lipowsky G, Izaurralde E, Bischoff FR, Schwarzmaier P, Hartmann E, Görlich D. Identification of a tRNA-specific nuclear export receptor. Mol Cell. 1998;1:359–369. doi: 10.1016/s1097-2765(00)80036-2. [DOI] [PubMed] [Google Scholar]
- Mahajan R, Delphin C, Guan T, Gerace L, Melchior F. A small ubiquitin-related polypeptide involved in targeting RanGAP1 to nuclear pore complex protein RanBP2. Cell. 1997;88:97–107. doi: 10.1016/s0092-8674(00)81862-0. [DOI] [PubMed] [Google Scholar]
- Malik HS, Eickbush TH, Goldfarb DS. Evolutionary specialization of the nuclear targeting apparatus. Proc Natl Acad Sci USA. 1997;94:13738–13742. doi: 10.1073/pnas.94.25.13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattaj IW, Englmeier L. Nucleocytoplasmic transport: the soluble phase. Annu Rev Biochem. 1998;67:265–306. doi: 10.1146/annurev.biochem.67.1.265. [DOI] [PubMed] [Google Scholar]
- Matunis MJ, Coutavas E, Blobel G. A novel ubiquitin-like modification modulates the partitioning of the Ran-GTPase-activating protein RanGAP1 between the cytosol and the nuclear pore complex. J Cell Biol. 1996;6:1457–1470. doi: 10.1083/jcb.135.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea PD, Turck CW, Gumbiner B. A homolog of the armadillo protein in Drosophila (plakoglobin) associated with E-cadherin. Science. 1991;254:1359–1361. doi: 10.1126/science.1962194. [DOI] [PubMed] [Google Scholar]
- Melchior F, Gerace L. Two-way trafficking with Ran. Trends Cell Biol. 1998;8:175–179. doi: 10.1016/s0962-8924(98)01252-5. [DOI] [PubMed] [Google Scholar]
- Melchior F, Guan T, Yokoyama N, Nishimoto T, Gerace L. GTP hydrolysis by Ran occurs at the nuclear pore complex in an early step of protein import. J Cell Biol. 1995a;131:571–581. doi: 10.1083/jcb.131.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior F, Paschal B, Evans J, Gerace L. Inhibition of nuclear protein import by nonhydrolyzable analogues of GTP and identification of the small GTPase Ran/TC4 as an essential transport factor. J Cell Biol. 1993;123:1649–1659. doi: 10.1083/jcb.123.6.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior F, Sweet DJ, Gerace L. Analysis of Ran/TC4 function in nuclear protein import. Methods Enzymol. 1995b;257:279–291. doi: 10.1016/s0076-6879(95)57032-2. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Imamoto N, Sekimoto T, Tachibana T, Seki T, Tada S, Enomoto T, Yoneda Y. Differential modes of nuclear localization signal (NLS) recognition by three distinct classes of NLS receptors. J Biol Chem. 1997;272:26375–26381. doi: 10.1074/jbc.272.42.26375. [DOI] [PubMed] [Google Scholar]
- Miyoshi Y, Iwao K, Nagasawa Y, Aihara T, Sasaki Y, Imaoka S, Murata M, Shimano T, Nakamura Y. Activation of the β-catenin gene in primary hepatocellular carcinomas by somatic alterations involving exon 3. Cancer Res. 1998;58:2524–2527. [PubMed] [Google Scholar]
- Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destree O, Clevers H. XTcf-3 transcription factor mediates β-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- Moore MS, Blobel G. The GTP-binding protein Ran/TC4 is required for protein import into the nucleus. Nature. 1993;365:661–663. doi: 10.1038/365661a0. [DOI] [PubMed] [Google Scholar]
- Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- Moroianu J, Blobel G, Radu A. Previously identified protein of uncertain function is karyopherin α and together with karyopherin β docks import substrate at nuclear pore complexes. Proc Natl Acad Sci USA. 1995;92:2008–2011. doi: 10.1073/pnas.92.6.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemitsu S, Albert I, Souza B, Rubinfeld B, Polakis P. Regulation of intracellular β-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc Natl Acad Sci USA. 1995;92:3046–3050. doi: 10.1073/pnas.92.7.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakielny S, Dreyfuss G. Import and export of the nuclear protein import receptor transportin by a mechanism independent of GTP hydrolysis. Curr Biol. 1998;8:89–95. doi: 10.1016/s0960-9822(98)70039-9. [DOI] [PubMed] [Google Scholar]
- Nakielny S, Fischer U, Michael WM, Dreyfuss G. RNA transport. Annu Rev Neurosci. 1997;20:269–301. doi: 10.1146/annurev.neuro.20.1.269. [DOI] [PubMed] [Google Scholar]
- Nigg EA. Nucleocytoplasmic transport: signals, mechanisms and regulation. Nature. 1997;386:779–787. doi: 10.1038/386779a0. [DOI] [PubMed] [Google Scholar]
- Nishimoto T, Eilen E, Basilico C. Premature chromosome condensation is a ts DNA-mutant of BHK cells. Cell. 1978;15:475–483. doi: 10.1016/0092-8674(78)90017-x. [DOI] [PubMed] [Google Scholar]
- Nishitani H, Ohtusbo M, Yamashita K, Iida H, Pines J, Yasudo H, Shibata Y, Hunter T, Nishimoto T. Loss of RCC1, a nuclear DNA-binding protein, uncouples the completion of DNA replication from the activation of cdc2 protein kinase and mitosis. EMBO J. 1991;10:1555–1564. doi: 10.1002/j.1460-2075.1991.tb07675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno M, Fornerod M, Mattaj IW. Nucleocytoplasmic transport: the last 200 nanometers. Cell. 1998;92:327–336. doi: 10.1016/s0092-8674(00)80926-5. [DOI] [PubMed] [Google Scholar]
- Ohtsubo M, Okazaki H, Nishimoto T. The RCC1 protein, a regulator for the onset of chromosome condensation locates in the nucleus and binds to DNA. J Cell Biol. 1989;109:1389–1397. doi: 10.1083/jcb.109.4.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsulic S, Peifer M. An in vivo structure-function study of armadillo, the β-catenin homologue, reveals both separate and overlapping regions of the protein required for cell adhesion and for wingless signaling. J Cell Biol. 1996;134:1283–1300. doi: 10.1083/jcb.134.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peifer M, Berg S, Reynoids AB. A repeating amino acid motif shared by proteins with diverse cellular roles. Cell. 1994;76:789–791. doi: 10.1016/0092-8674(94)90353-0. [DOI] [PubMed] [Google Scholar]
- Pollard VW, Michael WM, Nakielny S, Siomi MC, Wang F, Dreyfuss G. A novel receptor-mediated nuclear protein import pathway. Cell. 1996;86:985–994. doi: 10.1016/s0092-8674(00)80173-7. [DOI] [PubMed] [Google Scholar]
- Radu A, Blobel G, Moore MS. Identification of a protein complex that is required for nuclear protein import and mediates docking of import substrate to distinct nucleoporins. Proc Natl Acad Sci USA. 1995;92:1769–1773. doi: 10.1073/pnas.92.5.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren M, Drivas G, D’Eustachio P, Rush MG. Ran/TC4: a small nuclear GTP-binding protein that regulates DNA synthesis. J Cell Biol. 1993;120:313–323. doi: 10.1083/jcb.120.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rexach M, Blobel G. Protein import into nuclei: association and dissociation reactions involving transport substrate, transport factors, and nucleoporins. Cell. 1995;83:683–692. doi: 10.1016/0092-8674(95)90181-7. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Binding of GSK3β to the APC-β-catenin complex and regulation of complex assembly. Science. 1996;272:1023–1026. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P. Stabilization of β-catenin by genetic defects in melanoma cell lines. Science. 1997;275:1790–1792. doi: 10.1126/science.275.5307.1790. [DOI] [PubMed] [Google Scholar]
- Schneider S, Steinbeisser H, Warga RM, Hausen P. β-Catenin translocation into nuclei demarcates the dorsalizing centers in frog and fish embryos. Mech Dev. 1996;57:191–198. doi: 10.1016/0925-4773(96)00546-1. [DOI] [PubMed] [Google Scholar]
- Sekimoto T, Imamoto N, Nakajima K, Hirano T, Yoneda Y. Extracellular signal-dependent nuclear import of Stat1 is mediated by nuclear pore-targeting complex formation with NPI-1, but not Rch1. EMBO J. 1997;16:7067–7077. doi: 10.1093/emboj/16.23.7067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekimoto T, Nakajima K, Tachibana T, Hirano T, Yoneda Y. Interferon-γ-dependent nuclear import of Stat1 is mediated by the GTPase activity of Ran/TC4. J Biol Chem. 1996;271:31017–31020. doi: 10.1074/jbc.271.49.31017. [DOI] [PubMed] [Google Scholar]
- Shapiro L. The multi-talented β-catenin makes its first appearance. Structure. 1997;5:1265–1268. doi: 10.1016/s0969-2126(97)00278-5. [DOI] [PubMed] [Google Scholar]
- Siomi H, Dreyfuss G. A nuclear localization domain in the hnRNPA1 protein. J Cell Biol. 1995;129:551–559. doi: 10.1083/jcb.129.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stade K, Ford CS, Guthrie C, Weis K. Exportin 1 (Crm1p) is an essential nuclear export factor. Cell. 1997;90:1041–1050. doi: 10.1016/s0092-8674(00)80370-0. [DOI] [PubMed] [Google Scholar]
- Tachibana T, Hieda M, Sekimoto T, Yoneda Y. Exogenously injected nuclear import factor p10/NTF2 inhibits signal-mediated nuclear import and export of proteins in living cells. FEBS Lett. 1996;397:177–182. doi: 10.1016/s0014-5793(96)01180-5. [DOI] [PubMed] [Google Scholar]
- Tachibana T, Imamoto N, Seino H, Nishimoto T, Yoneda Y. Loss of RCC1 leads to suppression of nuclear protein import in living cells. J Biol Chem. 1994;269:24542–24545. [PubMed] [Google Scholar]
- Tsuji L, Takumi T, Imamoto N, Yoneda Y. Identification of novel homologues of mouse importin α, the α subunit of the nuclear pore-targeting complex, and their tissue-specific expression. FEBS Lett. 1997;416:30–34. doi: 10.1016/s0014-5793(97)01092-2. [DOI] [PubMed] [Google Scholar]
- Uchida S, Sekiguchi T, Nishitani H, Miyauchi K, Ohtsubo M, Nishimoto T. Premature chromosome condensation is induced by a point mutation in the hamster RCC1 gene. Mol Cell Biol. 1990;10:577–584. doi: 10.1128/mcb.10.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman KS, Powers MA, Forbes DJ. Nuclear export receptors: from importin to exportin. Cell. 1997;90:967–970. doi: 10.1016/s0092-8674(00)80361-x. [DOI] [PubMed] [Google Scholar]
- Weis K, Mattaj IW, Lamond AI. Identification of hSRP1 α as a functional receptor for nuclear localization sequences. Science. 1995;268:1049–1053. doi: 10.1126/science.7754385. [DOI] [PubMed] [Google Scholar]
- Yoneda Y, Imamoto-Sonobe N, Yamaizumi M, Uchida T. Reversible inhibition of protein import into the nucleus by wheat germ agglutinin injected into cultured cells. Exp Cell Res. 1987;173:586–595. doi: 10.1016/0014-4827(87)90297-7. [DOI] [PubMed] [Google Scholar]