Abstract
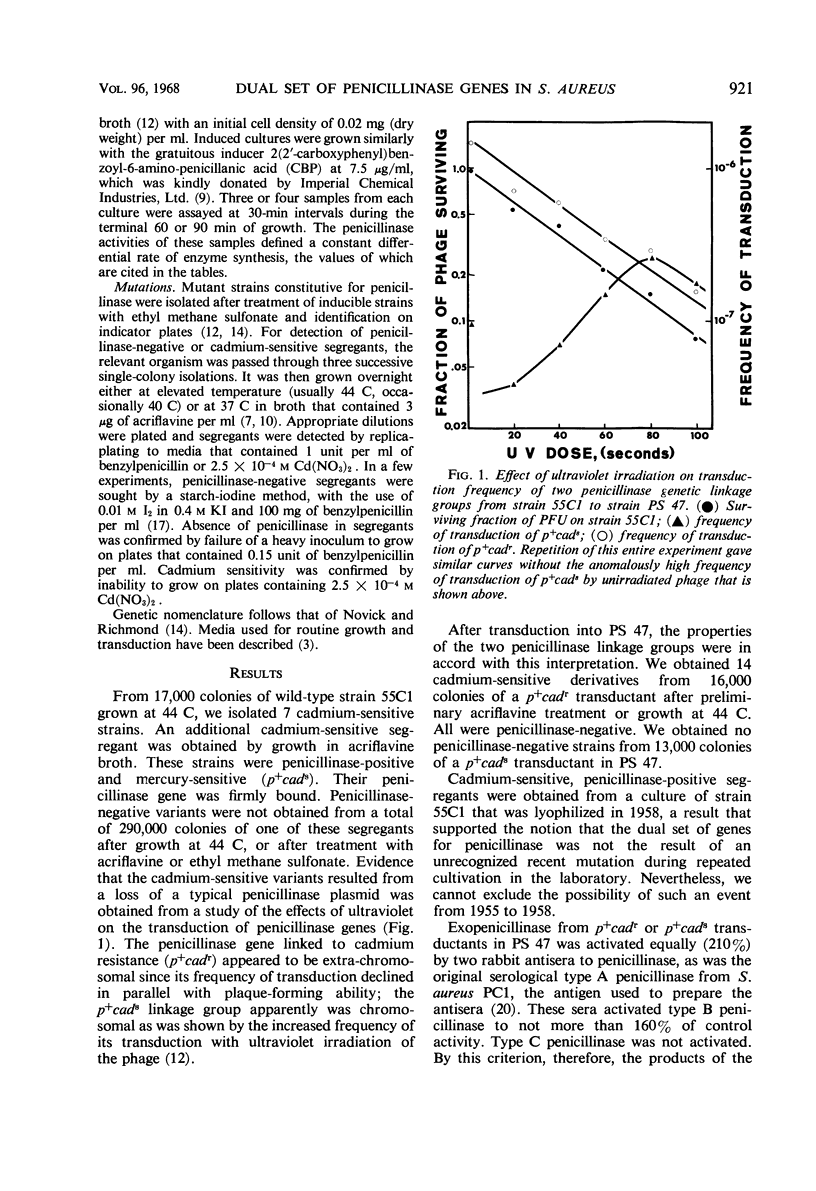
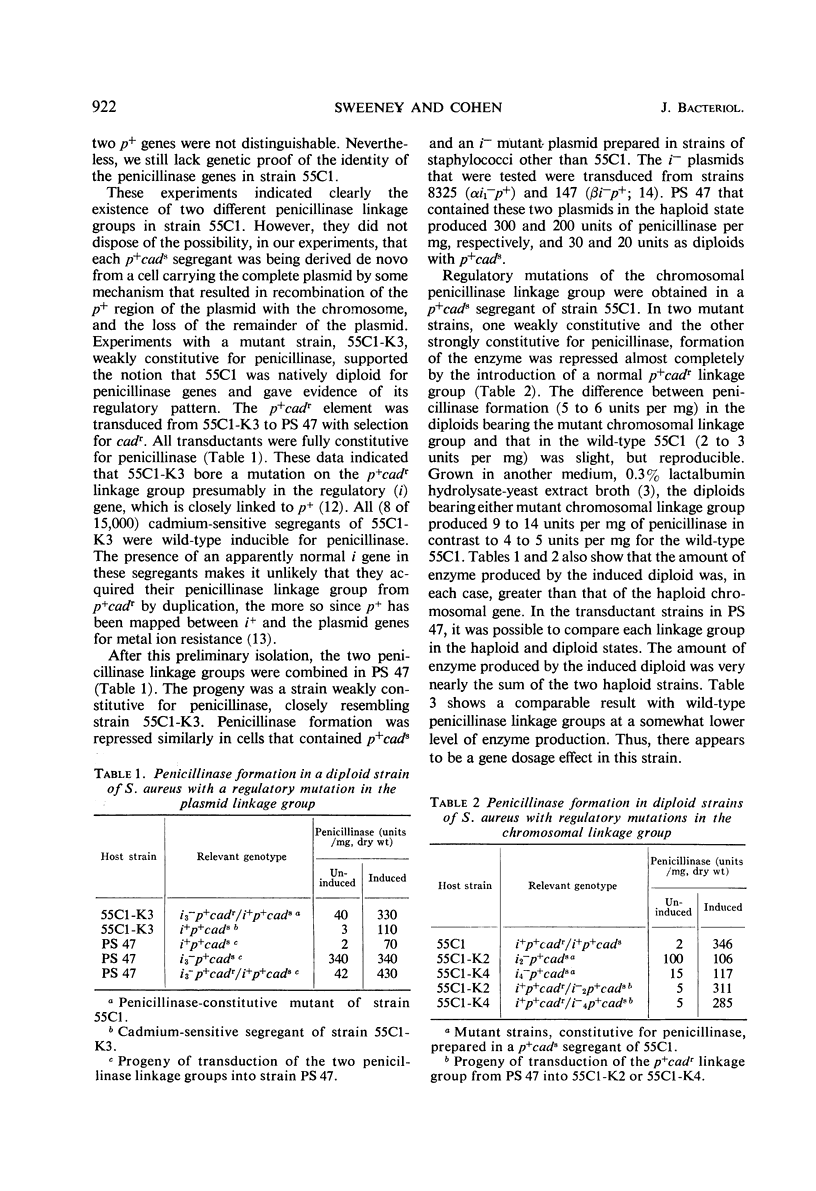
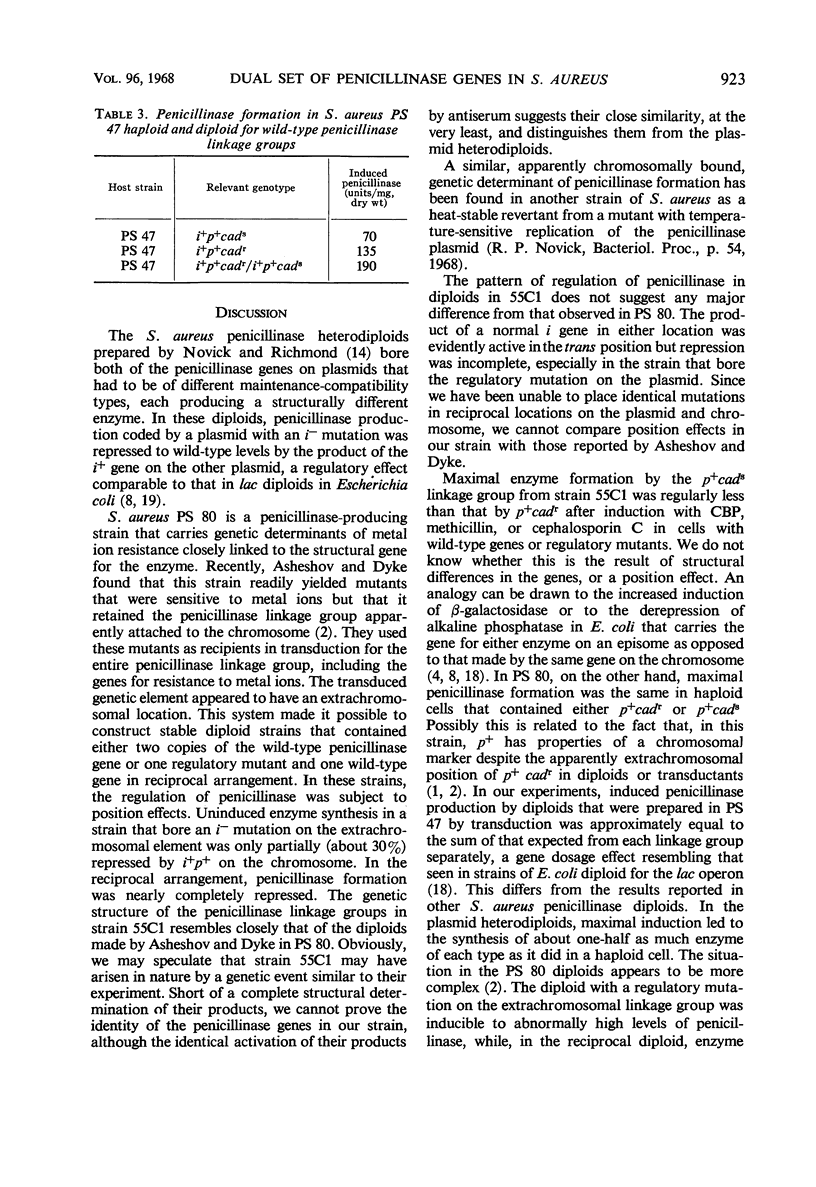
Staphylococcus aureus strain 55C1, isolated from a patient in 1955, contained two genetic linkage groups for penicillinase formation. One was linked to genes that control resistance to cadmium and mercuric ions; it had properties of a plasmidborne gene. The other was not linked to resistance to these metal ions; it had properties of a chromosomal gene. Penicillinase formation by cells that contained either linkage group was inducible by penicillins. Induced penicillinase in cells that contained both linkage groups equalled the sum of that produced in cells containing each group singly. Exopenicillinase produced by cells containing either gene was serological type A. Constitutive penicillinase formation resulting from regulator gene mutations in either linkage group was repressed to differing extents by a wild-type determinant in the trans position. The genetic structure and the regulation of penicillinase formation in strain 55C1 resembled in general those for penicillinase linkage groups which Asheshov and Dyke described for diploid mutant strains of S. aureus PS 80. There were differences in detail, however.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asheshov E. H. Chromosomal location of the genetic elements controlling penicillinase production in a strain of Staphylococcus aureus. Nature. 1966 May 21;210(5038):804–806. doi: 10.1038/210804a0. [DOI] [PubMed] [Google Scholar]
- Asheshov E. H., Dyke K. G. Regulation of the synthesis of penicillinase in diploids of Staphylococcus aureus. Biochem Biophys Res Commun. 1968 Feb 15;30(3):213–218. doi: 10.1016/0006-291x(68)90437-3. [DOI] [PubMed] [Google Scholar]
- Cohen S., Sweeney H. M. Constitutive penicillinase formation in Staphylococcus aureus owing to a mutation unlinked to the penicillinase plasmid. J Bacteriol. 1968 Apr;95(4):1368–1374. doi: 10.1128/jb.95.4.1368-1374.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAREN A., ECHOLS H. Genetic control of induction of alkaline phosphatase synthesis in E. coli. Proc Natl Acad Sci U S A. 1962 Aug;48:1398–1402. doi: 10.1073/pnas.48.8.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERONIMUS L. H., COHEN S. Induction of staphylococcal penicillinase. J Bacteriol. 1957 Jan;73(1):28–34. doi: 10.1128/jb.73.1.28-34.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN S. M. Mercury sensitivity of staphylococci. J Clin Pathol. 1962 May;15:249–251. doi: 10.1136/jcp.15.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASHIMOTO H., KONO K., MITSUHASHI S. ELIMINATION OF PENICILLIN RESISTANCE OF STAPHYLOCOCCUS AUREUS BY TREATMENT WITH ACRIFLAVINE. J Bacteriol. 1964 Jul;88:261–262. doi: 10.1128/jb.88.1.261-262.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- MAY J. W., HOUGHTON R. H., PERRET C. J. THE EFFECT OF GROWTH AT ELEVATED TEMPERATURES ON SOME HERITABLE PROPERTIES OF STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1964 Nov;37:157–169. doi: 10.1099/00221287-37-2-157. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P. ANALYSIS BY TRANSDUCTION OF MUTATIONS AFFECTING PENICILLINASE FORMATION IN STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1963 Oct;33:121–136. doi: 10.1099/00221287-33-1-121. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P., RICHMOND M. H. NATURE AND INTERACTIONS OF THE GENETIC ELEMENTS GOVERNING PENICILLINASE SYNTHESIS IN STAPHYLOCOCCUS AUREUS. J Bacteriol. 1965 Aug;90:467–480. doi: 10.1128/jb.90.2.467-480.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVICK R. P. Staphylococcal penicillinase and the new penicillins. Biochem J. 1962 May;83:229–235. doi: 10.1042/bj0830229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P. Penicillinase plasmids of Staphylococcus aureus. Fed Proc. 1967 Jan-Feb;26(1):29–38. [PubMed] [Google Scholar]
- Novick R. P., Roth C. Plasmid-linked resistance to inorganic salts in Staphylococcus aureus. J Bacteriol. 1968 Apr;95(4):1335–1342. doi: 10.1128/jb.95.4.1335-1342.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATTEE P. A., BALDWIN J. N. Transduction of resistance to chlortetracycline and novobiocin in Staphylococcus aureus. J Bacteriol. 1961 Dec;82:875–881. doi: 10.1128/jb.82.6.875-881.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce L. E., Meynell E. Mutation to high-level streptomycin-resistance in R+ bacteria. J Gen Microbiol. 1968 Jan;50(1):173–176. doi: 10.1099/00221287-50-1-173. [DOI] [PubMed] [Google Scholar]
- REVEL H. R. SYNTHESIS OF BETA-D-GALACTOSIDASE AFTER F-DUCTION OF LAC+GENES INTO ESCHERICHIA COLI. J Mol Biol. 1965 Jan;11:23–34. doi: 10.1016/s0022-2836(65)80168-1. [DOI] [PubMed] [Google Scholar]
- RICHMOND M. H. DOMINANCE OF THE INDUCIBLE STATE IN STRAINS OF STAPHYLOCOCCUS AUREUS CONTAINING TWO DISTINCT PENICILLINASE PLASMIDS. J Bacteriol. 1965 Aug;90:370–374. doi: 10.1128/jb.90.2.370-374.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHMOND M. H. WILD-TYPE VARIANTS OF EXOPENICILLINASE FROM STAPHYLOCOCCUS AUREUS. Biochem J. 1965 Mar;94:584–593. doi: 10.1042/bj0940584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond M. H. A second regulatory region involved in penicillinase synthesis in Staphylococcus aureus. J Mol Biol. 1967 Jun 14;26(2):357–360. doi: 10.1016/0022-2836(67)90305-1. [DOI] [PubMed] [Google Scholar]
- Richmond M. H. New type of restriction to the expression of a structural gene in bacteria. Nature. 1967 Dec 23;216(5121):1191–1192. doi: 10.1038/2161191a0. [DOI] [PubMed] [Google Scholar]



