Abstract
Chemotaxis of Escherichia coli toward phosphotransferase systems (PTSs)–carbohydrates requires phosphoenolpyruvate-dependent PTSs as well as the chemotaxis response regulator CheY and its kinase, CheA. Responses initiated by flash photorelease of a PTS substrates d-glucose and its nonmetabolizable analog methyl α-d-glucopyranoside were measured with 33-ms time resolution using computer-assisted motion analysis. This, together with chemotactic mutants, has allowed us to map out and characterize the PTS chemotactic signal pathway. The responses were absent in mutants lacking the general PTS enzymes EI or HPr, elevated in PTS transport mutants, retarded in mutants lacking CheZ, a catalyst of CheY autodephosphorylation, and severely reduced in mutants with impaired methyl-accepting chemotaxis protein (MCP) signaling activity. Response kinetics were comparable to those triggered by MCP attractant ligands over most of the response range, the most rapid being 11.7 ± 3.1 s−1. The response threshold was <10 nM for glucose. Responses to methyl α-d-glucopyranoside had a higher threshold, commensurate with a lower PTS affinity, but were otherwise kinetically indistinguishable. These facts provide evidence for a single pathway in which the PTS chemotactic signal is relayed rapidly to MCP–CheW–CheA signaling complexes that effect subsequent amplification and slower CheY dephosphorylation. The high sensitivity indicates that this signal is generated by transport-induced dephosphorylation of the PTS rather than phosphoenolpyruvate consumption.
INTRODUCTION
Chemotaxis of the enteric bacteria Escherichia coli and Salmonella typhimurium provides a paradigm for mechanistic analysis of how phosphorylation circuits mediate sensory responses (Bray, 1998). The motility of these bacteria consists of an alternating pattern of swimming runs and tumbles. A counterclockwise (CCW) rotating flagellar bundle drives swimming runs. Clockwise (CW) rotation of a presently undetermined number of flagella leads to bundle breakup, generating tumbling events that randomize cell orientation (Macnab and Ornston, 1977). Chemotactic migration is effected primarily by an increase of swimming runs up positive gradients (Berg and Brown, 1972). The bacteria use a temporal gradient-sensing mechanism. The chemotactic response to a sudden change in chemoeffector concentration consists of a subsecond excitation phase followed by slower adaptation back to prestimulus behavior (Macnab and Kosh-land, 1972).
The central chemotaxis circuit consists of the response regulator protein CheY, its kinase (CheA), and a catalyst of its autophosphatase activity (CheZ). CheY shuttles between methyl-accepting chemotaxis protein (MCP) complexes and flagellar motors. The MCP family of transmembrane chemoreceptors processes responses to attractants (oxygen, amino acids, and periplasmic sugar-binding proteins) as well as to repellents (leucine, weak acids/bases, extremes of pH, and temperature). The autophosphorylating histidine kinase CheA, together with the linker CheW, is stably associated with the MCPs, forming signaling complexes (Gegner et al., 1992). Analogous histidyl–aspartyl “two-component” phosphorelays and type I chemoreceptors mediate signal transduction processes in a wide range of species (Appleby et al., 1996; Stock and Surette, 1996). CheA phosphorylates CheY on an aspartyl residue (Sanders et al., 1989). Phosphorylated CheY (CheY.P) dissociates rapidly from the receptors (Schuster et al., 1993) and binds to flagellar motors, enhancing CW rotation (Welch et al., 1993). Configurational changes in MCPs triggered by binding of attractant ligands to the MCP periplasmic domain inhibit CheA activity, generating a positive, CCW motor response that promotes smooth-swimming. Withdrawal of MCP attractant ligands or addition of repellent ligands stimulates CheA kinase, generating negative, CW motor responses (Larsen et al., 1974), hence tumbling. The response to amino acid attractants is exquisitely sensitive (Segall et al., 1986). Aggregation of MCP signaling complexes may amplify individual ligand–MCP associations to achieve this high sensitivity (Bray, 1998).
Chemotaxis toward carbohydrates uses two pathways. In one pathway, carbohydrates use periplasmic binding protein components of the ATP-binding cassette transporters that bind MCPs when complexed with their sugar ligands. This association triggers smooth-swim responses independently of transport into the cell (Hazelbauer and Adler, 1971). The second pathway uses the phosphoenolpyruvate (PEP)-dependent carbohydrate phosphotransferase systems (PTSs), in which chemotaxis is inextricably linked to transport of the substrate (Lengeler and Jahreis, 1996, and references therein). PTSs consist of membrane-bound and substrate-specific Enzyme II (EII) complexes that accept phosphate from a cytoplasmic donor phosphorelay to phosphorylate the substrate as it is transported. The relay consists of Enzyme I (EI), a PEP-dependent histidine kinase, and a phosphohistidine carrier protein (HPr). EI and HPr are common to all EIIs, of which there are at least 15 in E. coli (Postma et al., 1996).
The chemotactic response range and threshold of PTS substrates has been characterized by swarm agar and capillary assays. The positive, response thresholds varied with the Km values of the substrate for its EII, regardless of the specific substrate–EII combination examined. Neither binding of a PTS substrate to its EII nor intracellular accumulation and subsequent metabolism of its phosphorylated form can by themselves trigger a chemotactic response (Adler et al., 1973; Adler and Epstein, 1974; Lengeler, 1975; Lengeler et al., 1981; Pecher et al., 1983).
The role of the MCP–Che circuitry during PTS-dependent chemotaxis has been explored. Responses to PTS stimuli and their subsequent adaptation do not depend on MCP methylation as established by study of bacteria tethered by a single flagellum to glass coverslips (Niwano and Taylor, 1982). Gutted strains lacking all Che proteins except trace amounts of CheZ (Abouhamed et al., 1998) responded to the PTS substrate mannose only during plasmid-based expression of CheA, CheW, and CheY, but the response was CW instead of CCW (Rowsell et al., 1995). EI, but not EI.P, inhibited CheA autophosphorylation in vitro; however, half-maximal inhibition was obtained at an EI/CheA ratio fivefold greater than that present in the cell (Lux et al., 1995).
Thus, inhibition of CheY phosphorylation by CheA attributable to accumulation of unphosphorylated EI during transport could underlie PTS chemotaxis but may be supplemented or modulated in important ways by additional processes. Changes in PEP levels, which also occur during transport (Lowry et al., 1971), could play this role. PEP was found to stimulate CheA autophosphorylation in vitro at physiological (1 mM) concentration (Lux et al., 1995). Furthermore, because the pyruvate generated from PEP feeds via acetyl CoA into the tricarboxylic acid cycle, transport of PTS substrates is likely to affect levels of acetyl.AMP metabolites via acetyl CoA, as well as tricarboxylic acid cycle intermediates such as fumarate. These have recently been implicated in CheY acetylation (Ramakrishnan et al., 1998) and control of motor switching (Montrone et al., 1996; Prasad et al., 1998), respectively.
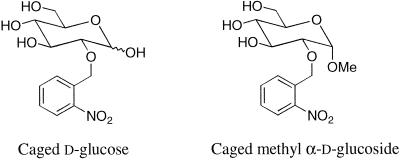
Mutant screens have been invaluable for analysis of chemotactic signal pathways, but PTS chemotactic mutants with associated defects in cellular metabolism may have been lethal and thus difficult to isolate. Biochemical data reveal molecular interactions, but assessment of their role in the intact cell requires behavioral assays. Behavioral capillary or swarm plate assays measure chemotactic response range but not kinetics, whereas flow cell-based assays of tethered bacteria measure adaptation, but only for responses that last several seconds. None of these assays permits quantification of the timing and amplitude of chemotactic signals. Photolysis of photolabile (caged) precursors by near-UV flash irradiation provides a potent means for in vivo perturbation, which in conjunction with appropriate time-resolved assays has been exploited for detailed mechanistic analysis of signaling pathways (Somlyo et al., 1988; Corrie et al., 1993; Ogden and Capiod, 1997). In a genetically characterized organism such as E. coli, this approach may also be coupled with analysis of mutants to temporally isolate, map, and characterize relatively ill-defined pathways, as illustrated here for the PTS chemotactic signaling pathway. Processing of PTS-dependent signals was time-resolved by coupling photolysis of caged precursors of d-glucose (d-glc) and its nonmetabolizable analog methyl α-d-glucopyranoside (Me α-glc) (Figure 1) to computer-assisted motion analysis of the response (Khan et al., 1993, 1995). Available data indicate that chemotactic responses triggered by these sugars are representative of responses obtained for all PTS substrates studied thus far (Adler et al., 1973; Lengeler et al., 1981).
Figure 1.
Chemical structures of the caged sugars used in this study.
The temporal resolution of this assay revealed that MCP signaling complexes are also used by PTS substrates to relay chemotactic signals with rapidity and sensitivity comparable to amino acid attractants. The high sensitivity renders implausible the idea that the signal to the MCP complexes derives from perturbation of PEP or associated metabolite levels, but may be reconciled with interaction of PTS Enzyme I with the MCP-associated CheA.
MATERIALS AND METHODS
Caged Compounds
A caged fluorophore, the 1-(2-nitrophenyl)ethyl ether of 8-hydroxypyrene-1,3,6-trisulfonic acid (caged HPTS) was used to estimate the magnitude of the concentration jumps achieved in flash photorelease assays to within 20% (Jasuja et al., 1999). Caged l-aspartate [aspartic acid β-(2,6-dinitrobenzyl) ester] and caged l-serine [N-1-(2-nitrophenyl)ethoxycarbonyl-l-serine] were used to measure excitation responses to aspartate and serine, attractant ligands for the major MCPs Tar and Tsr, respectively. The synthesis and photochemical properties of the caged HPTS, l-aspartate, and l-serine reagents have been described (Khan et al., 1993; Jasuja et al., 1999).
The synthesis and photochemical properties of caged d-glc [2-O-(2-nitrobenzyl)-d-glucose] used in this study have been described (Corrie, 1993), and caged Me α-glc was prepared by modification of the caged d-glc synthesis, as described below. A solution of methyl 3,4,6-tri-O-acetyl-2-O-(2-nitrobenzyl)-α-d-glucopyranoside (Corrie, 1993) (0.53 g, 1.16 mmol) in methanol (5.1 ml) was treated with 2 M aqueous NaOH (1.88 ml). After 1 h at room temperature, the solution was stirred with MeOH-washed Dowex 50 (Sigma, Dorset, United Kingdom; H+ form; 3.41 g) to neutralize the alkali, and then filtered. The filtrate was evaporated under reduced pressure, and the residue was flash-chromatographed (MeOH:CHCl3, 6:94 vol/vol ratio; Merck, Dorset, United Kingdom; 40–63 μm silica gel). Pure fractions recovered from chromatography were combined and evaporated under reduced pressure, and the residue was dissolved in water and lyophilized to give caged Me α-glc [methyl 2-O-(2-nitrobenzyl)-α-d-glucopyranoside] as a pale yellow foam (0.27 g, 70%); (Found: [FAB mass spectrometry] [M + Na]+ 352.1020. [C14H19NO8 + Na]+ requires M+, 352.1020); UV: λmax (H2O)/nm 265 (ε 5300 M−1cm−1); δH (400 MHz; D2O; acetone standard) 8.09 (1 H, d, J 8.0 Hz, ArH-3), 7.72–7.78 (2 H, m, ArH), 7.53–7.63 (1 H, m, ArH), 5.06 (2 H, ABq, J 13.2 Hz, ArOCH2) 4.87 (1 H, d, J1,2 3.7 Hz, H-1), 3.85 (1 H, dd, J6,6′ 12.3 Hz, J5,6 2.2 Hz, H-6), 3.73 (1 H, dd, J5.6′ 4.6 Hz, H-6′) superimposed on 3.73 (1 H, t, J2,3 = J3,4 9.6 Hz, H-3), 3.61 (1 H, ddd, J4,5 9.6 Hz, H-5), 3.50 (1 H, dd, H-2), 3.40 (1 H, t, H-4), 3.36 (3 H, s, OMe).
The release rate of the glucoside upon flash photolysis was inferred from the decay rate of the photochemically generated aci-nitro intermediate, as described for the caged d-glc (Corrie, 1993). As for the caged d-glc, flash photolysis (pH 7.0, 20°C, 150 mM Na phosphate) showed biphasic decay, with rates of 97 and 7 s−1 and relative amplitudes of 2.6:1. To determine the product quantum yield, QP, of photolysis, aliquots (0.5 ml) of a solution of caged Me α-glc and caged d-glc (each 50 μM) with dithiothreitol (2 mM) in 10 mM sodium phosphate (Sigma) (pH 7.0) were exposed for varying times (8–24 s) to light from a xenon arc lamp (Photochemical Research Associates, London, Ontario, Canada) that passed through a Hoya Optics (Fremont, CA) U340 filter before illuminating the cell. The irradiated samples were kept at room temperature overnight to allow the anomers of the residual caged d-glc to reequilibrate (Corrie, 1993) and were analyzed by reversed-phase HPLC [Merck Lichrosphere RP8 column (catalog no. 50832); mobile phase 10 mM sodium phosphate, pH 7.0, plus 25% MeOH (vol/vol); flow rate 1.5 ml min−1; UV detection at 254 nm]. Caged Me α-glc eluted at 15.6 min and caged d-glc eluted as a double peak at 6.6 and 7.5 min. The extents of conversion for caged Me α-glc and caged d-glc were 35.1–68.7 and 39.6–75.3%, respectively (means of three determinations at each time point), with caged d-glc converted 1.07-fold more efficiently than caged Me α-glc. The QP for caged d-glc is 0.63 (Corrie, 1993), and the value for caged Me α-glc was therefore 0.59.
Growth Media and Chemicals
Bacteria were grown at 35°C in Luria broth (plus 10 mM d-glc and 2.5 mM CaCl2) for P1 transduction, and in tryptone broth for behavioral assays. Tryptone swarm agar (0.35%) plates were used for motility selection, and minimal medium (Adler, 1973) swarm agar (0.27%) plates (Difco) were used for diagnosing phenotypes.
The bacteria were washed thrice and resuspended in motility buffer before experiments (10 mM sodium/potassium phosphate, pH 7.0, 10 mM potassium chloride, 0.1 mM EDTA, 5 mM lithium lactate, 125 μM methionine). For flash photorelease assays, this buffer also contained 5 mM dithiothreitol. Stocks of lactate, methionine, and dithiothreitol were stored at −20°C and added to the motility buffer before experiments. d-glc, Me α-glc (< 0.01% d-glc contamination), l-aspartate, and l-leucine were purchased from Sigma (St. Louis, MO).
Bacterial Strains
The bacterial strains used in this work are listed in Table 1. Mutant strains used for analysis of PTS-mediated chemotactic responses were constructed in E. coli K12 strain JWL184–1. Mutations were moved via P1 transduction (Arber, 1960) as modified (Lengeler, 1975).
Table 1.
Strains
| Strain | Relevant genotype | Source, derivation, or reference |
|---|---|---|
| JWL184-1 | MglP− mal+ GalP63 | Lengeler et al. (1981) |
| RP2859 | Δ(cheRcheB)2241 | Parkinson and Houts (1982) |
| RP4971 | Δ(cheB)63 | J.S. Parkinson (unpublished data) |
| RP5099 | zea::Tn10-3 | J.S. Parkinson (unpublished data) |
| RP9351 | Δ(cheW-tap)2217 Δ(cheZ)67-25 zea::Tn10-3 | Liu and Parkinson (1989) |
| JLV37-115 | as JWL184-1 but cheZ | A.P. Vogler (unpublished data) |
| JLV92 | as JWL184-1 but ptsH5 | Grübl et al. (1990) |
| LJ120 | as K-12 but Δ(ptsG) Camr | K. Jahreis (unpublished data) |
| JWL191 | as JWL184-1 but ptsI191 malA1 | Lengeler et al. (1981) |
| TP2865 | Δ(crr) Kanr | Lévy et al. (1990) |
| LLR103 | as JWL184-1 but Δ(crr) Kanr | P1(TP2865) × JWL184-1 to Kanr |
| LLR104 | as JWL184-1 but Δ(ptsG) Camr | P1(LJ120) × JWL184-1 to Camr |
| LLR413 | as RP4971 zea::Tn10-3 | P1(RP5099) × RP4971 to Tetr |
| LLR414 | as RP2859 zea::Tn10-3 | P1(RP5099) × RP2859 to Tetr |
| KLR202 | as JWL184-1 but Δ(cheW-tap)2217Δ(cheZ)67-25 zea::Tn10-3 | P1(RP9351) × JWL184-1 to Tetr |
| KLR203 | as JWL184-1 but Δ(cheB)63 zec::Tn10-3 | P1(LLR413) × JWL184-1 to Tetr |
| KLR204 | as JWL184-1 but Δ(cheRcheB)2241 zea::Tn10-3 | P1(LLR414) × JWL184-1 to Tetr |
Behavioral Assays
Flash Photorelease Assays.
Flash photorelease assays were performed using shuttered (30 ms duration), near-UV (330–380 nm) epi-illumination, as described previously (Khan et al., 1993). Video records were digitized at 30 frames/s (VP320 digitizer, Motion Analysis Inc., Santa Rosa, CA) and analyzed off-line (SunSparc2 workstation, ExpertVision version 1.4 motion analysis software; M. Motion Analysis, Santa Rosa, CA) using frame-to-frame rate of change of direction (rcd) and linear speed (spd) operators. The instrumentation, software, and operators have been described (Khan et al., 1993, 1995). The population rcd responses were fitted using routines available in Sigmaplot (Jandel Scientific Inc., San Rafael, CA). Excitation response rates, kex, were determined from single exponential fits. When a lag was evident, as in near-threshold responses, the response half-time, t1/2 (= ln2/kex), was determined by a logistic fit.
Flow Cell Assays.
A continuous laminar flow cell (Berg and Block, 1984) was used for tethered cell experiments. Cells were sheared (21-gauge needles) and tethered on flagellar antibody-coated coverslips essentially as described (Khan et al., 1993). Buffer exchange was effected via an eight-way valve positioned close to the inlet of the flow cell. Continuous flow was maintained at 0.24 ml/min. Flow cell wash-in/wash-out kinetics were determined by exchange of buffers containing the hydrophilic chromophore HPTS (Molecular Probes, Eugene, OR).
Wash-in of buffer containing an attractant (d-glc or l-aspartate) or repellent (l-leucine) stimulus was maintained for 4 min followed by flow-in of buffer for an equivalent time. A sample was subjected to three to five such exchange cycles per experiment. Transition times (i.e., time period from flow-in to the first post-stimulus reversal) (Berg and Tedesco, 1975) were taken as a measure of adaptation. These were determined from video playback off-line using a stopwatch. They depended on the duration between successive jumps. For intervals <2 min per wash-in/wash-out cycle, transition times decreased with successive jumps, implying that this duration was not sufficient to attain a metabolic steady state (Lowry et al., 1971; Weigel et al., 1982).
RESULTS
Rapid Processing of PTS Chemotactic Signals
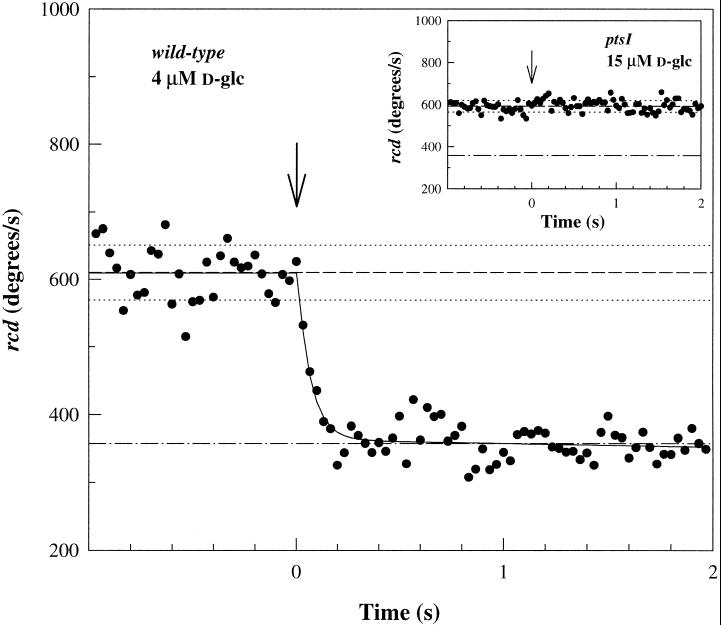
Excitation responses to step stimuli of d-glc were time-resolved by computerized motion analysis of swimming cell responses to flash photolysis of caged d-glc. In strain JWL184–1, which lacks periplasmic binding protein-mediated chemotaxis toward d-glc, photorelease of d-glc (4–40 μM) elicited rapid excitation responses (Figure 2). There was no detectable response in ptsI or ptsH mutant strains lacking EI (Figure 2, inset) or HPr, respectively, consistent with results from capillary assays (Lengeler et al., 1981). Responses of the mutants to photoreleased aspartate were similar to those measured for other E. coli strains (Jasuja et al., 1999). These results implied that the PTS machinery mediated the responses observed in the JWL184–1 parent strain.
Figure 2.
PTS-dependent excitation response to d-glc photorelease. Excitation response of strain JWL184–1 (wild type for PTS chemotaxis) to photorelease of 4 ± 0.9 μM d-glc. The saturation response is well-fit by a single exponential (kex = 14.9 ± 0.9 s−1). Inset: Nonresponsivity of mutant strain JWL191 (ptsI) lacking EI to 15 μM d-glc photorelease. Arrows denote photolyzing flash. The prestimulus population rcd (see MATERIALS AND METHODS) means (dashed lines) ± frame-to-frame SD (dotted lines) are shown. Dashed–dotted lines indicate the rcd value for complete smooth-swimming.
Extracellular Photorelease of Nanomolar Glucose Elicits Detectable PTS Chemotactic Responses
A general concern with use of caged compounds is that secondary products of the photolysis reaction may not be chemically or biologically inert. A further concern for this particular application was that the response might be due to photolysis of caged d-glc that had permeated into the cytoplasm. The control experiments with the mutant strains alleviated these concerns, but left open the possibility that the wild-type (JWL184-1) responses were due to PTS-specific uptake and subsequent intracellular photorelease of caged d-glc. Responses to intracellular photorelease should be independent of rates of PTS transport, provided the substrate has equilibrated between the extracellular and intracellular phases. Therefore, EII mutants with impaired rates of glucose transport were tested to determine whether responses were due to intracellular photorelease. Experimental cultures were incubated with caged glucose for times sufficient to allow its equilibration between the extracellular and intracellular phases (30 min). Longer incubation times did not measurably increase response strength. Mutant strains LLR103 and LLR104 lacked the soluble cytoplasmic (EIIAGlc) or the transmembrane (EIIBCGlc) component of the d-glc–specific EII, respectively. They responded to d-glc photorelease, but response thresholds were an order of magnitude greater than the nanomolar threshold seen for JWL184–1 (see below). These increased thresholds were commensurate with low-affinity transport of d-glc by the mannose EII (Lengeler et al., 1981) or EIIANag/EIIBCGlc chimeras (Vogler et al., 1988). Thus, the observed responses were due to transmembrane transport of extracellularly photoreleased d-glc.
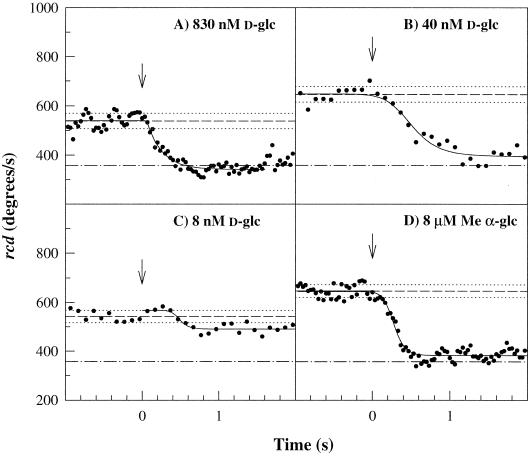
Saturation smooth-swim responses obtained on photorelease of 0.8 μM d-glc (Figure 3A) had single exponential form but a slower rate (kex) than those observed on release of 4 μM d-glc. At 0.04 μM, the response just failed to saturate and also deviated from a single exponential (Figure 3B). A still fivefold lower concentration elicited responses close to the detection threshold (Figure 3C). Such threshold responses were characterized by a marked lag phase. This indicated that at low concentrations the PTS signal pathway contained more than one rate-limiting process. Furthermore, these data showed importantly that the PTS chemotactic response had a nanomolar detection threshold rather than the micromolar level determined by capillary assays (Adler and Epstein, 1974; Lengeler et al., 1981).
Figure 3.
PTS chemotactic response sensitivity. Wild-type JWL184–1 responses. (A) 0.83 ± 0.09 μM d-glc; kex = 4.9 ± 0.4 s−1. (B) 40 ± 3 nM d-glc. (C) 4 ± 0.3 nM d-glc. (D) 8 ± 0.2 μM α-methyl glucoside. Note the lag preceding the rcd decrease, evident in c and less so in b and d. t1/2 values were ∼500 ms for b and c and 250 ms for d, respectively. Arrows and reference lines are as in Figure 2.
The response threshold for Me α-glc was 40-fold higher than that for d-glc, consistent with the lower affinity of the d-glc PTS for Me α-glc. Photorelease of 8 μM Me α-glc evoked a slower response (Figure 3D) than that for a comparable concentration jump of photoreleased d-glc (Figure 2). Its form deviated from a single exponential and was similar to that seen for 50 nM d-glc photorelease. Responses to photorelease of higher concentrations followed single exponential excitation kinetics, whereas responses to lower concentrations showed a lag that increased with decreasing concentrations. This concentration dependence was qualitatively similar to that observed for d-glc. In contrast, single exponential kinetics characterized the responses to amino acid attractants down to the smallest measurable values (Jasuja et al., 1999).
PTS Chemotactic Signals Are Transmitted via the MCP-Che Phosphorelay
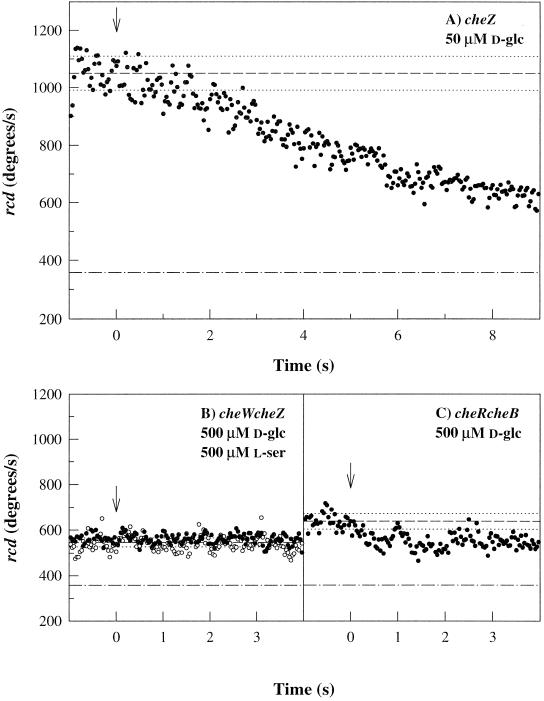
PTS chemotaxis requires CheA and CheY (Rowsell et al., 1995). This might be because the chemotactic signal generated by PTS substrates is relayed to the flagellar motors via the Che phosphorylation cascade. Alternatively, phosphorylated CheY may be required for PTS chemotactic signal reception by flagellar motors. To distinguish between these possibilities, excitation responses of a cheZ-negative mutant to d-glc photorelease were measured. The mutant response (Figure 4A) was dramatically slowed by comparison to the wild-type response. It was comparable to responses of cheZ mutants to serine photorelease (Khan et al., 1993). Thus, a decrease in CheY.P levels is implicated in the mechanism by which the PTS signal effects motor response. This signal cannot act via CheZ, because cheZ mutants still respond.
Figure 4.
PTS-dependent excitation responses of che mutant strains. (A) cheZ mutant JLV37–115; 50 μM d-glc photorelease. (B) cheW cheZ mutant (KLR202); 500 μM photorelease of l-serine (○) and d-glc (●). (C) cheR cheB (KLR204) mutant; 500 μM d-glc photorelease. Arrows and reference lines are as in Figure 2.
How might the PTS and Che phosphorelays communicate? Decrease in CheY.P levels may be brought about by inhibition of CheA kinase activity or by direct interaction of the PTS signal with CheY.P. In the former alternative, PTS signal processing should be affected by deletion of the MCPs, because the activity of soluble CheA is negligible relative to MCP-bound CheA (Gegner et al., 1992; Schuster et al., 1993). Unfortunately, mutant strains with the MCPs deleted have a smooth-swimming phenotype (Khan et al., 1993). Alternatively, therefore, mutant strains with altered MCP-bound CheA activity were examined. Deletion of the linker CheW or the MCP-modifying enzymes, the methyltransferase CheR and/or the methylesterase CheB, are known to impair MCP signaling triggered by amino acids. These deletions also affected PTS chemotactic signaling.
CheW is required for interaction of the MCPs with CheA. Hence, cheW cheZ double-deletion mutants have close to wild-type swim-tumble bias (Figure 4B). This double mutant did not respond to photorelease of up to 0.5 mM d-glc or 0.5 mM serine or, in tethered cell assays, concentration jumps up to 1 mM serine. Because cheZ mutants responded strongly to 50 μM d-glc photorelease (Figure 4A), the lack of response in the double mutant must be due to the cheW deletion. These data are consistent with the report that CheW is required, in addition to CheA and CheY, to enable gutted strains to respond to the PTS substrate mannose (Rowsell et al., 1995). In the latter case, CheW is needed for interaction of the PTS signal with CheA. The present experiments show that the physiologically relevant inhibition of MCP-bound CheA activity by PTS signals also requires CheW.
In tethered cell assays, cheB mutants could be transiently driven into complete CCW rotation by step increases of attractant amino acids (≤20 μM serine; ≤400 μM aspartate). These abnormally large increases were presumably needed to overcome the reduced sensitivity (Segall et al., 1986) and extreme CW bias of cheB mutants. d-glc concentration jumps up to 1 mM failed to elicit a measurable response. Similarly, photorelease of 0.5 mM d-glc elicited barely detectable responses from cheB mutants (Lux, unpublished results) or cheR cheB mutants, which had close to wild-type swim-tumble bias (Figure 4C). This reduced sensitivity was not due to increased MCP methylation levels, because elevation or reduction of these levels by aspartate (1 mM) or leucine (10 mM), respectively (Springer et al., 1979), was without effect. It may result, therefore, from the absence of the deaminase activity of CheB rather than its methylesterase activity. In any case, the absence of CheB places MCP–CheA complexes in a conformation that interferes not only with transmembrane signaling by periplasmic ligands but also interaction with the cytoplasmic PTS chemotactic signal.
PTS Chemotactic Excitation and Adaptation Kinetics Scale with Signal Strength
Complete smooth-swimming responses were obtained at 50–100 nM photoreleased d-glc, but response rates continued to increase with d-glc concentration. Cell densities in the experimental samples used for photorelease assays prevented measurement of chemotactic adaptation because the extracellularly photoreleased d-glc was rapidly depleted by PTS-mediated uptake. Therefore, adaptive transition times (tr) (Berg and Tedesco, 1975) were measured in tethered cell assays to assess the signal strength obtained for concentration increases >100 nM.
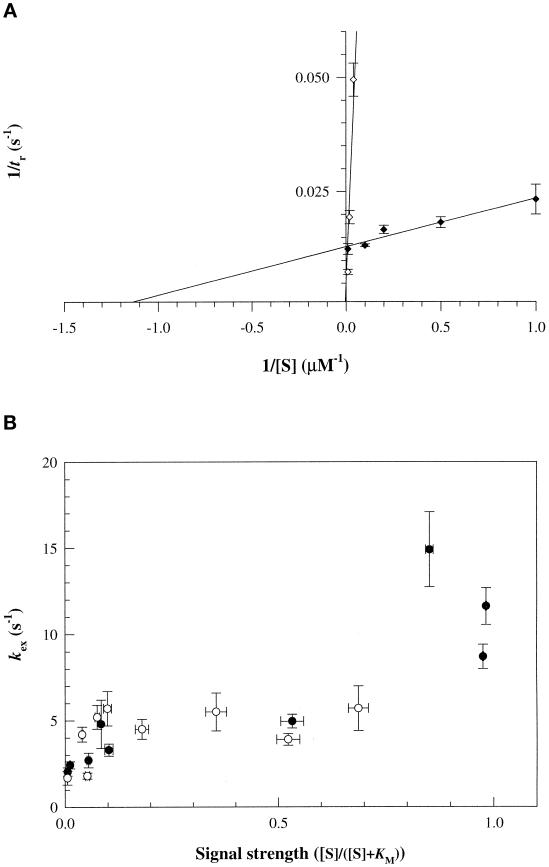
Transition times increased with d-glc or Me α-glc concentration. An apparent Km of 0.9 ± 0.5 μM was obtained for d-glc from reciprocal plots (Figure 5A). This strain has an apparent Km for transport through EIIGlc of 5 μM (Lengeler et al., 1981). Values for other E. coli strains ranging from 3 to 20 μM have been reported. The data for Me α-glc were consistent with the 40-fold difference in response thresholds determined in photorelease assays, as well as with differences between the d-glc and Me α-glc transport Km values (Adler and Epstein, 1974; Stock et al., 1982; Misset et al., 1983; Grenier et al., 1986).
Figure 5.
Adaptation and excitation kinetics of the positive PTS signal. d-glc (closed circles); Me α-glc (open circles). (A) Reciprocal plots of JWL184–1 tethered cell transition times, tr (±SE), versus substrate concentration. Responses at other d-glc or Me α-glc concentrations were normalized by responses to 10 μM d-glc determined for each experiment. The latter had a mean tr of 75 ± 1.8 s. Each data point typically represents results of two independent experiments (20–50 cells/experiment). (B) Excitation response rates, kex, determined from photorelease assays, plotted against signal strength.
The chemotactic Km values were used to define signal strength. This was {[S]/([S]+ Km)}, where [S] is photoreleased sugar concentration. Excitation response rates increased with signal strength. Responses at high signal strength (>0.8) had mean response rates (11.7 ± 3.1 s−1) indistinguishable from those measured for the MCP attractant ligand aspartate (Jasuja et al., 1999). Rates of responses to d-glc and Me α-glc were superimposable (Figure 5B).
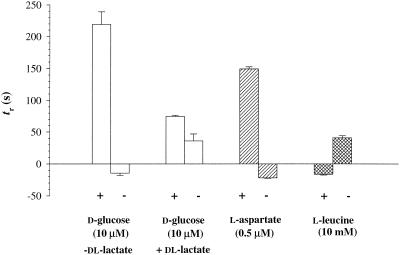
Responses to Withdrawal of PTS Substrates Depend on Metabolic State
CW responses to withdrawal of d-glc were not observed under our standard buffer conditions, which contained lactate. Instead, a weak positive CCW response, whose duration did not depend on concentration, was occasionally observed (Figure 6). Weak CW responses were obtained during d-glc withdrawal in buffers lacking lactate; however, addition of lactate to such buffers also caused CCW responses, and its withdrawal caused CW responses. This suggested that the bacteria were partially deenergized in these buffers and that responses to both glucose and lactate under these conditions reflected changes in their energy levels. Negative CW responses to withdrawal of amino acid attractants or addition of repellents were obtained in tethered cell assays, as expected (Larsen et al., 1974).
Figure 6.
Tethered cell responses to d-glc withdrawal. Transition times, tr (±SE), on addition (+) or withdrawal (−) are shown. Positive and negative values denote CCW and CW responses, respectively. Results obtained with a single experimental culture split into two halves, one energized by dl-lactate and the other not. Tethered cells lacking lactate were partially deenergized because they responded positively to its addition and negatively to its withdrawal. Similar results were obtained in additional experiments (n > 5 for each condition). Responses to addition or withdrawal of the attractant aspartate (0.5 μM) or the repellent leucine (10 mM), periplasmic ligands for the MCPs Tar and Tsr, respectively, measured in separate experiments, are also shown. These responses were insensitive to the presence of lactate. In each case, 20–50 cell populations were analyzed.
DISCUSSION
Previous knowledge, summarized in INTRODUCTION, indicated that CheA could be inhibited and stimulated by unphosphorylated EI and PEP, respectively, and additionally identified further control points downstream in the Che signal pathway that could be affected by the PTS transport-induced drop in PEP levels. These possibilities were distinguished and the nature of the PTS signal clarified by time-resolved quantification of the excitation response. Mutant strains and rapid photorelease of PTS chemoattractants were used to dissect the pathway and characterize its sensitivity. Excitation and adaptation kinetics of PTS-mediated chemotactic responses triggered by photorelease of d-glc and the nonmetabolizable Me α-glc were compared to assess whether perturbation of metabolite levels affects response. The PTS signal was found to have nanomolar sensitivity for d-glc. It was processed with a rate comparable to signals generated by MCP attractants over most of the response range. These findings provide novel insight into the in vivo operation of the PTS phosphorelay during chemotaxis.
Coupling between the PTS and Che Phosphorelays
EI and HPr negative mutants did not respond to photorelease of d-glc. EIIAGlc and EIIBCGlc transport mutants had elevated response thresholds. Hence the responses observed in wild-type bacteria were due to extracellular photorelease of the sugars that required subsequent PTS-dependent transport to effect a chemotactic response.
The slower kinetics of the excitation response in cheZ mutants showed that the parameter sensed by flagellar motors was the decrease in CheY.P levels. Thus the PTS signal does not act via an independent pathway on a step affecting CheY.P binding to, or action on, the motor, nor does it affect CheZ-dependent dephosphorylation of CheY. cheR cheB and cheW cheZ mutants have impaired MCP-based signaling. These strains hardly responded to PTS-dependent chemotactic signals generated by d-glc photorelease. These observations, together with the kinetics of the cheZ mutant response, argued against the possibility that these signals acted directly to accelerate CheY.P dephosphorylation. They indicated instead that the signals inhibited the CheA kinase activity of MCP signaling complexes.
Thus, the mutant analysis provides evidence for a single PTS chemotactic signal that is transmitted via MCP signaling complexes to effect a decrease in CheY.P levels, hence CCW motor response.
Timing and Amplification in the PTS Chemotactic Signal Pathway
Rapidity, kex = 4.4 ± 0.9 to 11.7 ± 3.1 s−1 over the 0.1 to 0.9 range of signal strength (Figure 5B), and high sensitivity, i.e. detection of Km/100 concentration differences (10 nM for d-glc), constitute our two major findings regarding the physiology of the PTS chemotactic response. These properties constrain possibilities regarding the nature of the PTS signal.
Rapid, reversible histidine phosphorylations/dephosphorylations, with rates of 106–108 M−1s−1 (Anderson et al., 1993; Meadow and Roseman, 1996), characterize the PTS phosphorelay. These rapid kinetics are consistent with structural data showing that the phosphorylatable histidine residues are located on exposed surface loops and do not require large conformational changes for accessibility (McEvoy and Dahlquist, 1997). Flux at steady state in the absence of substrate transport is much slower than the phosphotransfer rates. It is limited by the slow (3.4 × 103 M−1s−1 [Chauvin et al., 1994]) dimerization of EI monomers, maintaining the PTS phosphoenzymes predominantly (>80%) in their phosphorylated form (Hoving et al., 1981; Nelson et al., 1986).
Transport results in redistribution of the PTS components toward their nonphosphorylated forms at a rate determined by the concentration of substrate. Km values determined for chemotactic adaptation provide further support for the premise that the substrate concentration dependence for chemotaxis reflects that for transport (Lengeler et al., 1981). Adoption of this premise accounts simply for the shift from biphasic to single exponential decay kinetics. Given a maximal transport rate (Vmax) of 70 ± 20 μmol·g−1 dry wt·min−1 (Adler and Epstein, 1974; Stock et al., 1982), PTS phosphotransfers will exceed the rate for CheY.P dephosphorylation at 200 nM extracellular d-glc (Table 2) and will not limit signaling for this and greater concentration jumps. This is consistent with the observation that single exponential excitation kinetics with a rate equal to that obtained for signaling by MCP periplasmic ligands are obtained over most of the response range, with a lag evident for 50 nM and lower d-glc concentration jumps (Figures 3 and 5B).
Table 2.
PTS concentration and rate changes during chemotactic excitation
| Response | [S] (nM) | t1/2 (s) | v (μM·s−1) | ΔEI | ΔPEP |
|---|---|---|---|---|---|
| Close to threshold | 10 | 0.5 | 2.5 | 0.25 | 0.0025 |
| Close to saturation | 50 | 0.34 | 12 | 0.45 | 0.005 |
| Monoexponential kinetics | >200 | <0.1 | >40 |
Fractional changes in PEP and phosphorelay components were computed from initial rates of transport, v = {[S]·Vmax/([S]+ Km)} μM·s−1; where [S] = extracellularly photoreleased glucose; Km was determined from Figure 5A. The fractional change, ΔC = (v·t1/2)/CT; where CT, the total concentration, is 5, 25–100, and 25–50 μM for [EI], [HPr], and [EIIA], respectively (Scholte et al., 1982; Mattoo and Waygood, 1983), and 0.1–1 mM for [PEP] (Lowry et al., 1971). Therefore, ΔHPr and ΔEIIAGlc are one-fifth to one-twentieth the ΔEI. This assumes that the components are initially present almost entirely in their phosphorylated forms and that back reactions are negligible during the excitation time period (see RESULTS). The latter assumption is likely to be valid for Δ[HPr] and Δ[EIIA], but will provide an underestimate for Δ[EI] or Δ[PEP]. Vmax, hence v, were expressed as rates of micromolar change in intracellular concentration per second (μM·s−1). Reported values expressed as μmol·g−1 dry wt·min−1 were converted using cytoplasmic volume/gram dry weight = (number of bacteria/gram dry weight) (volume/bacterium) = (6 × 1012) (1.4 × 10−12) ml/g = 8.4 ml/g·Vmax for PTS d-glc transport = 200 μM·s−1. In contrast, Vmax for aspartate transport <1 μM·s−1 (Kay, 1971). CheY.P dephosphorylation rate = (kc + k−c) [CheY.P] = (10 s−1) (2 μM) = 20 μM·s−1 (Jasuja et al., 1999). Thus, this will become the single rate-limiting process for PTS chemotactic signaling for 0.2 μM and greater concentration jumps.
From initial transport rates, estimated from concentration jumps producing threshold and saturation responses, respectively, it may be estimated that PEP levels will change imperceptibly (<0.2%) during chemotactic excitation (Table 2). It is difficult to imagine how such small decreases in PEP levels can be converted to appropriate changes in MCP signaling activity. PEP levels do drop over 1–3 min (Lowry et al., 1971), in turn affecting transport rates (Weigel et al., 1982), but these changes are too slow to be relevant for chemotactic signal processing. Furthermore, subsequent metabolism of d-glc might be expected to retard the drop in PEP levels induced by its transport. This will not occur for the nonmetabolizable Me α-glc. The superimposition of plots of excitation response rates versus chemotactic signal strength for both sugars (Figure 5B) therefore also argues against a role for perturbation of PEP levels in chemotactic signaling.
In contrast, large changes in EI levels will occur during chemotactic excitation. Fractional increases in concentration of the dephosphorylated forms of other PTS components, HPr and EIIAGlc, which are present at an order of magnitude higher concentration, will be correspondingly less (Table 2). Ignorance of the basal leakage rate prevents an accurate estimate of the fractional increase in unphosphorylated [EI]; however, this will be substantial and will occur over times comparable to the excitation time, even for near-threshold responses. Macromolecular crowding, as suggested earlier (Lux et al., 1995), and/or CheW–MCP association could increase the affinity of unphosphorylated EI for CheA, allowing substantial inhibition of CheA activity to be achieved. In addition, subsequent amplification will be necessary to transduce the corresponding change in CheA activity to the observed motile responses (see Discussion in Lux et al., 1995).
Cessation of transport caused by substrate withdrawal will induce redistribution of the PTS components toward their phosphorylated forms. This process will be limited by the slow EI dimerization and should therefore elicit, at best, a weak negative chemotactic signal. The CW response observed in tethered cells (Rowsell et al., 1995) may not be due to PTS-generated signals but rather to perturbation of cellular energy levels because it seems to require partial deenergization of the bacteria. In the presence of lactate, a negative response was not observed.
Changes in PEP levels may also be involved in adaptation (Lux et al., 1995). The fact that both d-glc and Me α-glc have adaptation kinetics commensurate with the corresponding transport Km values indicates that adaptation cannot result solely from reequilibration of PEP pools, nor can it be due solely to MCP methylation (Niwano and Taylor, 1982). Multiple adaptive processes may be operative. Assessment of their contribution over physiologically relevant time scales will require time-resolved analysis of small stimuli.
Concluding Comment
Why are chemotactic responses to PTS substrates coupled to transport, unlike responses to amino acids? The answer may lie in the fact that transport of sugars/carbohydrates is intimately related to cellular energy metabolism. The free-energy change resulting from cleavage of the PEP phosphate bond is one of the highest known (Atkinson and Morton, 1960). This is used via the PTS cascade to scavenge these compounds from the medium much more rapidly than amino acids (Table 2 legend), whose uptake does not immediately affect cellular physiology. Distinct machinery has therefore evolved to allow amino acids to effect rapid chemotactic responses. Coupling of the PTS and Che phosphorelays affords an elegant solution where rapid PTS phosphotransfer reactions, already in place for transport, provide a time-resolved readout of the extracellular substrate concentration. This readout is amplified and relayed to flagellar motors using mechanisms intrinsic to the MCP–Che machinery (Figure 7). ATP-driven ATP-binding cassette transporters also mediate high-affinity sugar transport (Boos and Lucht, 1996). In this case the solution is direct interaction of the periplasmic binding proteins with the MCPs, because perturbations of intracellular ATP levels are prohibitive. In both cases, the MCP signaling complex emerges as the key element in chemotactic signal processing, a role that may be related to its importance in signal amplification.
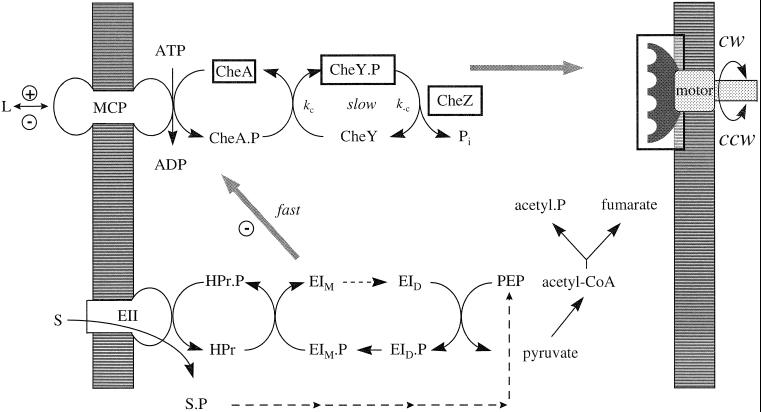
Figure 7.
Properties of the PTS chemotactic pathway. The PTS is poised far from equilibrium because of the slow dimerization of EI monomers (EID to EIM, dashed arrow), obligatory for phosphorylation by PEP. The positive chemotactic signal triggered by transport of PTS substrates (S) may originate either from a decrease in phosphorylation levels of the donor phosphorelay or from changes in metabolite levels caused by PEP consumption. It may act on CheA, CheZ, CheY.P, or the motor switch (boxed items). The present study sought to distinguish between these possibilities. The analysis of mutant responses showed that the PTS signal acted solely to inhibit MCP-bound CheA. The high response sensitivity argued that it is generated by the dephosphorylation of PTS phosphorelay components, most likely EI. PEP levels decrease little, and their subsequent restoration by metabolism of d-glc (dashed arrows, small arrowheads) does not affect signaling. The response kinetics implied that signaling (gray arrows) was limited, as for periplasmic MCP ligands (L), by CheY.P dephosphorylation (i.e., kc + k−c) over most of the PTS chemotactic response range if PTS dephosphorylation kinetics were determined by the transport rate, consistent with known transport and biochemical data. Rephosphorylation of the PTS phosphorelay on withdrawal of PTS substrates will be limited by EI dimerization and may not be rapid enough to stimulate CheA (+), in contrast to withdrawal of MCP attractant ligands. Evidence for such a signal was not obtained.
ACKNOWLEDGMENTS
We thank David R. Trentham (D.R.T.) for initiating collaborative efforts between J.E.T.C and S.K. and for encouragement and advice; John S. Parkinson (University of Utah) and Knut Jahreis (Universität Osnabrück) for strains; Kevin J. Welham (University of London) for mass spectroscopy; Ravi Jasuja for assistance with photorelease assay calibration; and the Medical Research Council Biomedical NMR Centre for access to facilities. This work was supported by grants from the National Institute for General Medical Sciences (GM-43919 to S.K.), the North Atlantic Treaty Organization (CRG-940021 to S.K./D.R.T.), and the Deutsche Forschungsgemeinschaft (SFB-171, TPC-3 to J.W.L.)
REFERENCES
- Abouhamed WN, Bray D, Schuster M, Boesch KC, Silversmith RE, Bourret RB. Computer-aided resolution of an experimental paradox in bacterial chemotaxis. J Bacteriol. 1998;180:3757–3764. doi: 10.1128/jb.180.15.3757-3764.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler J. A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J Gen Microbiol. 1973;74:77–91. doi: 10.1099/00221287-74-1-77. [DOI] [PubMed] [Google Scholar]
- Adler J, Epstein W. Phosphotransferase-system enzymes as chemoreceptors for certain sugars in Escherichia coli chemotaxis. Proc Natl Acad Sci USA. 1974;71:2895–2899. doi: 10.1073/pnas.71.7.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler J, Hazelbauer GL, Dahl MM. Chemotaxis toward sugars in Escherichia coli. J Bacteriol. 1973;115:824–847. doi: 10.1128/jb.115.3.824-847.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JW, Pullen K, Georges F, Klevit RE, Waygood EB. The involvement of the arginine 17 residue in the active site of the histidine-containing protein, HPr, of the phosphoenolpyruvate: sugar phosphotransferase system of Escherichia coli. J Biol Chem. 1993;268:12325–12333. [PubMed] [Google Scholar]
- Appleby JL, Parkinson JS, Bourret RB. Signal transduction via the multi-step phosphorelay: not necessarily a road less traveled. Cell. 1996;86:845–848. doi: 10.1016/s0092-8674(00)80158-0. [DOI] [PubMed] [Google Scholar]
- Arber W. Transduction of chromosomal genes and episomes in Escherichia coli. Virology. 1960;11:273–288. doi: 10.1016/0042-6822(60)90066-0. [DOI] [PubMed] [Google Scholar]
- Atkinson MR, Morton RK. Free energy and the biosynthesis of phosphates. In: Florkin M, editor. Comparative Biochemistry. II. H.S. Klasen, New York: Academic Press; 1960. pp. 1–95. [Google Scholar]
- Berg HC, Block SM. A miniature flow cell designed for rapid exchange of media under high-power microscope objectives. J Gen Microbiol. 1984;130:2915–2920. doi: 10.1099/00221287-130-11-2915. [DOI] [PubMed] [Google Scholar]
- Berg HC, Brown DA. Chemotaxis in Escherichia coli analyzed by three-dimensional tracking. Nature. 1972;239:500–504. doi: 10.1038/239500a0. [DOI] [PubMed] [Google Scholar]
- Berg HC, Tedesco PM. Transient response to chemotactic stimuli in Escherichia coli. Proc Natl Acad Sci USA. 1975;72:3235–3239. doi: 10.1073/pnas.72.8.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boos W, Lucht JL. Periplasmic binding protein-dependent ABC transporters. In: Neidhardt FC, Curtiss R III, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE, editors. Escherichia coli and Salmonella: Cellular and Molecular Biology. 2nd ed. Vol. 1. Washington, DC: American Society for Microbiology; 1996. pp. 1175–1209. [Google Scholar]
- Bray D. Signaling complexes: biophysical constraints on intracellular communication. Annu Rev Biophys Biomol Struct. 1998;27:59–75. doi: 10.1146/annurev.biophys.27.1.59. [DOI] [PubMed] [Google Scholar]
- Chauvin F, Brand L, Roseman S. Sugar transport by the bacterial phosphotransferase system. Characterization of the Escherichia coli enzyme I monomer/dimer transition kinetics by fluorescence anisotropy. J Biol Chem. 1994;269:20270–20274. [PubMed] [Google Scholar]
- Corrie JET. Synthesis, photochemistry and enzymology of 2-O-(2-nitrobenzyl)-d-glucose, a photolabile derivative of glucose. J Chem Soc Perkin Trans. 1993;1:2161–2166. [Google Scholar]
- Corrie JET, DeSantis A, Katayama Y, Khodakhah K, Messenger JB, Ogden DC, Trentham DR. Postsynaptic activation at the squid giant synapse by photolytic release of L-glutamate from a “caged” L-glutamate. J Physiol. 1993;465:1–8. doi: 10.1113/jphysiol.1993.sp019662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegner JA, Graham DR, Roth AF, Dahlquist FW. Assembly of an MCP receptor, CheW, and kinase CheA complex in the bacterial chemotaxis signal transduction pathway. Cell. 1992;70:975–982. doi: 10.1016/0092-8674(92)90247-a. [DOI] [PubMed] [Google Scholar]
- Grenier FC, Waygood EB, Saier MH., Jr The bacterial phosphotransferase system: kinetic characterization of the glucose, mannitol, glucitol, and N-acetyl glucosamine systems. J Cell Biochem. 1986;31:97–105. doi: 10.1002/jcb.240310203. [DOI] [PubMed] [Google Scholar]
- Grübl G, Vogler AP, Lengeler JW. Involvement of the histidine protein (HPr) of the phosphotransferase system in chemotactic signaling of Escherichia coli K12. J Bacteriol. 1990;172:5871–5876. doi: 10.1128/jb.172.10.5871-5876.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelbauer GL, Adler J. Role of the galactose binding protein in chemotaxis of Escherichia coli toward galactose. Nature. 1971;230:101–105. doi: 10.1038/newbio230101a0. [DOI] [PubMed] [Google Scholar]
- Hoving H, Lolkema JS, Robillard GT. Escherichia coli phosphoenolpyruvate-dependent phosphotransferase system: equilibrium kinetics and mechanism of Enzyme I phosphorylation. Biochemistry. 1981;20:87–93. doi: 10.1021/bi00504a015. [DOI] [PubMed] [Google Scholar]
- Jasuja R, Keyoung J, Reid GP, Trentham DR, Khan S. Chemotactic responses of Escherichia coli to small jumps of photoreleased L-aspartate. Biophys J. 1999;76:1706–1709. doi: 10.1016/S0006-3495(99)77329-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay WW. Two aspartate transport systems in Escherichia coli. J Biol Chem. 1971;216:7373–7382. [PubMed] [Google Scholar]
- Khan S, Castellano F, Spudich JL, McCray JA, Goody RS, Reid GP, Trentham DR. Excitatory signaling in bacteria probed by caged chemoeffectors. Biophys J. 1993;65:2368–2382. doi: 10.1016/S0006-3495(93)81317-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S, Spudich JL, McCray JA, Trentham DR. Chemotactic signal integration in bacteria. Proc Natl Acad Sci USA. 1995;92:9757–9761. doi: 10.1073/pnas.92.21.9757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen SH, Reader RW, Kort EN, Tso W-W, Adler J. Change in direction of flagellar rotation is the basis of chemotactic response in Escherichia coli. Nature. 1974;249:74–77. doi: 10.1038/249074a0. [DOI] [PubMed] [Google Scholar]
- Lengeler J. Mutations affecting transport of the hexitols d-mannitol, d-glucitol and d-galactitol in Escherichia coli K-12: isolation and mapping. J Bacteriol. 1975;124:26–38. doi: 10.1128/jb.124.1.26-38.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengeler J, Auburger AM, Mayer R, Pecher A. The phosphoenolpyruvate-dependent carbohydrate: phosphotransferase system enzymes II as chemoreceptors in chemotaxis of Escherichia coli K12. Mol Gen Genet. 1981;183:163–170. doi: 10.1007/BF00270156. [DOI] [PubMed] [Google Scholar]
- Lengeler JW, Jahreis K. Phosphotransferase systems or PTS as carbohydrate transport and as signal transduction systems. In: Konings WN, Kaback HR, Lolkema JS, editors. Handbook of Biological Physics. Vol. 2. Amsterdam: Elsevier Science; 1996. pp. 573–598. [Google Scholar]
- Lévy S, Zeng G-Q, Danchin A. Cyclic AMP synthesis in Escherichia coli strains bearing known deletions in the pts phosphotransferase operon. Gene. 1990;86:27–33. doi: 10.1016/0378-1119(90)90110-d. [DOI] [PubMed] [Google Scholar]
- Liu J, Parkinson JS. Role of CheW protein in coupling membrane receptors to the intracellular signaling system of bacterial chemotaxis. Proc Natl Acad Sci USA. 1989;86:8703–8707. doi: 10.1073/pnas.86.22.8703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Carter J, Ward JB, Glaser L. The effect of carbon and nitrogen sources on the level of metabolic intermediates in Escherichia coli. J Biol Chem. 1971;246:6511–6521. [PubMed] [Google Scholar]
- Lux R, Jahreis K, Bettenbrock K, Parkinson JS, Lengeler JW. Coupling the PTS and the MCP-dependent signaling pathways of Escherichia coli. Proc Natl Acad Sci USA. 1995;92:11583–11587. doi: 10.1073/pnas.92.25.11583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnab RM, Koshland DE., Jr The gradient-sensing mechanism in bacterial chemotaxis. Proc Natl Acad Sci USA. 1972;69:2509–2512. doi: 10.1073/pnas.69.9.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnab RM, Ornston MK. Normal-to-curly flagellar transitions and their role in bacterial tumbling. Stabilization of an alternative quartenary structure by mechanical force. J Mol Biol. 1977;112:1–30. doi: 10.1016/s0022-2836(77)80153-8. [DOI] [PubMed] [Google Scholar]
- Mattoo RL, Waygood EB. Determination of the levels of HPr and Enzyme I of the phosphoenolpyruvate-sugar phosphotransferase system in Escherichia coli and Salmonella typhimurium. Can J Biochem. 1983;61:29–37. doi: 10.1139/o83-005. [DOI] [PubMed] [Google Scholar]
- McEvoy MM, Dahlquist FW. Phosphohistidines in bacterial signaling. Curr Opin Struct Biol. 1997;7:793–797. doi: 10.1016/s0959-440x(97)80148-0. [DOI] [PubMed] [Google Scholar]
- Meadow ND, Roseman S. Rate and equilibrium constants for phosphoryltransfer between active site histidines of Escherichia coli HPr and the signal transducing protein IIIglc. J Biol Chem. 1996;271:33440–33445. doi: 10.1074/jbc.271.52.33440. [DOI] [PubMed] [Google Scholar]
- Misset O, Blaauw P, Postma PW, Robillard GT. Bacterial phosphoenolpyruvate-dependent phosphotransferase system. Mechanism of the transmembrane sugar translocation and phosphorylation. Biochemistry. 1983;22:6163–6170. doi: 10.1021/bi00295a019. [DOI] [PubMed] [Google Scholar]
- Montrone M, Oesterhelt D, Marwan W. Phosphorylation-independent chemoresponses correlate with changes in the cytoplasmic level of fumarate. J Bacteriol. 1996;178:6882–6887. doi: 10.1128/jb.178.23.6882-6887.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SO, Schuitema ARJ, Postma PW. The phosphoenolpyruvate: glucose phosphotransferase system of Salmonella typhimurium. The phosphorylated form of IIIglc. Eur J Biochem. 1986;154:337–341. doi: 10.1111/j.1432-1033.1986.tb09402.x. [DOI] [PubMed] [Google Scholar]
- Niwano M, Taylor BL. Novel sensory adaptation mechanism in bacterial chemotaxis to oxygen and phosphotransferase substrates. Proc Natl Acad Sci USA. 1982;79:11–15. doi: 10.1073/pnas.79.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden D, Capiod T. Regulation of Ca2+ release by InsP3 in single guinea pig hepatocytes and rat Purkinje neurones. J Gen Physiol. 1997;109:741–756. doi: 10.1085/jgp.109.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson JS, Houts SE. Isolation and behavior of Escherichia coli deletion mutants lacking chemotaxis functions. J Bacteriol. 1982;151:106–113. doi: 10.1128/jb.151.1.106-113.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecher A, Renner I, Lengeler J. The phosphoenolpyruvate-dependent carbohydrate: phosphotransferase system enzymes II, a new class of chemosensors in bacterial chemotaxis. In: Sund H, Veeger C, editors. Mobility and Recognition in Cell Biology. Berlin: Walter de Gruyter; 1983. pp. 517–531. [Google Scholar]
- Postma PW, Lengeler JW, Jacobson GR. Phosphoenolpyruvate: carbohydrate phosphotransferase systems. In: Neidhardt FC, Curtiss R III, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE, editors. Escherichia coli and Salmonella: Cellular and Molecular Biology. 2nd ed. Vol. 1. Washington, DC: American Society for Microbiology; 1996. pp. 1149–1174. [Google Scholar]
- Prasad K, Caplan SR, Eisenbach M. Fumarate modulates bacterial flagellar rotation by lowering the free energy difference between the clockwise and counterclockwise states of the motor. J Mol Biol. 1998;280:821–828. doi: 10.1006/jmbi.1998.1922. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan R, Schuster M, Bourret RB. Acetylation at Lys92 enhances signaling by the chemotaxis response regulator protein CheY. Proc Natl Acad Sci USA. 1998;95:4918–4923. doi: 10.1073/pnas.95.9.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowsell EH, Smith JM, Wolfe A, Taylor BL. CheA, CheW, and CheY are required for chemotaxis to oxygen and sugars of the phosphotransferase system in Escherichia coli. J Bacteriol. 1995;177:6011–6014. doi: 10.1128/jb.177.20.6011-6014.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders DA, Gillece-Castro BL, Stock AM, Burlingame AL, Koshland DE., Jr Identification of the site of phosphorylation of the chemotaxis response regulator protein, CheY. J Biol Chem. 1989;264:21770–21778. [PubMed] [Google Scholar]
- Scholte BJ, Schuitema ARJ, Postma PW. Characterization of factor IIIglc in catabolite repression-resistant (crr) mutants of Salmonella typhimurium. J Bacteriol. 1982;149:576–586. doi: 10.1128/jb.149.2.576-586.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster SC, Swanson RV, Alex LA, Bourret RB, Simon MI. Assembly and function of a quaternary signal transduction complex monitored by surface plasmon resonance. Nature. 1993;365:343–347. doi: 10.1038/365343a0. [DOI] [PubMed] [Google Scholar]
- Segall JE, Block SM, Berg HW. Temporal comparisons in bacterial chemotaxis. Proc Natl Acad Sci USA. 1986;83:8987–8991. doi: 10.1073/pnas.83.23.8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo AP, Walker JW, Goldman YE, Trentham DR, Kobayashi S, Kitazawa T, Somlyo AV. Inositol triphosphate, calcium and muscle contraction. Philos Trans R Soc Lond B Biol Sci. 1988;320:399–414. doi: 10.1098/rstb.1988.0084. [DOI] [PubMed] [Google Scholar]
- Springer MS, Goy MF, Adler J. Protein methylation in behavioral control mechanisms and in signal transduction. Nature. 1979;280:279–284. doi: 10.1038/280279a0. [DOI] [PubMed] [Google Scholar]
- Stock JB, Surette MG. Chemotaxis. In: Neidhardt FC, Curtiss R III, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE, editors. Escherichia coli and Salmonella: Cellular and Molecular Biology. 2nd ed. Vol. 1. Washington, DC: American Society for Microbiology; 1996. pp. 1103–1129. [Google Scholar]
- Stock JB, Waygood EB, Meadow ND, Postma PW, Roseman S. Sugar transport by the bacterial phosphotransferase system. The glucose receptors of the Salmonella typhimurium phosphotransferase system. J Biol Chem. 1982;257:14543–14552. [PubMed] [Google Scholar]
- Vogler AP, Brockhuizen CP, Schuitema A, Lengeler JW, Postma PW. Suppression of IIIGlc-defects by enzymes IINag and IIBgl of the PEP: carbohydrate phosphotransferase system. Mol Microbiol. 1988;2:719–726. doi: 10.1111/j.1365-2958.1988.tb00082.x. [DOI] [PubMed] [Google Scholar]
- Weigel N, Kukuruzinska MA, Nakazawa A, Waygood EB, Roseman S. Sugar transport by the bacterial phosphotransferase system. Phosphoryl transfer reactions catalyzed by enzyme I of Salmonella typhimurium. J Biol Chem. 1982;27:14477–14491. [PubMed] [Google Scholar]
- Welch M, Oosawa K, Aizawa S, Eisenbach M. Phosphorylation-dependent binding of a signal molecule to the flagellar switch of bacteria. Proc Natl Acad Sci USA. 1993;90:8787–8791. doi: 10.1073/pnas.90.19.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]