Abstract
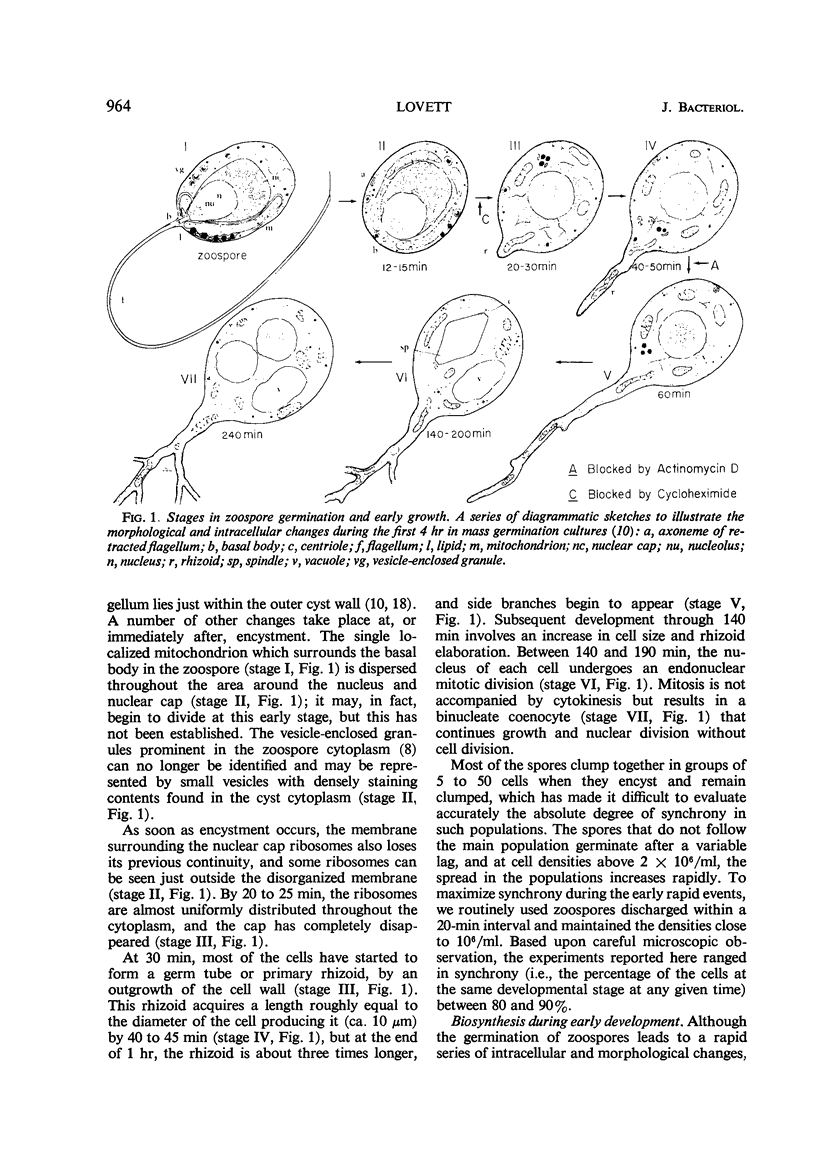
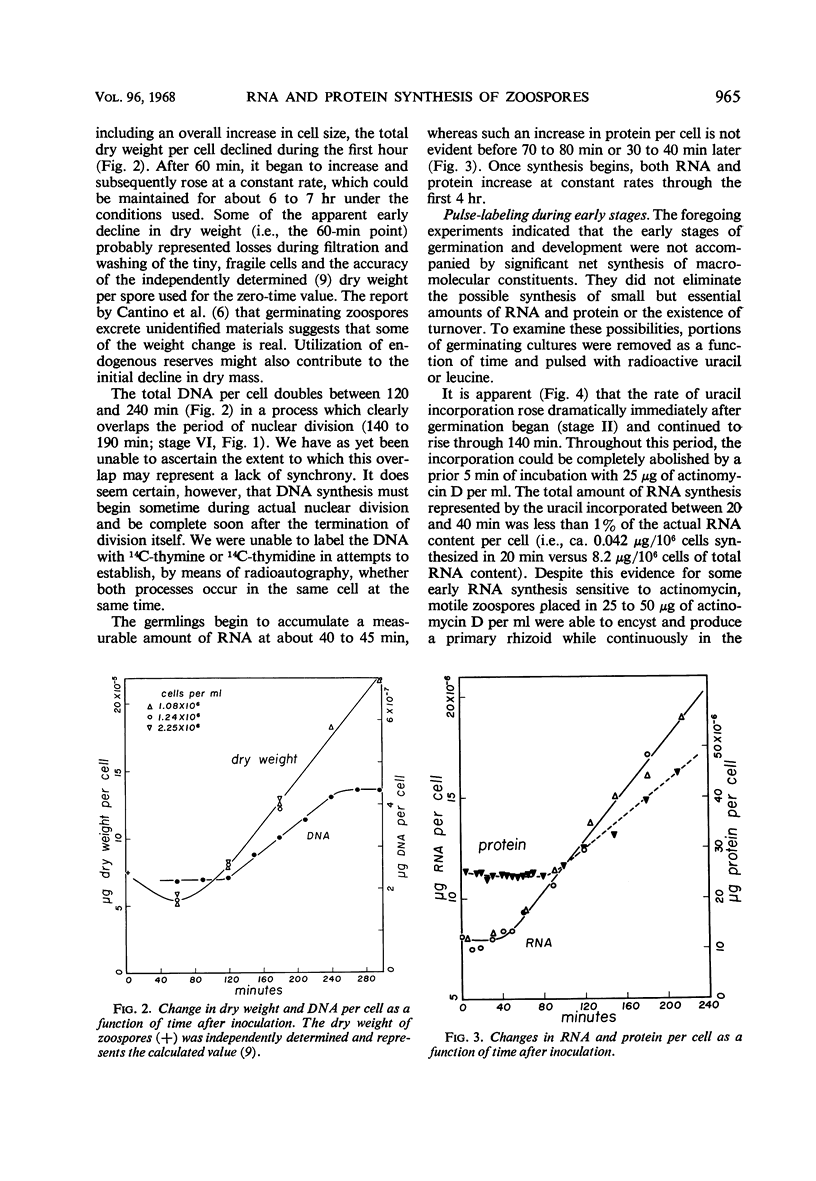
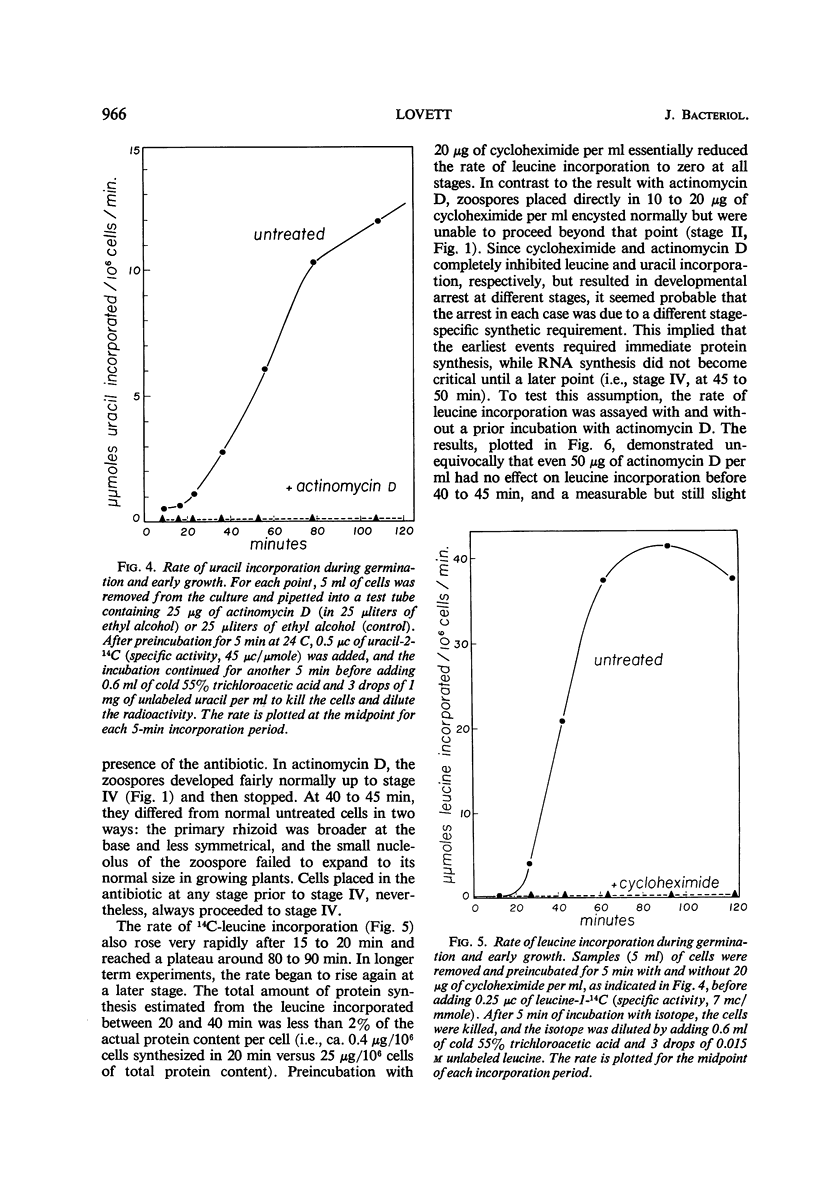
Freshly harvested zoospores of Blastocladiella emersonii begin to germinate about 15 min after inoculation into a defined growth medium at a density of 106 zoospores per ml. Flagellum retraction accompanies encystment, and dispersal of the ribosomal nuclear cap takes place shortly thereafter. The primary rhizoid begins to emerge at 25 to 30 min and starts to branch at ca. 60 min. The first nuclear division occurs between 120 and 190 min. The dry weight per cell increases linearly after 60 min, whereas the deoxyribonucleic acid per cell doubles between 120 and 240 min. A linear increase in total ribonucleic acid (RNA) is detectable beginning at 40 to 45 min, and in total protein beginning at 80 min; neither process is interrupted during nuclear division. Encystment and nuclear cap disorganization are associated with a sharp rise in the rates of precursor incorporation into RNA and protein. Cycloheximide at 20 μg/ml prevents leucine incorporation at all stages and inhibits development beyond the earliest encystment stage. Actinomycin D at 25 μg to 50 μg/ml prevents uracil incorporation, but it has no effect on leucine incorporation or development until 40 to 45 min. At the latter stage, actinomycin D causes a sharp developmental arrest and begins to inhibit leucine incorporation. It is concluded that early protein synthesis must occur on the ribosomes formed during the prior growth phase and conserved through the zoospore stage in the nuclear cap. The results further indicate that this synthesis is dependent upon messenger RNA already present in the zoospore before germination.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLONDEL B., TURIAN G. Relation between basophilia and fine structure of cytoplasm in the fungus Allomyces macrogynus Em. J Biophys Biochem Cytol. 1960 Feb;7:127–134. doi: 10.1083/jcb.7.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN D. D., LITTNA E. RNA SYNTHESIS DURING THE DEVELOPMENT OF XENOPUS LAEVIS, THE SOUTH AFRICAN CLAWED TOAD. J Mol Biol. 1964 May;8:669–687. doi: 10.1016/s0022-2836(64)80116-9. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CANTINO E. C., LOVETT J. S. NON-FILAMENTOUS AQUATIC FUNGI: MODEL SYSTEMS FOR BIOCHEMICAL STUDIES OF MORPHOLOGICAL DIFFERENTIATION. Adv Morphog. 1964;4:33–93. doi: 10.1016/b978-1-4831-9950-4.50005-9. [DOI] [PubMed] [Google Scholar]
- Gross P. R. The control of protein synthesis in embryonic development and differentiation. Curr Top Dev Biol. 1967;2:1–46. doi: 10.1016/s0070-2153(08)60282-3. [DOI] [PubMed] [Google Scholar]
- LOVETT J. S. CHEMICAL AND PHYSICAL CHARACTERIZATION OF "NUCLEAR CAPS" ISOLATED FROM BLASTOCLADIELLA ZOOSPORES. J Bacteriol. 1963 Jun;85:1235–1246. doi: 10.1128/jb.85.6.1235-1246.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lessie P. E., Lovett J. S. Ultrastructural changes during sporangium formation and zoospore differentiation in Blastocladiella Emersonii. Am J Bot. 1968 Feb;55(2):220–236. [PubMed] [Google Scholar]
- MALKIN L. I., GROSS P. R., ROMANOFF P. POLYRIBOSOMAL PROTEIN SYNTHESIS IN FERTILIZED SEA URCHIN EGGS: THE EFFECT OF ACTINOMYCIN TREATMENT. Dev Biol. 1964 Dec;10:378–394. doi: 10.1016/0012-1606(64)90051-x. [DOI] [PubMed] [Google Scholar]
- MONROY A., TYLER A. FORMATION OF ACTIVE RIBOSOMAL AGGREGATES (POLYSOMES) UPON FERTILIZATON AND DEVELOPMENT OF SEA URCHIN EGGS. Arch Biochem Biophys. 1963 Dec;103:431–435. doi: 10.1016/0003-9861(63)90433-8. [DOI] [PubMed] [Google Scholar]
- Murphy M. N., Lovett J. S. RNA and protein synthesis during zoospore differentiation in synchronized cultures of Blastocladiella. Dev Biol. 1966 Aug;14(1):68–95. doi: 10.1016/0012-1606(66)90006-6. [DOI] [PubMed] [Google Scholar]
- NEMER M. OLD AND NEW RNA IN THE EMBRYOGENESIS OF THE PURPLE SEA URCHIN. Proc Natl Acad Sci U S A. 1963 Aug;50:230–235. doi: 10.1073/pnas.50.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater D. W., Spiegelman S. An estimation of genetic messages in the unfertilized echinoid egg. Proc Natl Acad Sci U S A. 1966 Jul;56(1):164–170. doi: 10.1073/pnas.56.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spirin A. S., Nemer M. Messenger RNA in early sea-urchin embryos: cytoplasmic particles. Science. 1965 Oct 8;150(3693):214–217. doi: 10.1126/science.150.3693.214. [DOI] [PubMed] [Google Scholar]



