Abstract
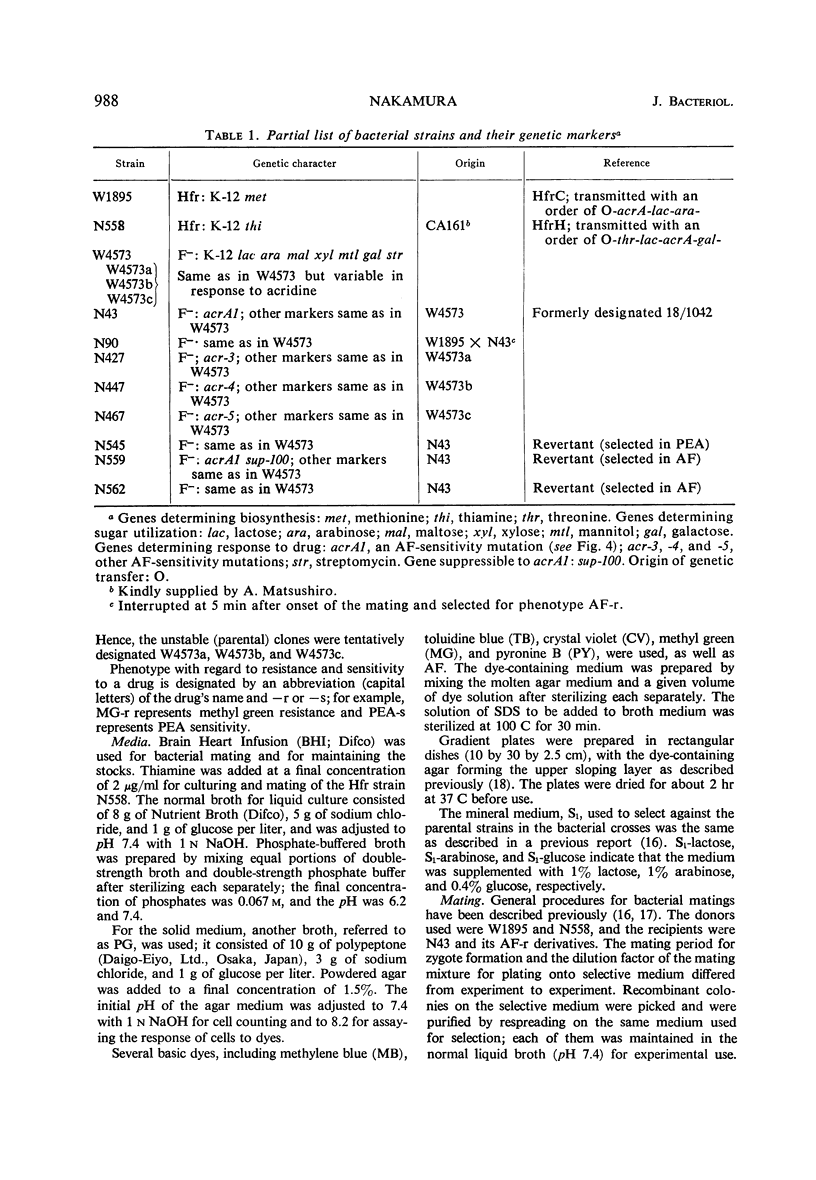
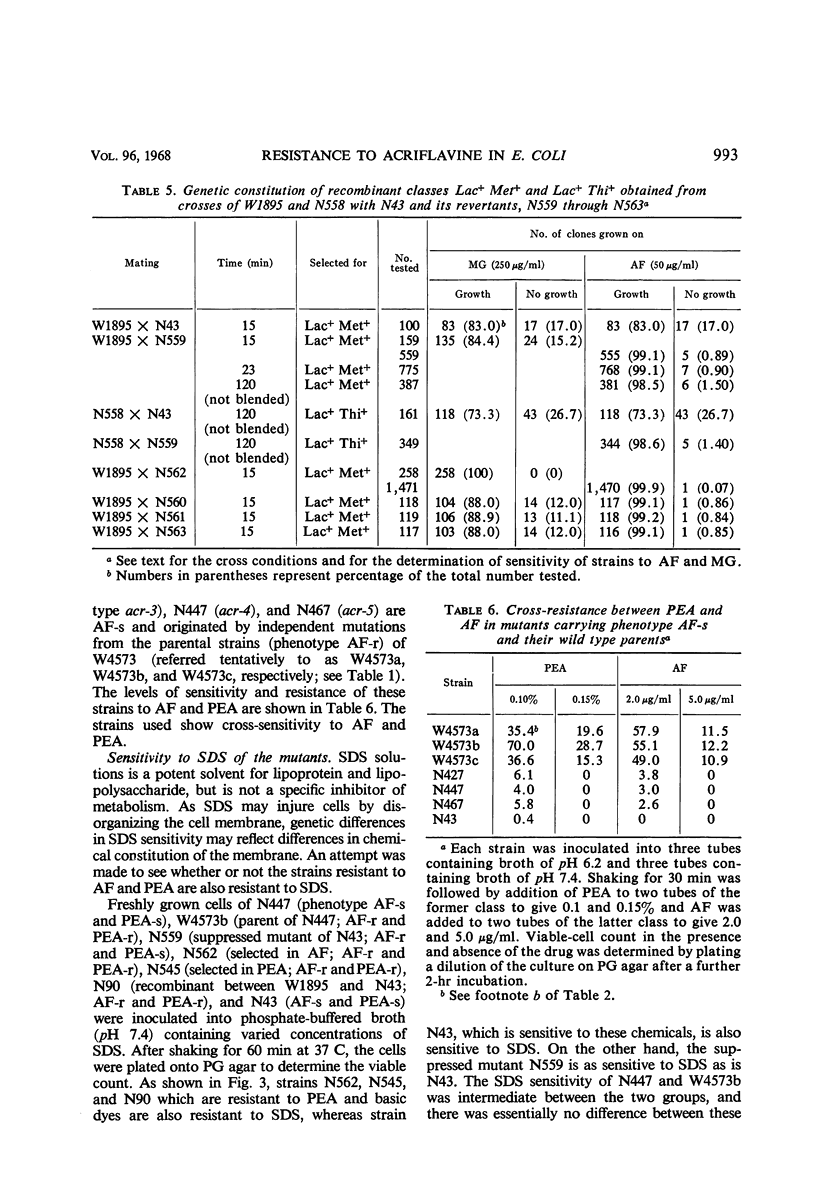
Wild-type strains of Escherichia coli K-12 are resistant to acriflavine. Gene acrA+ which determines resistance to acriflavine is located near the lac region of the chromosome. This gene determines not only resistance to basic dyes but also resistance to phenethyl alcohol. Acriflavine resistance was transmitted, together with phenethyl alcohol resistance, from a resistant Hfr strain to a sensitive recipient by mating. Reversion of the mutant gene acrA1 (phenotypically acriflavine-sensitive) to acriflavine resistance was accompanied by a change from phenethyl alcohol sensitivity to resistance, and conversely the revertants selected for phenethyl alcohol resistance were resistant to acriflavine. A suppressor mutation, sup-100, closely linked to the acr locus, suppresses the acrA1 gene (phenotypically acriflavine-resistant), but does not determine resistance to phenethyl alcohol and basic dyes other than acriflavine. The genetic change in the locus acrA1 to types resistant to basic dyes and phenethyl alcohol was accompanied by an increase in resistance to sodium dodecyl sulfate, a potent solvent of lipopolysaccharide and lipoprotein. It is suggested that gene acrA determines synthesis of a membrane substance. The system seemed to be affected strongly by the presence of inorganic phosphate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERRAH G., KONETZKA W. A. Selective and reversible inhibition of the synthesis of bacterial deoxyribonucleic acid by phenethyl alcohol. J Bacteriol. 1962 Apr;83:738–744. doi: 10.1128/jb.83.4.738-744.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E., Anderson T. F. The surface structure of Escherichia coli. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1592–1599. doi: 10.1073/pnas.54.6.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRICK F. H., BARNETT L., BRENNER S., WATTS-TOBIN R. J. General nature of the genetic code for proteins. Nature. 1961 Dec 30;192:1227–1232. doi: 10.1038/1921227a0. [DOI] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENBERG J., MANDELL J. D., WOODY P. L. A preliminary report--resistance and cross-resistance of Escherichia coli mutants to radiomimetic agents. Cancer Chemother Rep. 1961 Apr;11:51–56. [PubMed] [Google Scholar]
- HURWITZ J., FURTH J. J., MALAMY M., ALEXANDER M. The role of deoxyribonucleic acid in ribonucleic acid synthesis. III. The inhibition of the enzymatic synthesis of ribonucleic acid and deoxyribonucleic acid by actinomycin D and proflavin. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1222–1230. doi: 10.1073/pnas.48.7.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. W., DeMoss J. A. Effects of toluene on Escherichia coli. J Bacteriol. 1965 Nov;90(5):1420–1425. doi: 10.1128/jb.90.5.1420-1425.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILLEY B. D., BREWER J. H. The selective antibacterial action of phenylethyl alcohol. J Am Pharm Assoc Am Pharm Assoc. 1953 Jan;42(1):6–8. doi: 10.1002/jps.3030420103. [DOI] [PubMed] [Google Scholar]
- Lark K. G., Lark C. Regulation of chromosome replication in Escherichia coli: a comparison of the effects of phenethyl alcohol treatment with those of amino acid starvation. J Mol Biol. 1966 Sep;20(1):9–19. doi: 10.1016/0022-2836(66)90113-6. [DOI] [PubMed] [Google Scholar]
- Lester G. Inhibition of Growth, Synthesis, and Permeability in Neurospora crassa by Phenethyl Alcohol. J Bacteriol. 1965 Jul;90(1):29–37. doi: 10.1128/jb.90.1.29-37.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H. Acriflavine-binding capacity of Escherichia coli in relation to acriflavine sensitivity and metabolic activity. J Bacteriol. 1966 Nov;92(5):1447–1452. doi: 10.1128/jb.92.5.1447-1452.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H. Gene-Controlled Resistance to Acriflavine and Other Basic Dyes in Escherichia coli. J Bacteriol. 1965 Jul;90(1):8–14. doi: 10.1128/jb.90.1.8-14.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H. Phenethyl alcohol sensitivity in Escherichia coli. J Bacteriol. 1967 Mar;93(3):1183–1184. doi: 10.1128/jb.93.3.1183-1184.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonoyama M., Ikeda Y. Inhibition of RNA phage growth by phenetyl alcohol. Biochem Biophys Res Commun. 1964 Feb 18;15(1):87–91. doi: 10.1016/0006-291x(64)90108-1. [DOI] [PubMed] [Google Scholar]
- Prevost C., Moses V. Action of phyenethyl alcohol on the synthesis of macromolecules in Escherichia coli. J Bacteriol. 1966 Apr;91(4):1446–1452. doi: 10.1128/jb.91.4.1446-1452.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSENKRANZ H. S., CARR H. S., ROSE H. M. PHENETHYL ALCOHOL. I. EFFECT ON MACROMOLECULAR SYNTHESIS OF ESCHERICHIA COLI. J Bacteriol. 1965 May;89:1354–1369. doi: 10.1128/jb.89.5.1354-1369.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz H. S., Mednis A., Marks P. A., Rose H. M. Metabolic effects of phenethyl alcohol on mammalian cells. Biochim Biophys Acta. 1967 Dec 19;149(2):513–518. doi: 10.1016/0005-2787(67)90179-7. [DOI] [PubMed] [Google Scholar]
- Salton M. R. Structure and function of bacterial cell membranes. Annu Rev Microbiol. 1967;21:417–442. doi: 10.1146/annurev.mi.21.100167.002221. [DOI] [PubMed] [Google Scholar]
- Silver S., Wendt L. Mechanism of action of phenethyl alcohol: breakdown of the cellular permeability barrier. J Bacteriol. 1967 Feb;93(2):560–566. doi: 10.1128/jb.93.2.560-566.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino Y. Mutants of Escherichia coli sensitive to methylene blue and acridines. Genet Res. 1966 Feb;7(1):1–11. doi: 10.1017/s0016672300009423. [DOI] [PubMed] [Google Scholar]
- TREICK R. W., KONETZKA W. A. PHYSIOLOGICAL STATE OF ESCHERICHIA COLI AND THE INHIBITION OF DEOXYRIBONUCLEIC ACID SYNTHESIS BY PHENETHYL ALCOHOL. J Bacteriol. 1964 Dec;88:1580–1584. doi: 10.1128/jb.88.6.1580-1584.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDEL W. Bacterial viruses; with particular reference to adsorption/penetration. Annu Rev Microbiol. 1958;12:27–48. doi: 10.1146/annurev.mi.12.100158.000331. [DOI] [PubMed] [Google Scholar]
- WITKIN E. M. Modification of mutagenesis initiated by ultraviolet light through postteatment of bacteria with basic dyes. J Cell Comp Physiol. 1961 Dec;58(3):135–144. doi: 10.1002/jcp.1030580413. [DOI] [PubMed] [Google Scholar]
- WOODY-KARRER P., GREENBERG J. RESISTANCE AND CROSS-RESISTANCE OF ESCHERICHIA COLI S MUTANTS TO THE RADIOMIMETIC AGENT PROFLAVINE. J Bacteriol. 1964 Mar;87:536–542. doi: 10.1128/jb.87.3.536-542.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn R. K., Heicke B., Ochs H. G., Tiesler E., Forster W., Hanske W., Walter W., Hollstein H. In vitro inhibition of the synthesis of deoxyribonucleic acid by 2-phenyl-ethanol and some of its derivatives. Nature. 1966 Oct 15;212(5059):297–298. doi: 10.1038/212297a0. [DOI] [PubMed] [Google Scholar]
- Zampieri A., Greenberg J. Cross-resistance relationships in Escherichia coli between ultraviolet radiation and nitrous acid. J Bacteriol. 1964 May;87(5):1094–1099. doi: 10.1128/jb.87.5.1094-1099.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]



