Abstract
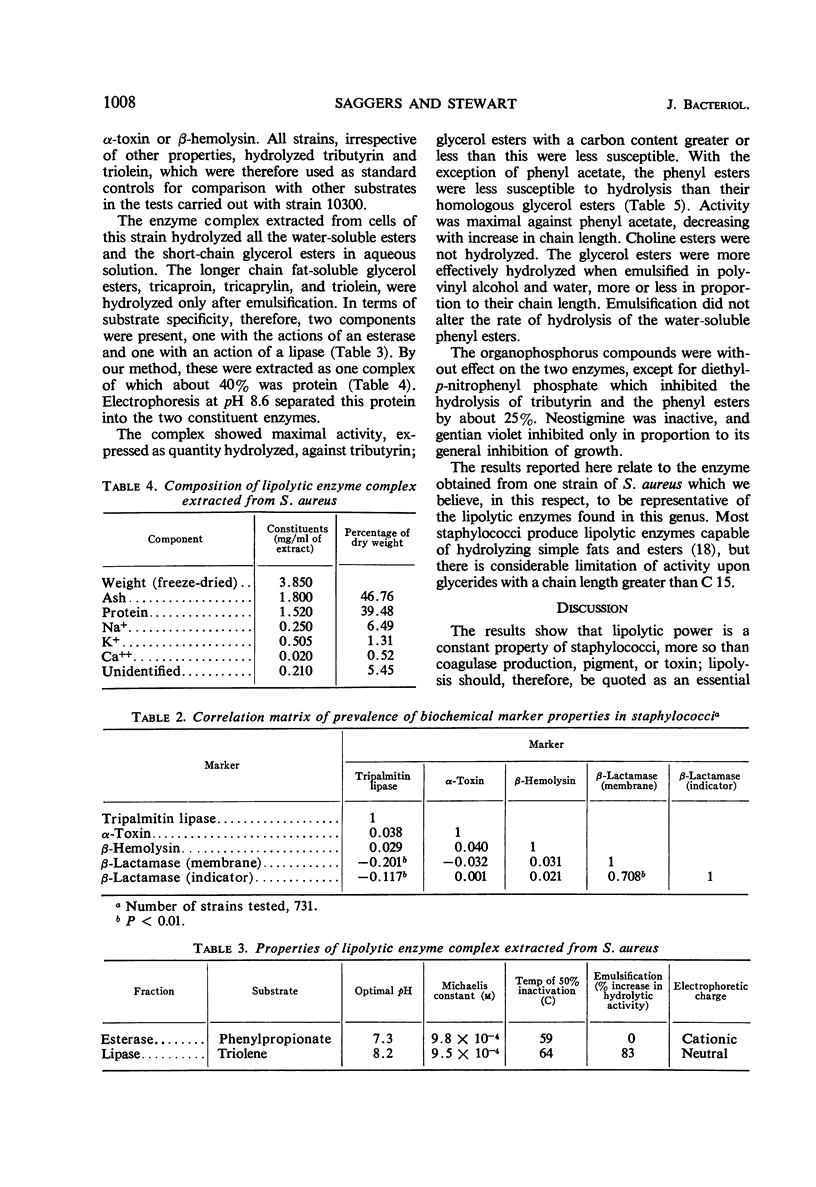
Staphylococci split a wide range of lipid substrates by production of an enzyme complex with two main components (i) a lipase acting optimally on fat-soluble glycerides, and (ii) an esterase acting optimally on water-soluble esters. The action is dependent upon carbon chain length, interfacial dispersion, solubility, and pH of substrate and end products. The esterase is less susceptible to organophosphorus inhibitors than mammalian esterases. There is no apparent correlation between lipolysis and markers of pathogenicity such as production of coagulase and toxin, but the possession of a flexible lipolytic mechanism might account for the persistence of staphylococci in the fatty secretions of mammalian skin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALDRIDGE W. N. Some esterases of the rat. Biochem J. 1954 Aug;57(4):692–702. doi: 10.1042/bj0570692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry J. A. Detection of Microbial Lipase by Copper Soap Formation. J Bacteriol. 1933 Apr;25(4):433–434. doi: 10.1128/jb.25.4.433-434.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIES M. E. A study of diffusible lipase produced by staphylococci and of its immunological activity. J Gen Microbiol. 1954 Aug;11(1):37–44. doi: 10.1099/00221287-11-1-37. [DOI] [PubMed] [Google Scholar]
- GILLESPIE W. A., ALDER V. G. Production of opacity in egg-yolk media by coagulase-positive staphylococci. J Pathol Bacteriol. 1952 Jan;64(1):187–200. doi: 10.1002/path.1700640119. [DOI] [PubMed] [Google Scholar]
- HOLT R. J., STEWART G. T. Techniques for the rapid and sensitive detection of penicillinase. J Clin Pathol. 1963 May;16:263–267. doi: 10.1136/jcp.16.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOHN J., REIS J. L. BACTERIAL NUCLEOTIDASES. J Bacteriol. 1963 Oct;86:713–716. doi: 10.1128/jb.86.4.713-716.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- NOVICK R. P., RICHMOND M. H. NATURE AND INTERACTIONS OF THE GENETIC ELEMENTS GOVERNING PENICILLINASE SYNTHESIS IN STAPHYLOCOCCUS AUREUS. J Bacteriol. 1965 Aug;90:467–480. doi: 10.1128/jb.90.2.467-480.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAGGERS B. A., STEWART G. T. POLYVINYL-PYRROLIDONE-IODINE: AN ASSESSMENT OF ANTIBACTERIAL ACTIVITY. J Hyg (Lond) 1964 Dec;62:509–518. doi: 10.1017/s0022172400040225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAH D. B., WILSON J. B. EGG YOLK FACTOR OF STAPHYLOCOCCUS AUREUS. II. CHARACTERIZATION OF THE LIPASE ACTIVITY. J Bacteriol. 1965 Apr;89:949–953. doi: 10.1128/jb.89.4.949-953.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart G. T. The lipases and pigments of staphylococci. Ann N Y Acad Sci. 1965 Jul 23;128(1):132–151. doi: 10.1111/j.1749-6632.1965.tb11635.x. [DOI] [PubMed] [Google Scholar]
- Trussell R. E., Weed L. A. The Lipolytic Action of Staphylococci on Some Pure Triglycerides. J Bacteriol. 1937 Apr;33(4):381–388. doi: 10.1128/jb.33.4.381-388.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]


