Abstract
Budding yeast adjusts to increases in external osmolarity via a specific mitogen-activated protein kinase signal pathway, the high-osmolarity glycerol response (HOG) pathway. Studies with a functional Hog1–green fluorescent protein (GFP) fusion reveal that even under nonstress conditions the mitogen-activated protein kinase Hog1 cycles between cytoplasmic and nuclear compartments. The basal distribution of the protein seems independent of its activator, Pbs2, and independent of its phosphorylation status. Upon osmotic challenge, the Hog1–GFP fusion becomes rapidly concentrated in the nucleus from which it is reexported after return to an iso-osmotic environment or after adaptation to high osmolarity. The preconditions and kinetics of increased nuclear localization correlate with those found for the dual phosphorylation of Hog1–GFP. The duration of Hog1 nuclear residence is modulated by the presence of the general stress activators Msn2 and Msn4. Reexport of Hog1 to the cytoplasm does not require de novo protein synthesis but depends on Hog1 kinase activity. Thus, at least three different mechanisms contribute to the intracellular distribution pattern of Hog1: phosphorylation-dependent nuclear accumulation, retention by nuclear targets, and a kinase-induced export.
INTRODUCTION
One of the key questions in signal transduction processes is how signals are distributed to localized physiological targets. For example, in the control of transcription by extracellular signals, it is evident that one of the activated signal components or one of its substrates has to travel from the cytoplasmic to the nuclear compartment. This question has become a focus in studies on mitogen-activated protein kinase (MAPK)1-dependent signal pathways. These, among eukaryotes, highly conserved signaling systems are organized in a core module composed of three sequentially acting kinases, a MAPK kinase kinase, MAPK kinase (MAPKK or MEK), and MAPK (Waskiewicz and Cooper, 1995). The MAPK is activated by phosphorylation of strictly conserved T and Y residues in the catalytic domain of the kinase (T-E/G/P-Y motif), which enables the kinase to phosphorylate a set of response-specific substrates (for review, see Su and Karin, 1996; Treisman, 1996). Because some of the MAPK substrates can be considered resident nuclear proteins, and because the MAPK cascade is activated in the cytoplasm or at the plasma membrane, it has been clear that at least one component of the signal pathway has to move to the nucleus. Indeed, analysis of cellular localization of higher eukaryote p42 and p44 MAPKs revealed that mitogenic stimulation by serum or α-thrombin seemed to considerably enhance nuclear transfer of both classical MAPK isoforms (Gonzalez et al., 1993; Lenormand et al., 1993). Because this event could be correlated with dual tyrosine and threonine phosphorylation (Chen et al., 1992), the existence of an import mechanism has been proposed that depends on the activation of the pathway.
MAPKs do not contain classical import signals, so other nonconventional and perhaps indirect mechanisms would have to be invoked to explain the movements of the protein. Attempts to find out whether the dual phosphorylation of the kinase was part of such an import signal yielded partly conflicting results. For example, the behavior of phosphorylation site mutants did not always support the notion that it was an essential feature for the cytoplasmic–nuclear transfer (Lenormand et al., 1993). More recent evidence, however, seemed to resolve this question, suggesting that the modification of the MAPK is indeed an important feature for determining the localization of the kinase (Khokhlatchev et al., 1998). These studies also opened the possibility that phosphorylation-induced dimerization constituted part of the nuclear import signal. Another line of investigations found that the activator of the MAPK, MEK, is actively excluded from the nucleus by an inherent nuclear export signal (NES) (Fukuda et al., 1996). This led to the proposal that cytoplasmic anchorage of the uninduced MAPK by MEK could contribute to the basal pattern of MAPK localization (Fukuda et al., 1997). Analogous to this situation, it has subsequently been proposed that nuclear accumulation might be based on a retention mechanism by nuclear factors (Gaits et al., 1998; Wilkinson and Millar, 1998). This concept originated from studies with the stress-induced Schizosaccharomyces pombe MAPK StyI/Spc1, a homologue of the mammalian stress kinase p38, but it has also gained some recent support for the classical MAPKs (Lenormand et al., 1998). The work on the S. pombe kinase showed that nuclear localization of the kinase can be observed after exposure to generic stress conditions. However, the nuclear localization of StyI/Spc1 depends not only on the phosphorylation status of the kinase but also on the presence of its target transcription factor, Atf1 (Gaits et al., 1998). These data gave the first hint that it may not be just nuclear import that is regulated but the ability to find and interact with appropriate substrates that serve as nuclear anchors.
Although budding yeast has been one of the classical model systems for studying MAPK signaling (Herskowitz, 1995), no major insights have yet been gained with respect to MAPK localization. Although one study has dealt with the localization of the filamentous growth and mating-specific MAPK Kss1 (Ma et al., 1995), no important regulatory features with regard to nuclear import were deduced at this time. Another environmentally controlled MAPK pathway is the so-called high-osmolarity glycerol response (HOG) system that allows yeast to maintain osmotic homeostasis (Boguslawski, 1992; Brewster et al., 1993). The MAPK Hog1 has been the prototype for the class of kinases related to p38. In contrast to its fission yeast (Degols et al., 1996) or mammalian counterparts (Kyriakis and Avruch, 1996), the HOG pathway in budding yeast appears to be activated exclusively by hyperosmotic stress (Schüller et al., 1994). Changes in external osmolarity are sensed by two transmembrane proteins that act independently but converge at the level of the MAPKK, Pbs2 (Maeda et al., 1994, 1995). One of the branches uses a phospho-relay mechanism to activate a redundant pair of MAPKKs, Ssk2 and Ssk22, that control dual phosphorylation of Hog1 via the Pbs2 kinase (Posas et al., 1996; Posas and Saito 1998). On the other hand, a low basal activity level of Hog1 and the transience of Hog1 activation seems to be assured by a set of MAPK-specific protein phosphatases (Maeda et al., 1993; Wurgler-Murphy et al., 1997).
Hog1 appears to coordinate the induction of a major part of the Saccharomyces cerevisiae osmostress protection mechanisms, but its immediate substrates are still unknown. Cells that are suddenly challenged by a hyperosmotic environment respond by the transient induction of several genes, such as CTT1 or GPD1, emphasizing that transcriptional control should play an important role in a cell’s short-term adjustment to osmostress (Albertyn et al., 1994; Schüller et al., 1994). Two redundantly acting transcription factors, Msn2 and Msn4, have been identified that play a crucial role in this response (Martinez-Pastor et al., 1996). Although these factors are activated by diverse stress situations, they also respond to osmotic instabilities by being concentrated in nucleus (Görner et al., 1998). Hog1 has no control over the nuclear accumulation of these factors in response to hyperosmotic conditions but appears to modulate their efficiency as activators. In addition, factors other than Msn2 are able to mediate transcriptional responses to osmostress. Although their nature still needs to be defined, it seems that they are not members of the ATF family (Hohmann, 1997).
In the present investigation we have concentrated on the question of whether Hog1 might be the factor that establishes the connection between the cyto-plasmic part of the signal pathway and its nuclear response system. To this effect we followed the intracellular localization of a functional Hog1–green fluorescent protein (GFP) fusion from nonstressed conditions through the acute stress phase until cells have become adapted. Our data emphasize that several transport and retention phenomena can be dissociated that depend on different cellular mechanisms: 1) continuous cycling between the cytoplasm and the nucleus, 2) phosphorylation-dependent nuclear accumulation, 3) nuclear retention, and 4) MAPK activity-dependent export during adaptation. Importantly, we show that all the associated changes in the nuclear–cytoplasmic equilibrium occur independently of de novo protein synthesis.
MATERIALS AND METHODS
Yeast Strains and General Methods
The yeast strains used in this study are summarized in Table 1. They all have W303-1A genetic background. The complete coding region of the PBS2 gene was deleted with HIS3 by the microhomology PCR method (Manivasakam et al., 1995). Deletion was verified by PCR analysis of chromosomal DNA using specific primers. General cloning methods are described by Sambrook et al. (1989). Yeast media, growth conditions, and procedures were used as presented by Rose et al. (1990).
Table 1.
Yeast strains and plasmids used
| Strain, plasmid | Genotype | Source |
|---|---|---|
| Strains | ||
| W303-1A | MATa ade2-1 can1-100 his3-11 leu2-3 trp1-1 ura3-1 | R. Rothstein |
| K4327 | MATa ade2-1 can1-100 his3-11 leu2-3 trp1-1 ura3-1 hog1∷TRP1 | Cvrckova et al., 1995 |
| VRY10 | MATa ade2-1 can1-100 his3-11 leu2-3 trp1-1 ura3-1 pbs2∷HIS3 | This study |
| YM24 | MATa ade2-1 can1-100 his3-11 leu2-3 trp1-1 ura3-1 msn2∷HIS3 msn4∷TRP1 | Estruch and Carlson, 1993 |
| Plasmids | ||
| YCp111 | LEU2 CEN4 ARS1 | Gietz and Sugino, 1988 |
| pVR65-WT | LEU2 CEN4 ARS1 HOG1-GFP | This study |
| pVR65-2x | LEU2 CEN4 ARS1 HOG1-2xGFP | This study |
| pVR65-3x | LEU2 CEN4 ARS1 HOG1-3xGFP | This study |
| pVR65-T/A | LEU2 CEN4 ARS1 HOG1T/A-GFP (HOG1 with T174A mutation) | This study |
| pVR65-Y/F | LEU2 CEN4 ARS1 HOG1Y/F-GFP (HOG1 with T174F mutation) | This study |
| pVR65-K/R | LEU2 CEN4 ARS1 HOG1K/R-GFP (HOG1 with K52R mutation) | This study |
| pVR65-NES | LEU2 CEN4 ARS1 HOG1-NES-GFP (HOG1-GFP fused to NES) | This study |
| YCp22 | TRP1 CEN4 ARS1 | Gietz and Sugino, 1988 |
| YCp33 | URA3 CEN4 ARS1 | Gietz and Sugino, 1988 |
| pVR15 | TRP1 CEN4 ARS1 PBS2 | This study |
| pVR15-GFP | TRP1 CEN4 ARS1 PBS2-GFP | This study |
| pVR20 | TRP1 CEN4 ARS1 PBS2S/A,T/A (PBS2 with S174A and T178A mutations) | This study |
| pVR15K/M | TRP1 CEN4 ARS1 PBS2K/M (PBS2 with K389M mutation) | This study |
| YEp112 | TRP1 2μm | Gietz and Sugino, 1988 |
| pVR28 | TRP1 2μm PBS2-GFP | This study |
| pGSS21 | URA3 2μm PGAL1-SSK2ΔN (SSK2 from M1173 to D1579) | Wurgler-Murphy et al., 1997 |
Plasmids
Plasmids used in this study are summarized in Table 1. The HOG1 coding region including the endogenous promoter was PCR amplified from plasmid pVR50 (wild-type [WT] HOG1 gene in vector YCp111) to introduce a NotI site at the very C-terminus of the HOG1 ORF using universal primer and oligonucleotide VR25(WH) (5′AAATGGATCCATTAGCGGCCGCTCTGTTGGAACTCAT TAGC) (BamHI and NotI sites underlined) and cloned as a 1.8-kb HindIII–BamHI fragment into YCp111 to produce plasmid pVR50–NotI. Hog1–GFP (pVR65–WT) was generated by ligating an enhanced GFP NotI cassette (Görner et al., 1998) into the NotI site of plasmid pVR50–NotI. Hog1T174A–GFP (pVR65–T/A), Hog1Y176F–GFP (pVR65–Y/F), and Hog1K52R–GFP (pVR65–K/R) were constructed by replacing a 1.1-kb SalI–SalI fragment of pVR65–WT with the corresponding fragments of the mutant genes described by Schüller et al. (1994). Protein kinase A inhibitor NES (Wen et al., 1995) was inserted as an oligonucleotide adaptor (CATGAATGAATTAGCCTTGAAATTAGCAGGTCTTGATATCAACAAGATGCATGC) into the NcoI site within the N-terminal sequence of GFP of pVR65 generating Hog1–NES–GFP (pVR65–NES). The same NcoI site was used to construct Hog1 fused to more than one GFP. An NcoI fragment derived from the plasmid containing in-frame fusion of two GFPs was introduced into the NcoI site of Hog1–GFP resulting in plasmids containing Hog1 fusions with two or three tandem repeats of GFP. Pbs2p–GFP was generated by cloning a 1.3-kb NheI–SphI fragment of pVR15 (a 2.9-kb SpeI–SacI fragment containing the complete PBS2 coding region with endogenous promoter and terminator sequence cloned into an XbaI–SacI digest of YCp22) into phage vector M13mp18, isolation of single-stranded DNA, and introduction of an XbaI site immediately in front of the stop codon by in vitro mutagenesis using oligonucleotide 5′-TGGATATTAACGCTATCTAGATAAACCACCCATATG (XbaI site underlined). The 0.9-kb Eco47III–SphI fragment was cloned back into pVR15 to produce pVR15–XbaI. The GFP coding region was amplified by PCR to introduce XbaI sites at both ends and was cloned into the XbaI site of pVR15–XbaI, generating a Pbs2p–GFP fusion (pVR15–GFP). A multicopy version of this construct was produced by cloning of a 2.9-kb SalI–SacI fragment of pVR15–GFP into corresponding sites of plasmid YEp112 (pVR28). Pbs2pS514A and Pbs2pT518A were constructed by overlapping PCR technique (Higuchi, 1990) using oligonucleotide VR12 (5′-GGTGTTTCTGGTAATTTGGTGGCAGCACTGGCCAAGGCAAATATTGGTTGTCG) and its complement VR13. Pbs2pK389 M (pVR15K/M) was prepared by replacing a 2.2-kb ClaI–SacI fragment of pVR15 with the corresponding fragment of pPBM23 (a kind gift from H. Saito, Harvard Medical School, Boston, MA).
Fluorescence Microscopy
Cells were grown to logarithmic phase (OD600 ∼ 0.8–1.0) in synthetic complete medium omitting components used as selective markers. DAPI (final concentration, 2 μg/ml) was added as DNA dye 15 min before microscopy. The proportion of cells with nuclear signal accumulation was determined by counting cells with distinctly enhanced nuclear fluorescence. The number of cells with accumulated nuclear signal was expressed relative to the sum of cells counted showing fluorescence (total number of cells examined, >300) after growth under iso-osmotic conditions or after exposure to 0.4 M NaCl (if not indicated otherwise) for 5 min. For time course experiments cells were scored in a similar manner using the camera pictures of samples taken at the time points indicated. To examine the effects of inhibitors of protein synthesis on Hog1–GFP localization, cycloheximide (final concentration, 0.1 mg/ml) was added to the cultures 2 h before exposure to hyperosmotic stress. Living cells were viewed with a Zeiss (Thornwood, NY) Axioplan 2 fluorescence microscope, and images were scanned with a Quantix charge-coupled device camera (Photometrics, Tucson, AZ) using IPLab software (Signal Analytics, Vienna, VA). Pictures were assembled with Adobe (Mountain View, CA) Photoshop 4.0 software.
Protein Extract Preparation and Western Blot Analysis
Cells were grown in appropriate synthetic medium to OD600 ∼ 0.8–1.0. When they were hyperosmotically stressed, NaCl (final concentration, 0.4 M) was added at room temperature for the period indicated. To prepare protein extracts, cultures were rapidly cooled down in ice water and harvested by centrifugation. Cells were suspended in ice-cold lysis buffer (0.1 M 2-[N-morpholino]ethanesulfonic acid, 10 mM EGTA, 1% DMSO, 1 mM DTT, pH 6.5) containing Complete protease inhibitor mix (Boehringer Mannheim, Mannheim, Germany) and phosphatase inhibitors (0.1 mM Na vanadate, 10 mM NaF). The cell pellet was then resuspended in ice-cold lysis buffer and vortexed with glass beads three times for 5 min each. Samples were centrifuged at 14,000 rpm two times for 10 min each. All operations were performed at 4°C. Protein concentration was determined in the supernatants by using a Bio-Rad (Hercules, CA) protein assay kit before they were frozen in liquid nitrogen. Extracts (100 μl) were boiled with 50 μl of 4× sample buffer (0.12 M Tris, 20% [vol/vol] glycerol, 0.286 M 2-mercaptoethanol, 0.086 mM bromphenol blue, 5% SDS, pH 6.8) for 3 min. Samples were resolved by 10% SDS-PAGE (Bio-Rad Mini Protean) and transferred onto nitrocellulose membranes (Schleicher & Schüll, Keene, NH) in a SemiDry blotting apparatus (Bio-Rad). Phosphorylated Hog1–GFP was immunodetected by phospho-specific p38 MAPK (T180/Y182) antibody (New England Biolabs, Beverly, MA), and GFP protein was immunodetected with GFP polyclonal antibody (Clontech, Palo Alto, CA). Immunoblots were developed by using an HRP-conjugated protein ECL detection kit (Amersham, Arlington Heights, IL). Probes for immunodetection were removed by incubation in stripping buffer according to recommendations of the suppliers before applying another primary antibody to the same membrane.
RESULTS
Hog1–GFP and Pbs2–GFP Fusions Are Functional
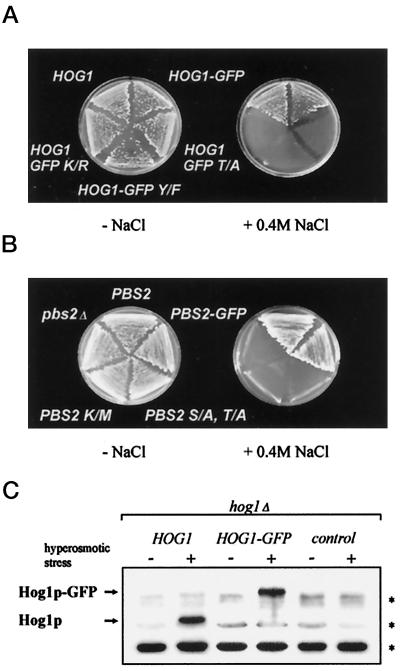
In an attempt to investigate the intracellular location of the Hog1 MAPK during different environmental conditions, we fused its gene to sequences encoding a version of GFP. To verify that the fusion protein has preserved the biological function of Hog1, we transformed a hog1Δ strain with the gene and checked for viability under hyperosmotic stress conditions (Figure 1A). Our results show that the HOG1–GFP fusion gene just like a WT HOG1 gene can complement the lethality of a hog1 null mutation under persistant high-osmolarity conditions. We found that an antibody specifically recognizing the phosphorylated form of mammalian p38/RK MAPK also detects the activated form of Hog1 and Hog1–GFP (Figure 1C) but not products containing a single amino acid substitution at the modification sites. Hog1–GFP is noticably modified only during the appropriate hyperosmotic stimulus when it is also mainly located in the nucleus (see below). Thus, the GFP part of the fusion protein does not seem to interfere with the activation and function of the kinase. In fact, we were able to obtain Hog1 fusions with two or more tandem repeats of GFP that on a physiological level behaved exactly like the single GFP fusion (our unpublished results; also see Figure 5). In a similar experiment we attached the GFP sequence to the C-terminal coding region of PBS2, the gene encoding the direct activator of Hog1. The PBS2–GFP fusion gene complemented as well as the WT gene a pbs2 defect for growth on high salt (Figure 1B). We conclude that in both cases GFP fluorescence provides a valid marker for the intracellular localization of the kinases.
Figure 1.
Expression of Hog1–GFP and Pbs2–GFP complement hyperosmotic sensitivity of corresponding genomic mutants. (A) Strain K4327 (hog1Δ) was transformed with centromer plasmid pVR50 (HOG1), pVR65–WT (HOG1–GFP), pVR65–T/A (HOG1–GFP T/A), pVR65–Y/F (HOG1–GFP Y/F), or pVR65–K/R (HOG1–GFP K/R). The expression of HOG1 alleles was driven by their endogenous promoter. Transformants were grown on selective medium at 30°C without or with 0.4 M NaCl. (B) Strain VRY 10 (pbs2Δ) was transformed with pVR15 (PBS2), pVR15–GFP (PBS2–GFP), pVR20 (PBS2 S/A, T/A), or pVR15K/M (PBS2 K/M) and YCp22 (control plasmid, pbs2Δ). Expression of PBS2 alleles was driven by their endogenous promoter. Transformants were grown on selective medium at 30°C in the absence or presence of 0.4 M NaCl. (C) Antibody against an active human p38 MAPK specifically recognizes an activated form of yeast Hog1. The protein extracts from strain K4327 (hog1Δ) transformed with a centromer plasmid bearing a WT HOG1 (pVR50), HOG1–GFP fusion gene (pVR65–WT), or control plasmid (YCp111) were tested. Cells were grown in selective medium. For hyperosmotic stress, NaCl was added to a final concentration of 0.4 M for 5 min before preparation of protein extracts, which were analyzed by Western blotting using antibody against active p38 MAPK. Asterisks indicate the positions of a set of proteins from yeast crude extract (e.g., those with slightly higher mobility than Hog1) that are nonspecifically recognized by anti-active human p38 antibody.
Figure 5.
Intracellular distribution and phosphorylation of enlarged Hog1 kinases. (A) Fluorescence images of hog1 cells transformed with a plasmid expressing Hog1 fused to three tandemly repeated GFP polypeptides. Cells were treated and processed as in Figure 8. GFP images were taken under normal growth conditions 5 min after osmotic stress and 5 min after return to iso-osmotic medium. (B) Western analysis from yeast cells containing a HOG1 fusion with one, two, and three copies of GFP. The top panel was been probed with anti-active p38 antibody; the bottom panel was probed with antibodies against GFP. The apparent sizes for marker proteins are indicated.
The Nuclear Concentration of a Hog1–GFP Fusion Increases Specifically upon Hyperosmotic Stress
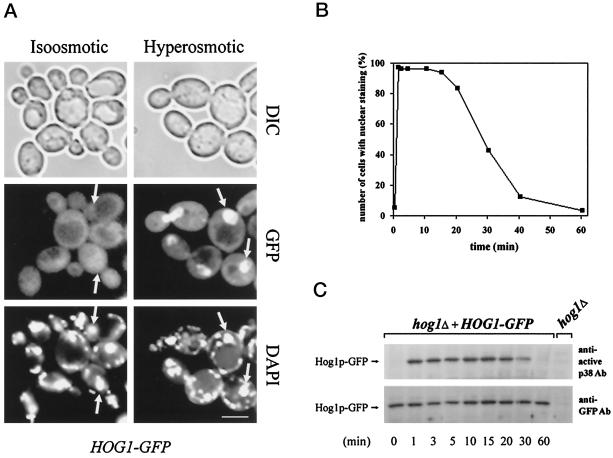
The subcellular distribution of Hog1–GFP was first analyzed in a hog1Δ strain (Figure 2). Under standard growth conditions, Hog1–GFP appears uniformly distributed throughout the cell (with the exception of the vacuole). When stressed with hyperosmotic agents (0.4 M NaCl or 1 M sorbitol), the signal rapidly changed, with GFP fluorescence now being concentrated in nuclei (Figure 2A; our unpublished results). This change in the localization pattern was observed in the vast majority (>90%) of cells (Figure 2B). Unlike StyI/Spc1 in fission yeast and p38/RK in higher eukaryotes, in the budding yeast the HOG pathway appears to become activated exclusively by hyperosmotic stress; it is not directly involved in transmission of any other stress signals (Schüller et al., 1994). To examine whether the nuclear translocation of Hog1–GFP is controlled with the same specificity, we examined its cellular distribution under heat stress (37°C) after exposure to a weak organic acid (10 mM sorbic acid), oxidative stress (0.4 mM H202), and adjustment to 7% ethanol. None of these stress conditions induces the nuclear accumulation of Hog1–GFP, even though the same conditions cause nuclear translocation of the general stress activator Msn2 (Görner et al., 1998). This observation is consistent with the interpretation that rapid nuclear import of Hog1–GFP is limited to those occasions that normally activate the kinase, suggesting that Hog1–GFP nuclear accumulation is a HOG pathway-mediated event.
Figure 2.
Hog1–GFP nuclear accumulation in response to hy-perosmotic stress correlates with its dual phosphorylation status. (A) Hyperosmotic stress induces nuclear accumulation of Hog1. Logarithmically growing strain K4327 (hog1Δ) transformed with pVR65–WT (HOG1–GFP) was examined by fluorescence or light microscopy under iso-osmotic (selective medium) or hyperosmotic growth conditions (5 min after addition of NaCl into selective medium to a final concentration of 0.4 M). In agreement with the observations of others (Stade et al., 1997), DAPI used for staining of nuclei preferentially stains mitochondrial DNA in living cells. Positions of nuclei are indicated by arrows. DIC, differential interference contrast. Bar, 5 μm. (B) The kinetics of Hog1 nuclear accumulation and dual phosphorylation are similar. Strain K4327 (hog1Δ) was transformed with centromer plasmid pVR65–WT (HOG1–GFP). Cells were grown in selective medium to logarithmic phase (iso-osmotic conditions). A control sample of unstressed cells was taken (time 0 min) followed by addition of NaCl to a final concentration of 0.4 M. Then cells were incubated for various times and examined by fluorescence microscopy. To determine the activation profile of Hog1–GFP, samples were taken at the time points indicated, and protein extracts were prepared. Western blots were analyzed with anti-active p38 MAPK antibody and with anti-GFP antibody (C). In a control experiment, no band corresponding to the position of the Hog1–GFP-specific band was detected with anti-GFP antibody when protein extract from hog1Δ strain was used.
Correlation between Phosphorylation and Nuclear Accumulation of Hog1–GFP
It has been noted before that the dual phosphorylation of Hog1 happens extremely fast after yeast cells are osmotically challenged. In <1 min, the protein kinase seems to become quantitatively modified. The increase in phosphorylation, however, is transient and readjusts during adaptation to the low steady-state level observed before stress (Brewster et al., 1993; Maeda et al., 1995). To examine whether there is a correlation between Hog1 phosphorylation and nuclear accumulation, we quantified the kinetics of translocation by counting cells with predominantly nuclear fluorescence. As shown in Figure 2, the phosphorylation and activation profile of Hog1–GFP and the enhanced nuclear signal clearly follow the same time-dependent patterns: they both peak at 1 min, remain close to the maximum up to 15 min, and then slowly decline to reach a steady-state level within ∼1 h after stress. This observation suggests that only the phosphorylated form of Hog1 is able to accumulate in the nucleus and that Hog1’s activation might be a prerequisite for the apparently enhanced nuclear translocation.
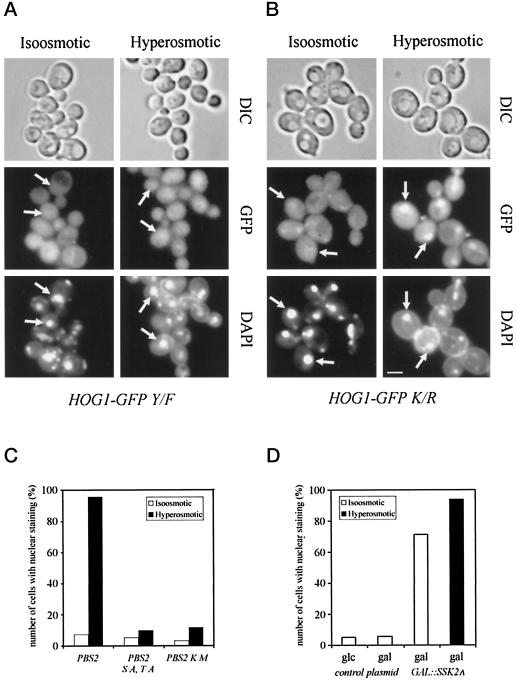
To test this assumption we generated versions of Hog1–GFP that had an amino acid substitution at one of the two the modification sites (T174A or Y176F). It is well documented that the same or corresponding amino acid residues are essential for the activity of Hog1 MAPK (Schüller et al., 1994) as well as for that of other MAPK family members (Gartner et al., 1992; Robbins et al., 1993). This result also holds true for the Hog1–GFP fusions, because the mutant constructs are unable to complement the high salt sensitivity of the hog1 mutant (Figure 1A). The fluorescence signals elicited by the T174A and the Y176F mutant show a mostly cytoplasmic distribution even after osmotic challenge (Figure 3A; our unpublished results). The loss of nuclear signal, however, is not just the consequence of a failure in signal transmission and activation of a component of the transport machinery, because the same result is obtained in a HOG-proficient background (our unpublished results). Because Hog1 is activated via the Pbs2 MAPKK, we assayed the nuclear signal of Hog1–GFP in different pbs2 mutants. Deletion of the PBS2 gene severely impaired nuclear accumulation (our unpublished results). Similar results were obtained with a Pbs2-K389M substitution, which results in a catalytically inactive kinase (Posas and Saito, 1997), and with a mutant that eliminates the activating phosphorylation sites S514 and T518 (Wurgler-Murphy et al., 1997) (Figure 3C).
Figure 3.
Phosphorylation, but not kinase activity is necessary for nuclear accumulation of Hog1. Strain K4327 (hog1Δ) was transformed with centromer plasmids (A) pVR65–Y/F containing HOG1 phosphorylation site mutant allele (HOG1–GFP Y/F, HOG1 with T174F mutation) or (B) pVR65–K/R containing HOG1 catalytic site mutant allele (HOG1–GFP K/R HOG1 with K52R mutation). Transformants were grown to logarithmic phase in selective medium (iso-osmotic). Where indicated, strains were stressed for 5 min by addition of NaCl to a final concentration of 0.4 M (hyperosmotic). Positions of nuclei are indicated by arrows. Bar, 5 μm. (C) Hog1–GFP nuclear accumulation is dependent on active Pbs2 MAPKK. The following strains were analyzed: strain VRY 10 (pbs2Δ) cotransformed with pVR65–WT (HOG1–GFP) and pVR15 (PBS2, endogenous promoter), pVR20 (PBS2S/A, T/A; PBS2 with S174A and T178A mutations), and pVR15K/M (PBS2K/M; PBS2 with K389M mutation). (D) The overexpression of the Ssk2ΔN allele induces nuclear accumulation of Hog1–GFP in nonstressed cells. Strain K4327 (hog1Δ) was cotransformed with centromer plasmid pVR65–WT (HOG1–GFP) and plasmid pGSS21 (2 μm PGAL1-SSK2ΔN, SSK2 from M1173 to D1579). Cells were grown in appropriate selective medium containing raffinose as carbon source, washed with water, and resuspended in fresh selective medium with galactose or glucose as carbon source (iso-osmotic). Where indicated, cells were stressed with 0.4 M NaCl for 5 min before microscopy (hyperosmotic). glc, glucose; gal, galactose.
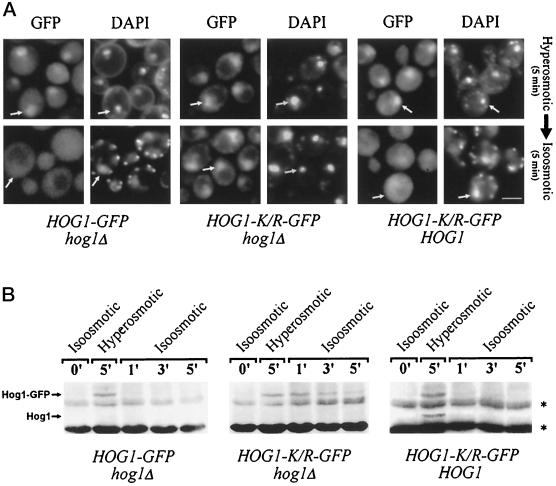
We further asked whether the Hog1 kinase requires its own enzymatic activity to experience the stress-related increase in nuclear localization. The K52R substitution disables the protein in its phospho-transfer function and thus produces a catalytically inactive kinase. However, the mutation does not prevent phosphorylation by the upstream kinase (Schüller et al., 1994). Hog1–GFP K52R is translocated to the nucleus as efficiently as WT protein after exposure of cells to hyperosmotic stress (Figure 3B; also see Figure 8A). This demonstrates that the intrinsic kinase activity of Hog1 is dispensable for nuclear accumulation.
Figure 8.
Hog1 nuclear export and dephosphorylation depend on Hog1 kinase activity. Strain K4327 (hog1Δ) was transformed with centromer plasmids containing pVR65–WT (HOG1–GFP) or HOG1–GFP K/R (pVR65–K/R) and strain W303-1A (WT) with HOG1–GFP K/R (pVR65–K/R). After exposure to hyperosmotic conditions (selective media with 0.4 M NaCl, 5 min), cells were returned rapidly to iso-osmotic conditions (selective medium without NaCl), and localization of Hog1–GFP derivatives was determined after 5 min (A; positions of nuclei are indicated by arrows; bar, 5 μm), or samples were taken at the time points indicated, and protein extracts were prepared (B). Western blots were analyzed with anti-active p38 MAPK antibody. Asterisks indicate the positions of proteins that are nonspecifically recognized by anti-active human p38 antibody.
After showing that a functional signal pathway is necessary for Hog1 nuclear accumulation, we wanted to address the question of whether activation of the kinase module is sufficient for this response or whether other osmotically induced events are also required. For this purpose we made use of a dominant activating allele of SSK2 that lacks the N-terminal regulatory domain. Expression of this constitutive kinase is incompatible with the survival of WT cells, but the lethality is suppressed in pbs2 or hog1 strains (Posas and Saito, 1997). In our case we generated the constitutive kinase from a galactose-regulated expression system. Galactose itself did not increase the number of stained nuclei. However, when the expression of Ssk2-ΔN was induced by addition of galactose in the absence of hyperosmotic stress, ∼80% of the cells exhibited a noticeably stronger Hog1–GFP signal in the nucleus (Figure 3D). Although the number of cells with distinct nuclear fluorescence can be further increased upon hyperosmotic stress, this result implies that induction of the kinase module is indeed sufficient for Hog1 nuclear accumulation.
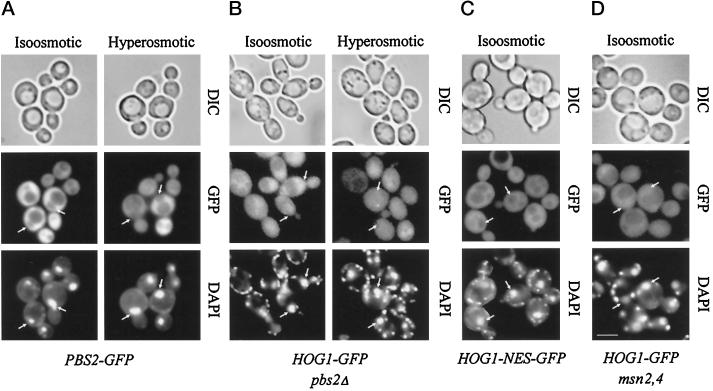
Pbs2, a Protein Excluded from the Nucleus, Does Not Affect Basal Nuclear–Cytoplasmic Shuttling of Hog1 during Noninducing Conditions
To investigate whether Hog1 was the only component of the MAPK cascade to appear in the nucleus, we examined the subcellular distribution of a Pbs2–GFP fusion. Microscopic examination showed that the GFP fluorescence was distributed evenly over the cytoplasmic compartment. This distribution did not change after application of high-osmolarity stress (Figure 4A). Furthermore, the nuclear compartment appeared free of GFP signal; as in the majority of cells the nuclear position could easily be distinguished as a dark area against a light background, an observation suggesting that Pbs2 may be actively excluded from nuclei. It has been proposed before that MEKs might serve as a cytoplasmic anchor for MAPK, preventing the nuclear appearance of the MAPK under noninducing conditions. We have tried to evaluate such a model for Pbs2 and Hog1 using our observations obtained in the absence of stress. If one approaches the Hog1–GFP localization data under this aspect, one finds that under iso-osmotic conditions the signal appears unchanged between a WT and pbs2Δ background (Figure 4B). It is also clear that neither Hog1–GFP nor its phosphorylation site–defective variant generates a nuclear exclusion signal comparable to that found with the Pbs2 fusion (compare Figures 3A and 4). This basal distribution of Hog1–GFP does not appear to be influenced by the size of the kinase. Fusions that contain two or even three tandem repeats of the GFP moiety (generating proteins of 105 and 135 kDa) show essentially the same distribution pattern as Hog1–GFP (Figure 5A).
Figure 4.
The MAPKK Pbs2 is not required for anchoring or basal nucleocytoplasmic cycling of Hog1. (A) Pbs2 appears constitutively localized in the cytoplasm. Strain VRY 10 (pbs2Δ) was transformed with pVR28 (2 μm PBS2–GFP), and transformants were grown in appropriate selective medium (iso-osmotic); where indicated, hyperosmotic stress was applied (0.4 M NaCl, 5 min; hyperosmotic). The positions of nuclei are indicated by arrows. (B) Strain VRY 10 (pbs2Δ) was transformed with pVR65-WT (HOG1–GFP). Cells with nuclear staining were counted after growing the strain to logarithmic phase in selective medium (iso-osmotic) and subjecting them to hyperosmotic conditions as described above. (C) Hog1–NES–GFP does not show basal nuclear staining under nonstress conditions. Strain K4327 (hog1Δ) was transformed with centromer plasmid pVR65–NES (HOG1–GFP fused to PKI NES), and cellular distribution of Hog1 was determined after growing them to logarithmic phase in appropriate selective medium (iso-osmotic). (D) Hog1 basal cycling is not dependent on Msn2 and Msn4 transcription factors. Strain YM24 (msn2,4Δ) was transformed with centromer plasmid pVR65 (HOG1–GFP), and cellular distribution of Hog1 was determined after growing cells to logarithmic phase in appropriate selective medium (iso-osmotic). Bar, 5 μm.
To test whether the observed differences between Hog1 and Pbs2 fusions are due to a technicality or whether they might reflect a substantial concentration of unphosphorylated Hog1–GFP in the nucleus, we inserted a generic NES between the Hog1 and the GFP sequence. Such a construct now elicited a pattern for Hog1 that was indistinguishable from the Pbs2–GFP pattern (Figure 4C). The Hog1–NES–GFP product is still physiologically intact, because it is able to rescue the osmotic sensitivity of a hog1 mutant. The fusion protein is also present at normal levels and appropriately phosphorylated after stress exposure, suggesting that the localization result is not due to secondary effects (our unpublished results). Together these experiments allow us to make three important conclusions. First, Hog1 probably cycles between the nucleus and the cytoplasm at all times. Second, the necessary transport steps in and out of the nucleus are independent of any phosphorylation events. Third, Pbs2’s putative function as a cytoplasmic anchor is not important for the basal distribution of Hog1. It is more likely that this distribution is achieved because nuclear export of Hog1 provides a rate-limiting step within the nuclear–cytoplasmic cycle.
Stress-specific Transcription Factors Are Not Essential for Hog1 Nuclear Accumulation but Determine Duration of Hog1 Nuclear Residence
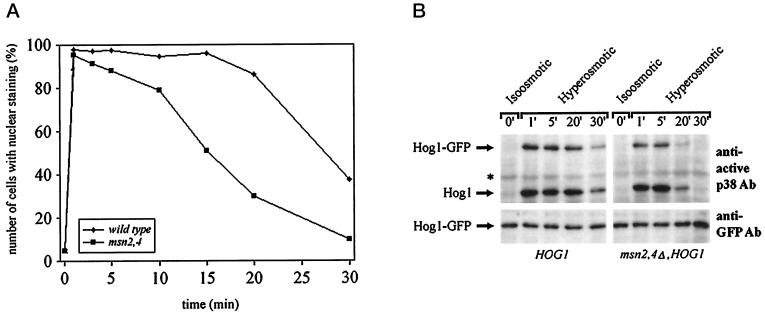
If cytoplasmic anchorage is not important for the intracellular distribution of Hog1, do nuclear retention signals play a role? Studies in S. pombe have suggested that a functional Atf transcription factor is essential for observing the nuclear accumulation of the p38 homologue StyI/Spc1 (Gaits et al., 1998). These data have been interpreted to the effect that the transcription factor serves as a nuclear anchor for the MAPK. Although the situation is more complicated in S. cerevisiae, we tested whether the general stress factors Msn2 and Msn4 influence the localization of the Hog1 kinase. A defect in these two ATF-unrelated factors greatly reduces the transcriptional induction of CTT1 in response to osmotic stress (Martinez-Pastor et al., 1996). Also, Msn2 and Msn4 change their intracellular distribution under such stress conditions, albeit independently of Hog1 (Görner et al., 1998). The msn2 msn4 double mutation did not cause a change in the basal distribution of Hog1–GFP (Figure 4D) and did not influence the level of Hog1–GFP (Figure 6). Also, shortly after stress induction most of the mutant cells did properly react by developing a distinct nuclear Hog1–GFP signal (Figure 6; our unpublished results). These results suggested either that Msn2 and Msn4 do not provide or establish a retention signal or that nuclear retention is not as important as has been implied by others. In such a case nuclear accumulation would be more a matter of changes in export–import efficiency after Hog1 becomes phosphorylated. However, when we investigated the phenomenon in more detail, we discovered that Msn2 and Msn4 are not completely uninvolved. After the localization of the GFP signal over time one could observe much faster fading of the nuclear signal in the mutants than in the WT cells. Approximately 50% of the WT cells retained a strong nuclear GFP signal up to 30 min after stress, whereas most msn2 msn4 cells already had lost their signal during that time span (Figure 6A). The more rapid loss of nuclear signal is paralleled by an earlier loss in dual phosphorylation (Figure 6B). We conclude that the nuclear fate of Hog1 is dependent on the integrity of the transcriptional stress response system, possibly because it provides or establishes nuclear anchorage for this kinase. The phosphorylation data suggest that in this case nuclear anchorage might protect Hog1 against the action of specific protein phosphatases.
Figure 6.
Duration of Hog1 nuclear accumulation is reduced in cells lacking Msn2 and Msn4 transcription factors. Logarithmically growing strain W303-1A (WT) and YM24 (msn2,4Δ) transformed with pVR65–WT (HOG1–GFP) were examined by fluorescence microscopy under iso-osmotic (selective medium) or hyperosmotic growth conditions (5 min after addition of NaCl into selective medium to a final concentration of 0.4 M) at the indicated time points (A) or by determining the phosphorylation profile of Hog1–GFP (B). Samples were taken at the time points indicated, and protein extracts were prepared. Western blots were analyzed with anti-active p38 antibody (asterisks indicate the positions of proteins that are nonspecifically recognized by antibody) or with anti-GFP antibody (bottom panel) to determine the protein level of Hog1–GFP.
Adaptation to Hyperosmotic Stress Correlates with Hog1–GFP Nuclear Export
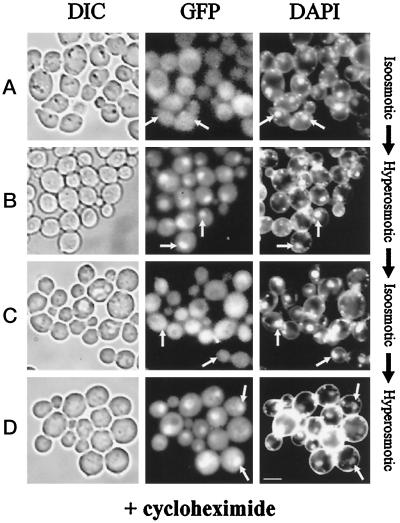
As described above, Hog1–GFP quickly accumulates in the nuclear compartment after hyperosmotic stress, a phenomenon that correlates well with the induction of kinase activation. In addition, when HOG pathway activity declines, the number of cells with nuclear GFP also decreases. Because the overall Hog1–GFP level remains constant under these conditions (see Figure 2C), the bulk of nuclear Hog1–GFP may not be degraded but reexported from the nucleus. It might even be ready to reenter the nucleus when cells are challenged again by hyperosmotic stress. To support this contention we asked whether protein synthesis is required to observe environment-related Hog1–GFP translocation events into and out of the nucleus. Cells expressing Hog1–GFP were preincubated with cycloheximide (0.1 mg/ml) for 2 h (Figure 7A). No induction of catalase activity can be observed under these conditions, implying efficient inhibition of protein synthesis (Schüller et al., 1994; our unpublished results). After preincubation, cells were exposed to a hyperosmotic environment (0.4 M NaCl). Cells accumulated Hog1–GFP in their nuclei, although protein synthesis was inhibited (Figure 7B). After 60 min of incubation at increased osmolarity, Hog1–GFP had disappeared from the nuclei with normal kinetics (Figure 7C; our unpublished results). After a second raise in osmolarity (0.8 M NaCl), Hog1–GFP quickly reaccumulated in nuclei (Figure 7D). Thus, de novo protein synthesis is dispensable not only for the initial nuclear entry of Hog1p–GFP but also for subsequent nuclear retention, nuclear export, and nuclear reentry.
Figure 7.
Cellular distribution of Hog1 during stress and adaptation is independent of de novo protein synthesis. Logarithmically growing strain K4327 (hog1Δ) transformed with centromer plasmid pVR65–WT (HOG1–GFP) was preincubated with cycloheximide (0.1 mg/ml) for 2 h before microscopy. Pictures were taken from cells incubated under iso-osmotic (selective medium) (A) or hyperosmotic conditions (5 min after addition of NaCl to a final concentration of 0.4 M) (B). (C) Cells from B were left to adapt for 60 min and then reexposed to hyperosmotic stress (5 min, final concentration of NaCl 0.8 M) (D). The positions of nuclei are indicated by arrows. Bar, 5 μm.
In a second experiment we used temporally limited exposure to hyperosmotic conditions. Five minutes after challenge with 0.4 M NaCl the cells were returned into the original medium. Although, as expected, most cells exhibited a nuclear GFP signal after stress exposure, they redistributed the protein to the cytoplasm after return to normalcy (Figure 8A). This export of GFP was remarkably fast, because the nuclear compartment seemed to be emptied of GFP well within 1 min, and it was not affected by the relative molecular mass of the protein, because multiply GFP-tagged Hog1 fusions (105 and 135 kDa, respectively) behaved in a similar manner (Figure 5; our unpublished results). A Western analysis with p38 antibodies revealed that at this time most of the protein was returned to its unphosphorylated state (Figure 8B). The temporal correlation of both phenomena suggests a tight causal relationship between the two events. Because the import of Hog1 does not require catalytically active protein (neither in cis nor in trans), we followed the fate of the catalytically inactive form under conditions that would normally support increased export of Hog1. The Hog1–K52R–GFP was expressed both in a hog1 and a WT background, and the protein was concentrated in the nucleus using exposure to 0.4 M NaCl. The cells were then centrifuged and resuspended in normal iso-osmotic medium. Under Hog1 kinase-deficient conditions the GFP signal remained in the nucleus long after return to iso-osmolarity (Figure 8A, center panels). In contrast, in a HOG1 strain, the mutant protein was exported with similar kinetics as the WT product (Figure 8A, right panels). A measurement of the phosphorylation status showed that, in the first case, Hog1–K52R–GFP remains phosphorylated over a considerable time frame, whereas in the second case it becomes almost instantly dephosphorylated (Figure 8B). We conclude that an active kinase has to be present to promote rapid nuclear export and that a causal relationship might exist between cytoplasmic redistribution and the dephosphorylation of the kinase.
DISCUSSION
In the work described here we have developed a yeast system in which the mechanisms required for cytoplasmic nuclear shuttling of a MAPK could be studied. To our knowledge this system actually provides one of the first instances in which such a kinase has been followed in vivo under normal physiological conditions. It should be noted that similar results using the same approach were recently reported from an independent investigation (Ferrigno et al., 1998). The key to these and our studies was the finding that a Hog1–GFP fusion correctly reflects the behavior of the normal kinase. The activation and function of the fusion protein were indistinguishable from those of its WT counterpart and thus provided an excellent cytological marker. The system enabled us to closely follow the kinetics of signal-dependent nuclear accumulation and redistribution. This seemed particularly relevant because the system works with a surprisingly fast reaction time, both during induction under hyperosmolarity as well as during adaptive conditions. Our additional finding that phospho-peptide antibodies developed against p38/RK recognize Hog1 enabled us to correlate the double phosphorylation status with the intracellular distribution of the protein. The finding that nuclear accumulation of a MAPK requires the activating phosphorylation events is perhaps not so surprising anymore. What this study offers, however, is undisputable evidence that phosphorylation of the protein is the key to its signal-induced nuclear accumulation.
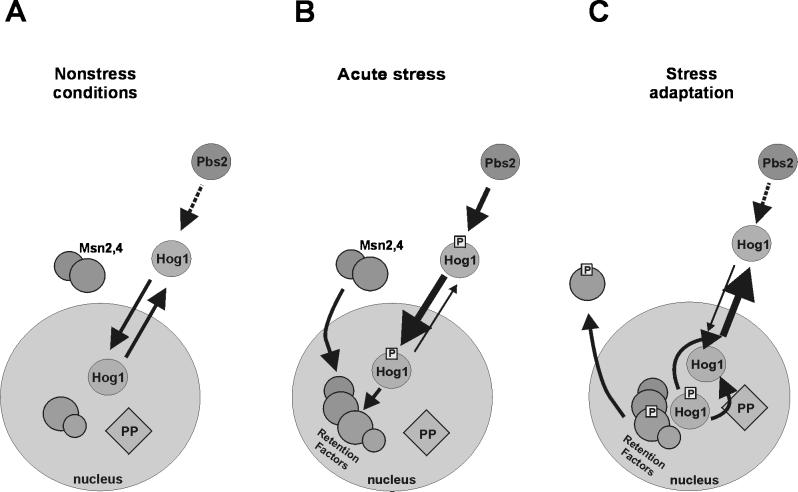
In our opinion several additional insights could be gained from our data, and their interpretation is summarized in the model presented in Figure 9. First of all, it appears that Hog1 undergoes continuous nuclear import and export even as catalytically inactive kinase. We believe in such a cycle because the fluorescence of the fusion protein is normally found throughout the cell, including the nucleus. Its distribution is quite in contrast to the signal generated by the activator of Hog1, Pbs2–GFP. Here the nucleus can be clearly visually identified as a dark, unstained region. If a generic NES is added to the Hog1–GFP fusion, a similar pattern of nuclear exclusion can be seen, suggesting that a more efficient export will change the equilibrium distribution of the kinase. Comparing the distribution pattern of WT Hog1 and several mutant versions of kinase did not reveal a significant difference, suggesting that the basal nuclear cytoplasmic equilibrium attained by import and export must happen independently of either substrate binding or kinase activity. In principle, the nuclear pore could perhaps accommodate proteins the size of a MAPK and allow free passage in either direction (Ohno et al., 1998). However, the different Hog1–GFP fusions tested should already fall well within the generally assumed size restrictions imposed by the nuclear pore, which means that either Hog1–GFP assumes a special conformation that allows diffusion-based exchange between the two compartments or that Hog1 localization relies on a carrier-dependent transport mechanism. MAPKs seem to lack generic import and export sequences, which has raised the question of adaptive factors that mediate between the kinase and the transport machineries. So what might the adaptor for inactive Hog1 be? For classical mammalian MAPKs it looks as if MEKs could provide a shuttling device at least for part of the journey (Fukuda et al., 1997; Jaaro et al., 1997). However, a pbs2Δ strain also exhibits the normal pattern of Hog1–GFP distribution under noninducing conditions. Hence, one can conclude that this protein acts neither as a cytoplasmic anchor nor as a shuttling device. If Pbs2 had a cytoplasmic retention or nuclear export role, then we might expect that even inactive Hog1–GFP accumulates in the nucleus in pbs2Δ strains. If Pbs2 were to enable the import of Hog1 not only by normal basal level phosphorylation but also by functioning as a carrier, then we could expect a nuclear exclusion signal of Hog1 in pbs2Δ strains. Neither result has been observed by us. In addition, our data rule out that one of the known stress-mediating transcription factors, Msn2, serves as an essential transport device. This was an intriguing possibility, because Msn2 itself shuttles between the nucleus and the cytoplasm. Although it has been speculated that MAPKs continuously cycle between the two compartments in the absence of an external stimulus, we believe that our experiments provide considerable evidence that such a view has some basis in reality (Figure 9A). Whether the kinase requires special factors for the transport remains to be answered. It should be noted that Ferrigno et al. (1998) recently reported that nmd5 mutants are defective in nuclear accumulation of activated Hog1. However, it remains to be seen whether the transport factor encoded by NMD5 interacts directly with the kinase and whether an nmd5-related defect can be extended to the distribution of the unmodified kinase.
Figure 9.
Model proposing how different regulatory mechanisms cooperate to determine Hog1 intracellular distribution. (A) Under favorable growth conditions Hog1 travels between cytoplasm and nucleus. This cycling is independent of activity and the presence of upstream components of the MAPK pathway. (B) During acute stress Hog1 rapidly accumulates in the nucleus. Hog1 phosphorylation by Pbs2 is essential for this process. Phosphorylated Hog1 is either more competent for nuclear import or less competent for nuclear export (as indicated by the thickness of the arrows). At the same time, some nuclear retention factors accumulate in the nuclear compartment. At this step, Hog1 kinase might modify stress response–specific targets. (C) Late phase of adaptation induces kinase activity-dependent nuclear export of Hog1. There are three potential mechanisms for which kinase activity is necessary. Hog1 might activate nuclear phosphatases; that might convert Hog1 into a more efficient export cargo. Alternatively, nuclear substrates phosphorylated by Hog1 might be exported, decreasing the concentration of nuclear retention factors. Finally, Hog1 might be required to enhance the activity of an export system. P, phosphate; PP, protein phosphatase.
If we take continuous shuttling of the MAPK for granted, what causes the difference between the basal and phosphorylation-induced signals? The large change in intracellular distribution can be explained by an increase in import efficiency and a decrease in export efficiency or by the induction of a nuclear retention mechanism (Figure 9B). Two observations have been made that mainly support either one or the other explanation. For the classical MAPK ERK2 it has been proposed that the modified protein undergoes a conformational change that allows it to dimerize. These dimers were characterized as constituting more efficiently transported entities than the unmodified monomers (Khokhlatchev et al., 1998). Thus, the authors of this work clearly favored an import control model. The p38 and Hog1 homologue of S. pombe, StyI/Spc1, accumulates in the nucleus after a rather generic stress exposure. It was found that the kinase was unable to give a nuclear signal when a supposedly resident nuclear substrate of the kinase was missing from the cells. Here, Gaits et al. (1998) championed the idea that the nuclear factor would provide an anchor to retain the activated kinase in the nucleus. In the absence of this anchor the kinase could not be concentrated and would be reexported from the nucleus.
Our data shed light and expand on both ideas. First, one should remember that the appearance of a strong nuclear Hog1 signal is very fast, being completed on the order of <1 min. Second, the absence of a major stress-related transcription complex has no effect on how quickly the nuclear signal appears. We therefore feel that the kinase really must attain a state that makes it either a more efficient import cargo or a less efficient export cargo. For example, the modified kinase could more easily associate with its import carrier. It could also more easily dissociate from such a complex in the nuclear compartment and resist being incorporated into an export-competent complex. Whether this situation is due to a dimerization event, as proposed for ERKs, we cannot answer conclusively. One might, however, note that we were unable to find any evidence for such dimers. For example, coprecipitation experiments with differently sized Hog1 kinases were negative (Reiser, unpublished results). Also, similar to results obtained with StyI/Spc1 in S. pombe, the active Hog1 kinase clearly did not support the nuclear accumulation of unphosphorylatable kinase forms, suggesting that at least with this type of MAPK, dimers might not so easily form in vivo as in vitro to be a major source of regulation.
Even though nuclear retention may not have a role in the initial phase of the response, it seems to be one important factor for the persistent nuclear residence of the kinase, because mutants deficient in Msn2 and Msn4 exhibit a lower half-life for the stress-induced nuclear signal. Superficially, this observation seems to be more in line with the S. pombe work, with the exception that in this case the authors (Gaits et al., 1998) reported a complete loss of nuclear MAPK signal in the absence of the anchoring transcription factor. This difference in results could be due to different technical approaches, because the S. pombe work was done with immunolocalization methods after fixation and by measuring only a few time points separated by at least 10 min. So it could be that an early and transient nuclear signal was missed under such conditions. On the other hand, it is also possible that we have missed the predominant nuclear retention factor, because the physiological direct target of Hog1 remains still at large. In principle, it could be just the concentration of nuclear substrates that determines how long the kinase remains in the nucleus. The concentration of these targets might decrease over time if they happen to become modified and exported as a consequence of modification. For example, the mammalian p38/RK target MAPK-activated protein kinase II is reexported to the cytoplasm after a p38-dependent phosphorylation event that uncovers a normally hidden NES (Engel et al., 1998). It is not unlikely that similar Hog1 substrates will be found in yeast (Figure 9C).
This finally brings up the question of how MAPKs get redistributed during adaptation. Our data make quite clear that this is not a passive event in reaction to the throttling of the initiating signal. Reexport is clearly happening as a regulated event, and it can happen fast. The export is independent of protein synthesis and allows Hog1 to reenter another activation cycle, meaning the kinase could be dephosphorylated and recruited into a Pbs2-dependent activation complex. Surprisingly, the export-initiating event requires Hog1 activity, because the catalytically inactive protein seems to linger in the nucleus much longer than the active kinase. However, it is not intrinsic kinase activity that is required for this action, because the export defect of the mutant protein can be complemented in trans. Thus Hog1 must actively induce a process that makes it competent for export. Because the export can happen so fast after return to iso-osmotic conditions, this should rule out that the loss of the nuclear signal is just the consequence of modification of putative retention factors. Our data rather suggest a close link between export and dephosphorylation of Hog1 (Figure 9C). Even if we are not yet in a position to distinguish between cause and effect, it is possible that Hog1 activates nuclear phosphatases to allow recognition by the export machinery. For example, the Hog1-specific phosphatase Ptp2 can indeed be found predominantly in the nucleus (our unpublished results; Ota, personal communication) and tightly associated with Hog1 (Wurgler-Murphy et al., 1997). It might be noteworthy that the localization of the phosphatase does not change upon stress stimulation (our unpublished results). Regulated export, on the other hand, may allow an efficient cytoplasmic system to rapidly cleanse the kinase from phosphorylation. We can imagine future experiments to settle this question.
ACKNOWLEDGMENTS
We thank H. Saito for providing plasmid constructs and I. Ota for permission to cite unpublished work. We are grateful to C. Schüller for help with microscopy and for comments on the manuscript, G. Griffioen, Martin Piskacek, Leos̆ Valás̆ek, M. Teige, and other members of the Ruis laboratory for helpful discussions, and H. Nierlich for excellent technical assistance. This work was supported by grants P12478 (to H.R.) and W001 (predoctoral fellowship to V.R.) of the Austrian Fonds zur Förderung wissenschaftlicker Forschung.
Abbreviations used:
- GFP
green fluorescent protein
- HOG
high-osmolarity glycerol response
- MAPK
mitogen-activated protein kinase
- MAPKK or MEK
MAPK kinase
- NES
nuclear export signal
- WT
wild-type
REFERENCES
- Albertyn J, Hohmann S, Thevelein JM, Prior BA. GPD1, which encodes glycerol-3-phosphate dehydrogenase, is essential for growth under osmotic stress in Saccharomyces cerevisiae, and its expression is regulated by the high-osmolarity glycerol response pathway. Mol Cell Biol. 1994;14:4135–4144. doi: 10.1128/mcb.14.6.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boguslawski G. PBS2, a yeast gene encoding a putative protein kinase, interacts with the RAS2 pathway and affects osmotic sensitivity of Saccharomyces cerevisiae. J Gen Microbiol. 1992;138:2425–2432. doi: 10.1099/00221287-138-11-2425. [DOI] [PubMed] [Google Scholar]
- Brewster JL, de Valoir T, Dwyer ND, Winter E, Gustin MC. An osmosensing signal transduction pathway in yeast. Science. 1993;259:1760–1763. doi: 10.1126/science.7681220. [DOI] [PubMed] [Google Scholar]
- Chen RH, Sarnecki C, Blenis J. Nuclear localization and regulation of erk- and rsk-encoded protein kinases. Mol Cell Biol. 1992;12:915–927. doi: 10.1128/mcb.12.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvrckova F, De Virgilio C, Manser E, Pringle JR, Nasmyth K. Ste20-like protein kinases are required for normal localization of cell growth and for cytokinesis in budding yeast. Genes & Dev. 1995;9:1817–1830. doi: 10.1101/gad.9.15.1817. [DOI] [PubMed] [Google Scholar]
- Degols G, Shiozaki K, Russell P. Activation and regulation of the Spc1 stress-activated protein kinase in Schizosaccharomyces pombe. Mol Cell Biol. 1996;16:2870–2877. doi: 10.1128/mcb.16.6.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel K, Kotlyarov A, Gaestel M. Leptomycin B-sensitive nuclear export of MAPKAP kinase 2 is regulated by phosphorylation. EMBO J. 1998;17:3363–3371. doi: 10.1093/emboj/17.12.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estruch F, Carlson M. Two homologous zinc finger genes identified by multicopy suppression in a SNF1 protein kinase mutant of Saccharomyces cerevisiae. Mol Cell Biol. 1993;13:3872–3881. doi: 10.1128/mcb.13.7.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrigno P, Posas F, Koepp D, Saito H, Silver PA. Regulated nucleo/cytoplasmic exchange of HOG1 MAPK requires the importin β homologs NMD5 and XPO1. EMBO J. 1998;17:5606–5614. doi: 10.1093/emboj/17.19.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Gotoh I, Gotoh Y, Nishida E. Cytoplasmic localization of mitogen-activated protein kinase kinase directed by its NH2-terminal, leucine-rich short amino acid sequence, which acts as a nuclear export signal. J Biol Chem. 1996;271:20024–20028. doi: 10.1074/jbc.271.33.20024. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Gotoh Y, Nishida E. Interaction of MAP kinase with MAP kinase kinase, its possible role in the control of nucleocytoplasmic transport of MAP kinase. EMBO J. 1997;16:1901–1908. doi: 10.1093/emboj/16.8.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaits F, Degols G, Shiozaki K, Russell P. Phosphorylation and association with the transcription factor Atf1 regulate localization of Spc1/StyI stress-activated kinase in fission yeast. Genes & Dev. 1998;12:1464–1473. doi: 10.1101/gad.12.10.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner A, Nasmyth K, Ammerer G. Signal transduction in Saccharomyces cerevisiae requires tyrosine and threonine phosphorylation of FUS3 and KSS1. Genes & Dev. 1992;6:1280–1292. doi: 10.1101/gad.6.7.1280. [DOI] [PubMed] [Google Scholar]
- Gietz RD, Sugino A. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-bp restriction sites. Gene. 1988;74:527–734. doi: 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]
- Gonzalez FA, Seth A, Raden DL, Bowman DS, Fay FS, Davis RJ. Serum-induced translocation of mitogen-activated protein kinase to the cell surface ruffling membrane and the nucleus. J Cell Biol. 1993;122:1089–1101. doi: 10.1083/jcb.122.5.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görner W, Durchschlag E, Martinez-Pastor MT, Estruch F, Ammerer G, Hamilton B, Ruis H, Schüller C. Nuclear localization of the C2H2 zinc finger protein Msn2p is regulated by stress and protein kinase A activity. Genes & Dev. 1998;12:586–597. doi: 10.1101/gad.12.4.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskowitz I. MAP kinase pathways in yeast: for mating and more. Cell. 1995;80:187–197. doi: 10.1016/0092-8674(95)90402-6. [DOI] [PubMed] [Google Scholar]
- Higuchi R. Recombinant PCR. In: Innis MA, Gelfand DH, editors. PCR Protocols, A Guide to Methods and Applications. J.J. Sninsky, New York: Academic Press; 1990. pp. 177–183. [Google Scholar]
- Hohmann S. Shaping up: the responses of yeast to osmotic stress. In: Hohmann S, Magar WH, editors. Yeast Stress Responses. Austin, TX: Landes Biosciences; 1997. pp. 101–145. [Google Scholar]
- Jaaro H, Rubinfeld H, Hanoch T, Seger R. Nuclear translocation of mitogen-activated protein kinase kinase (MEK1) in response to mitogenic stimulation. Proc Natl Acad Sci USA. 1997;94:3742–3747. doi: 10.1073/pnas.94.8.3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khokhlatchev AV, Canagarajah B, Wilsbacher J, Robinson M, Atkinson M, Goldsmith E, Cobb MH. Phosphorylation of the MAP kinase ERK2 promotes its homodimerization and nuclear translocation. Cell. 1998;93:605–615. doi: 10.1016/s0092-8674(00)81189-7. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J. Sounding the alarm, protein kinase cascades activated by stress and inflammation. J Biol Chem. 1996;271:24313–24316. doi: 10.1074/jbc.271.40.24313. [DOI] [PubMed] [Google Scholar]
- Lenormand P, Brondello JM, Brunet A, Pouyssegur J. Growth factor-induced p42/p44 MAPK nuclear translocation and retention requires both MAPK activation and neosynthesis of nuclear anchoring proteins. J Cell Biol. 1998;142:625–633. doi: 10.1083/jcb.142.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenormand P, Sardet C, Pages G, L’Allemain G, Brunet A, Pouyssegur J. Growth factors induce nuclear translocation of MAP kinases (p42mapk and p44mapk) but not of their activator MAP kinase kinase (p45mapkk) in fibroblasts. J Cell Biol. 1993;122:1079–1088. doi: 10.1083/jcb.122.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Cook JG, Thorner J. Phosphorylation and localization of Kss1, a MAP kinase of the Saccharomyces cerevisiae pheromone response pathway. Mol Biol Cell. 1995;6:889–909. doi: 10.1091/mbc.6.7.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T, Takekawa M, Saito H. Activation of yeast PBS2 MAPKK by MAPKKKs or by binding of an SH3- containing osmosensor. Science. 1995;269:554–558. doi: 10.1126/science.7624781. [DOI] [PubMed] [Google Scholar]
- Maeda T, Tsai AY, Saito H. Mutations in a protein tyrosine phosphatase gene (PTP2) and a protein serine/threonine phosphatase gene (PTC1) cause a synthetic growth defect in Saccharomyces cerevisiae. Mol Cell Biol. 1993;13:5408–5417. doi: 10.1128/mcb.13.9.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T, Wurgler-Murphy SM, Saito H. A two-component system that regulates an osmosensing MAP kinase cascade in yeast. Nature. 1994;369:242–245. doi: 10.1038/369242a0. [DOI] [PubMed] [Google Scholar]
- Manivasakam P, Weber SC, McElver J, Schiestl RH. Microhomology mediated PCR targeting in Saccharomyces cerevisiae. Nucleic Acids Res. 1995;23:2799–2800. doi: 10.1093/nar/23.14.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Pastor MT, Marchler G, Schüller C, Marchler-Bauer A, Ruis H, Estruch F. The Saccharomyces cerevisiae zinc finger proteins Msn2p and Msn4p are required for transcriptional induction through the stress response element (STRE) EMBO J. 1996;15:2227–2235. [PMC free article] [PubMed] [Google Scholar]
- Ohno M, Fornerod M, Mattaj IW. Nucleocytoplasmic transport, the last 200 nanometers. Cell. 1998;92:327–336. doi: 10.1016/s0092-8674(00)80926-5. [DOI] [PubMed] [Google Scholar]
- Posas F, Saito H. Osmotic activation of the HOG MAPK pathway via Ste11p MAPKKK, scaffold role of Pbs2p MAPKK. Science. 1997;276:1702–1705. doi: 10.1126/science.276.5319.1702. [DOI] [PubMed] [Google Scholar]
- Posas F, Saito H. Activation of the yeast SSK2 MAP kinase kinase kinase by the SSK1 two- component response regulator. EMBO J. 1998;17:1385–1394. doi: 10.1093/emboj/17.5.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posas F, Wurgler-Murphy SM, Maeda T, Witten EA, Thai TC, Saito H. Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1-YPD1-SSK1 “two-component” osmosensor. Cell. 1996;86:865–875. doi: 10.1016/s0092-8674(00)80162-2. [DOI] [PubMed] [Google Scholar]
- Robbins DJ, Zhen E, Owaki H, Vanderbilt CA, Ebert D, Geppert TD, Cobb MH. Regulation and properties of extracellular signal-regulated protein kinases 1 and 2 in vitro. J Biol Chem. 1993;268:5097–5106. [PubMed] [Google Scholar]
- Rose MD, Winston F, Hieter P. Methods in Yeast Genetics: A Laboratory Course Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1990. [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Schüller C, Brewster JL, Alexander MR, Gustin MC, Ruis H. The HOG pathway controls osmotic regulation of transcription via the stress response element (STRE) of the Saccharomyces cerevisiae CTT1 gene. EMBO J. 1994;13:4382–4389. doi: 10.1002/j.1460-2075.1994.tb06758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stade K, Ford CS, Guthrie C, Weis K. Exportin 1 (Crm1p) is an essential nuclear export factor. Cell. 1997;90:1041–1050. doi: 10.1016/s0092-8674(00)80370-0. [DOI] [PubMed] [Google Scholar]
- Su B, Karin M. Mitogen-activated protein kinase cascades and regulation of gene expression. Curr Opin Immunol. 1996;8:402–411. doi: 10.1016/s0952-7915(96)80131-2. [DOI] [PubMed] [Google Scholar]
- Treisman R. Regulation of transcription by MAP kinase cascades. Curr Opin Cell Biol. 1996;8:205–215. doi: 10.1016/s0955-0674(96)80067-6. [DOI] [PubMed] [Google Scholar]
- Waskiewicz AJ, Cooper JA. Mitogen and stress response pathways, MAP kinase cascades and phosphatase regulation in mammals and yeast. Curr Opin Cell Biol. 1995;7:798–805. doi: 10.1016/0955-0674(95)80063-8. [DOI] [PubMed] [Google Scholar]
- Wen W, Meinkoth JL, Tsien RY, Taylor SS. Identification of a signal for rapid export of proteins from the nucleus. Cell. 1995;82:463–473. doi: 10.1016/0092-8674(95)90435-2. [DOI] [PubMed] [Google Scholar]
- Wilkinson MG, Millar JB. SAPKs and transcription factors do the nucleocytoplasmic tango. Genes & Dev. 1998;12:1391–1397. doi: 10.1101/gad.12.10.1391. [DOI] [PubMed] [Google Scholar]
- Wurgler-Murphy SM, Maeda T, Witten EA, Saito H. Regulation of the Saccharomyces cerevisiae HOG1 mitogen-activated protein kinase by the PTP2 and PTP3 protein tyrosine phosphatases. Mol Cell Biol. 1997;17:1289–1297. doi: 10.1128/mcb.17.3.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]