Abstract
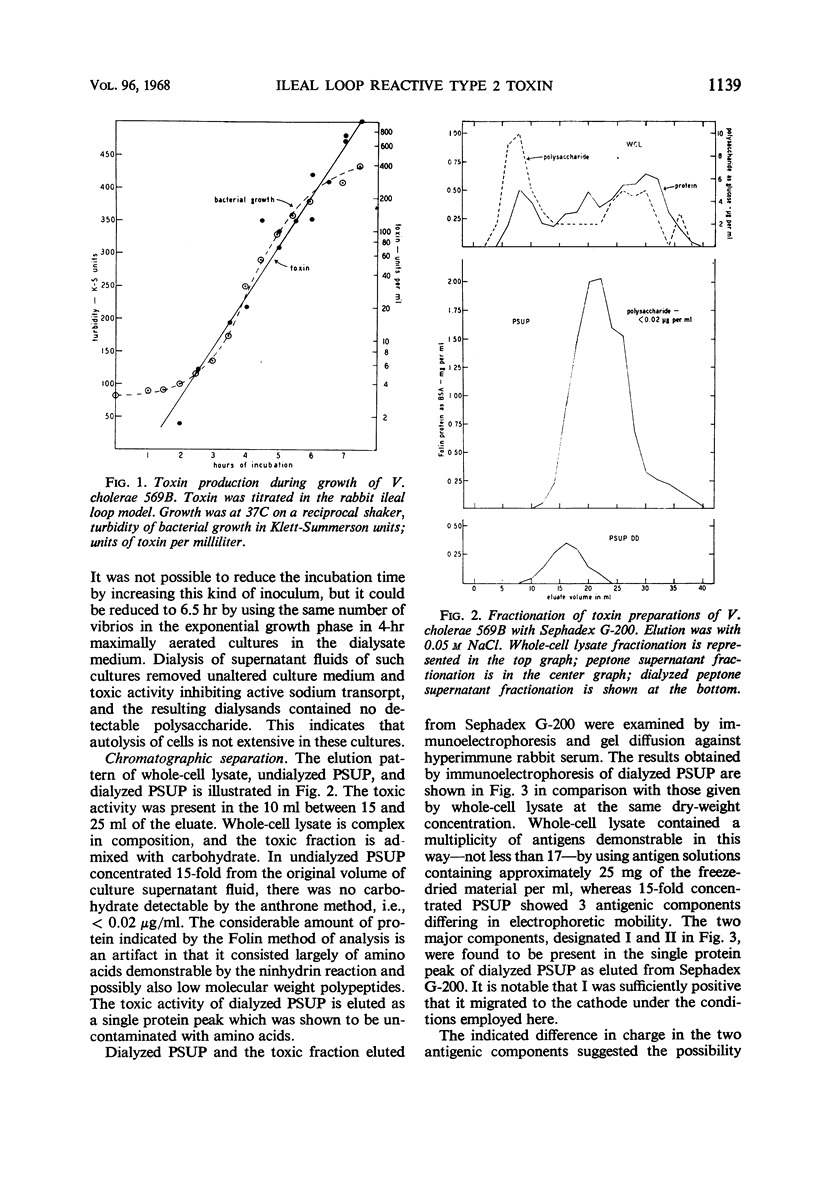
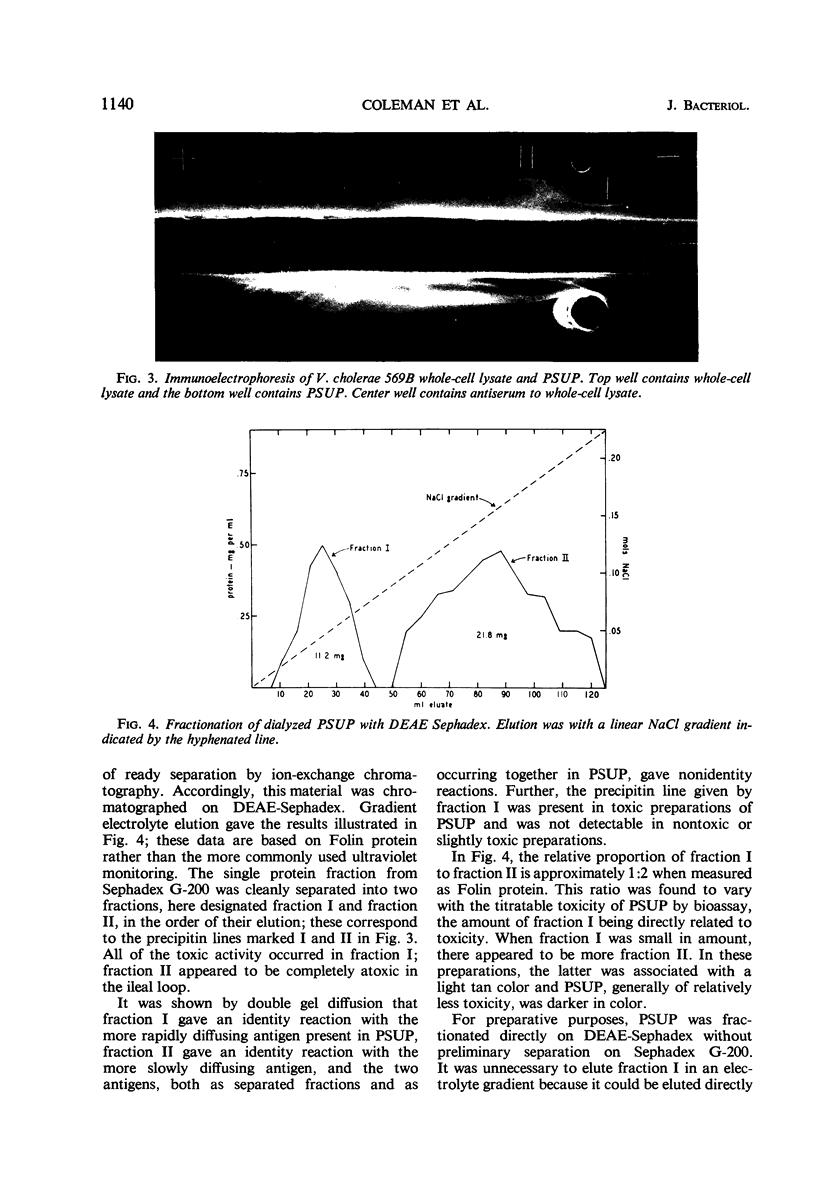
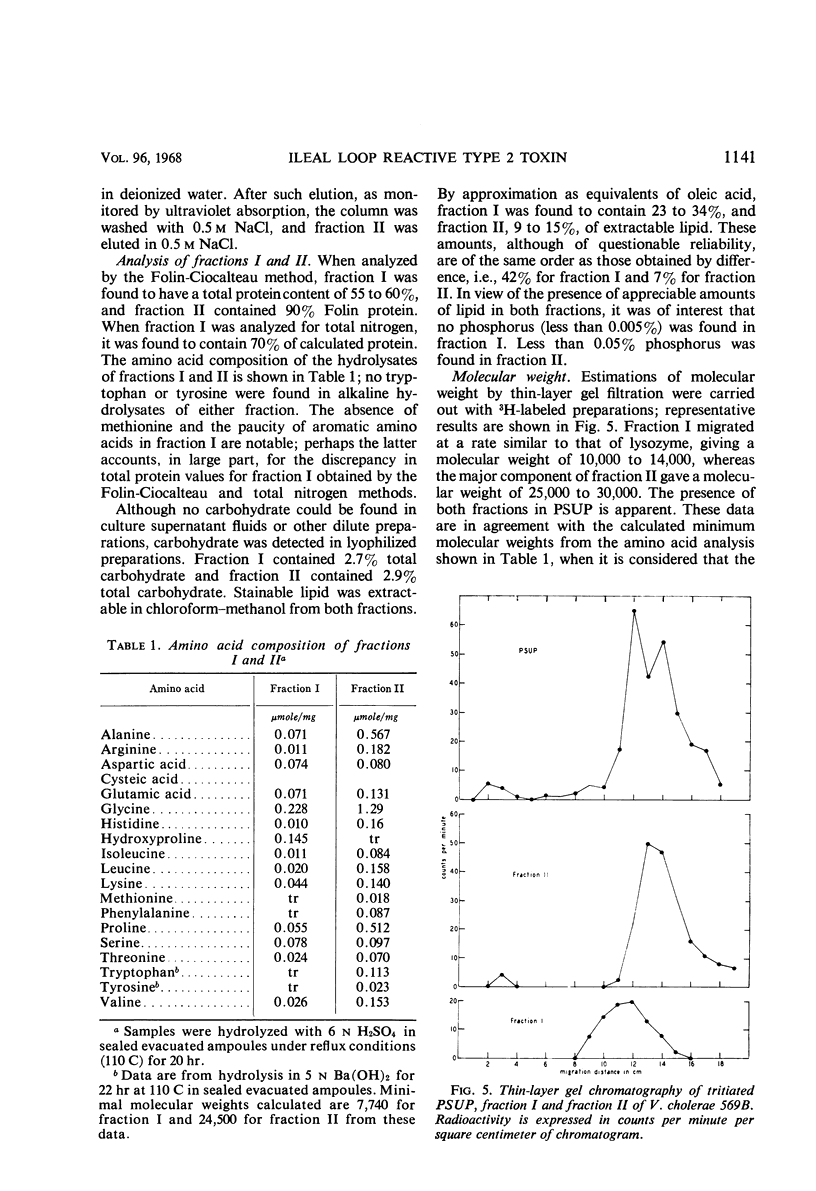
Details for the preparation and partial purification of culture supernatant fluids of Vibrio cholerae (V. comma) 569B which retain rabbit ileal loop fluid-accumulating activity are presented. These preparations were fractionated on Sephadex G-200 and on diethylaminoethyl-Sephadex. On the latter, two fractions were obtained by elution with a linear sodium chloride gradient. The fraction designated “fraction I” retains the toxic activity as demonstrated in the rabbit ileal loop model. Chemical and immunological properties of this active fraction are described.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows W., Musteikis G. M. Cholera infection and toxin in the rabbit ileal loop. J Infect Dis. 1966 Apr;116(2):183–190. doi: 10.1093/infdis/116.2.183. [DOI] [PubMed] [Google Scholar]
- Carpenter C. C., Sack R. B., Feeley J. C., Steenberg R. W. Site and characteristics of electrolyte loss and effect of intraluminal glucose in experimental canine cholera. J Clin Invest. 1968 May;47(5):1210–1220. doi: 10.1172/JCI105810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K. Y., Salton M. R. Fatty acid composition of bacterial membrane and wall lipids. Biochim Biophys Acta. 1966 Feb 1;116(1):73–79. doi: 10.1016/0005-2760(66)90093-2. [DOI] [PubMed] [Google Scholar]
- DE S. N. Enterotoxicity of bacteria-free culture-filtrate of Vibrio cholerae. Nature. 1959 May 30;183(4674):1533–1534. doi: 10.1038/1831533a0. [DOI] [PubMed] [Google Scholar]
- DUTTA N. K., PANSE M. V., KULKARNI D. R. Role of cholera a toxin in experimental cholera. J Bacteriol. 1959 Oct;78:594–595. doi: 10.1128/jb.78.4.594-595.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwert M. E., Leitch G. J., Burrows W. Increased serum glutamic-oxalacetic transaminase and lactic dehydrogenase levels produced by cholera infection and cell-free toxin in the rabbit. J Infect Dis. 1967 Jun;117(3):265–272. doi: 10.1093/infdis/117.3.265. [DOI] [PubMed] [Google Scholar]
- Kasai G. J., Burrows W. The titration of cholera toxin and antitoxin in the rabbit ileal loop. J Infect Dis. 1966 Dec;116(5):606–614. doi: 10.1093/infdis/116.5.606. [DOI] [PubMed] [Google Scholar]
- Leitch G. J., Iwert M. E., Burrows W. Experimental cholera in the rabbit ligated ileal loop: toxin-induced water and ion movement. J Infect Dis. 1966 Jun;116(3):303–312. doi: 10.1093/infdis/116.3.303. [DOI] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. II. Prog Allergy. 1962;6:30–154. doi: 10.1159/000313795. [DOI] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- SCHEIDEGGER J. J. Une micro-méthode de l'immuno-electrophorèse. Int Arch Allergy Appl Immunol. 1955;7(2):103–110. [PubMed] [Google Scholar]



