Abstract
Uridine diphosphate d-glucose dehydrogenase (EC 1.1.1.22) from Aerobacter aerogenes has been partially purified and its properties have been investigated. The molecular weight of the enzyme is between 70,000 and 100,000. Uridine diphosphate d-glucose is a substrate; the diphosphoglucose derivatives of adenosine, cytidine, guanosine, and thymidine are not substrates. Nicotinamide adenine dinucleotide (NAD), but not nicotinamide adenine dinucleotide phosphate, is active as hydrogen acceptor. The pH optimum is between 9.4 and 9.7; the Km is 0.6 mm for uridine diphosphate d-glucose and 0.06 mm for NAD. Inhibition of the enzyme by uridine diphosphate d-xylose is noncooperative and of mixed type; the Ki is 0.08 mm. Thus, uridine diphosphate d-glucose dehydrogenase from A. aerogenes differs from the enzyme from mammalian liver, higher plants, and Cryptococcus laurentii, in which uridine diphosphate d-xylose functions as a cooperative, allosteric feedback inhibitor.
Full text
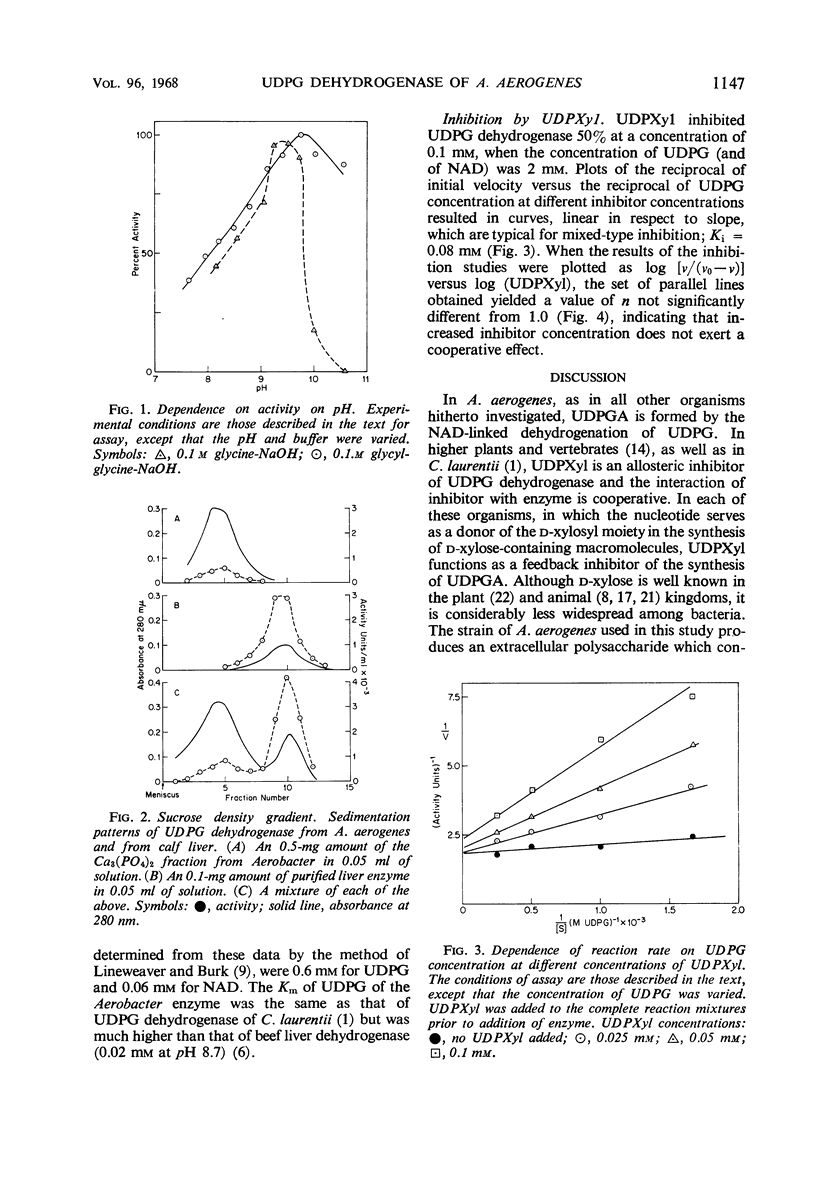
PDF

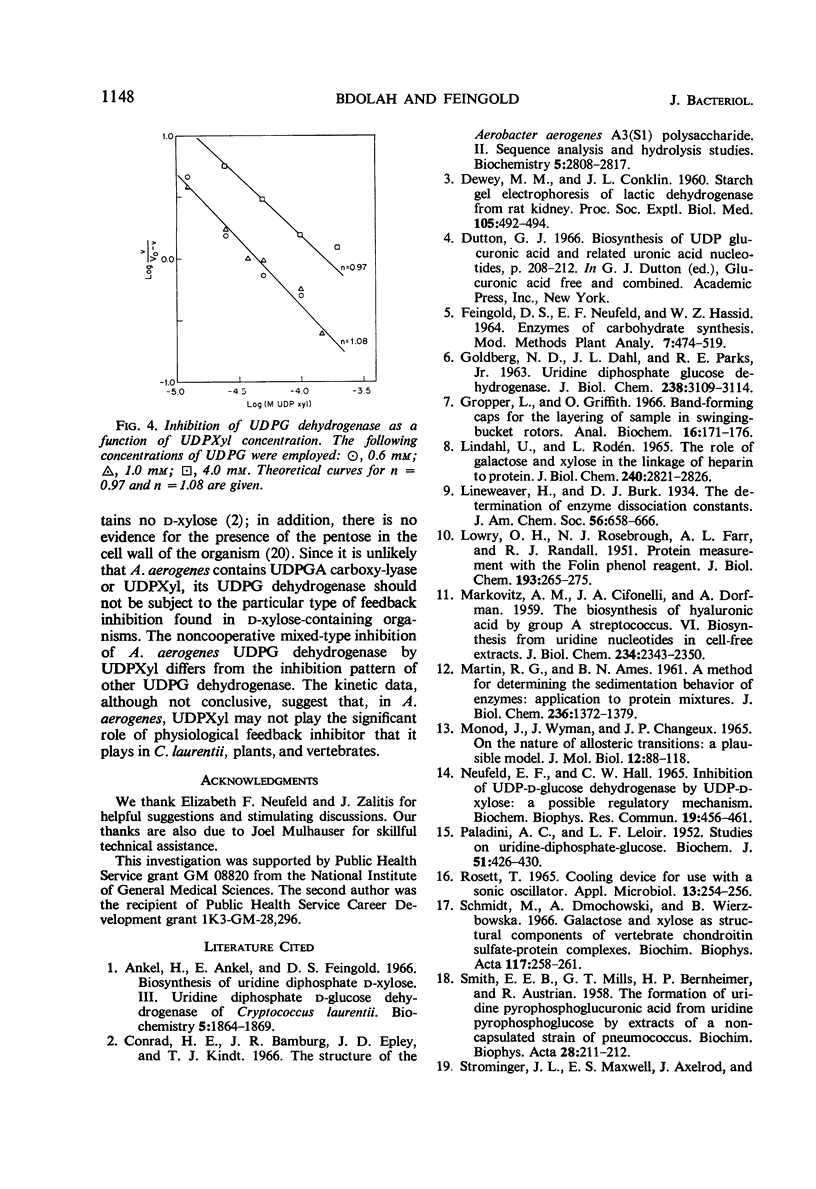

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELROD J., KALCKAR H. M., MAXWELL E. S., STROMINGER J. L. Enzymatic formation of uridine diphosphoglucuronic acid. J Biol Chem. 1957 Jan;224(1):79–90. [PubMed] [Google Scholar]
- Ankel H., Ankel E., Feingold D. S. Biosynthesis of uridine diphosphate D-xylose. 3. Uridine diphosphate D-glucose dehydrogenase of Cryptococcus laurentii. Biochemistry. 1966 Jun;5(6):1864–1869. doi: 10.1021/bi00870a012. [DOI] [PubMed] [Google Scholar]
- Conrad H. E., Bamburg J. R., Epley J. D., Kindt T. J. The structure of the Aerobacter aerogenes A3(S1) polysaccharide. II. Sequence analysis and hydrolysis studies. Biochemistry. 1966 Sep;5(9):2808–2817. doi: 10.1021/bi00873a005. [DOI] [PubMed] [Google Scholar]
- DEWEY M. M., CONKLIN J. L. Starch gel electrophoresis of lactic dehydrogenase from rat kidney. Proc Soc Exp Biol Med. 1960 Dec;105:492–494. doi: 10.3181/00379727-105-26153. [DOI] [PubMed] [Google Scholar]
- GOLDBERG N. D., DAHL J. L., PARKS R. E., Jr URIDINE DIPHOSPHATE GLUCOSE DEHYDROGENASE. PH DEPENDENCE OF THE REACTIONS WITH THE 5-FLUOROURACIL AND 6-AZAURACIL ANALOGUES OF URIDINE DIPHOSPHATE GLUCOSE. J Biol Chem. 1963 Sep;238:3109–3114. [PubMed] [Google Scholar]
- Gropper L., Griffith O. Band-forming caps for the layering of sample in swinging-bucket rotors. Anal Biochem. 1966 Jul;16(1):171–176. doi: 10.1016/0003-2697(66)90094-7. [DOI] [PubMed] [Google Scholar]
- LINDAHL U., RODEN L. THE ROLE OF GALACTOSE AND XYLOSE IN THE LINKAGE OF HEPARIN TO PROTEIN. J Biol Chem. 1965 Jul;240:2821–2826. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARKOVITZ A., CIFONELLI J. A., DORFMAN A. The biosynthesis of hyaluronic acid by group A Streptococcus. VI. Biosynthesis from uridine nucleotides in cell-free extracts. J Biol Chem. 1959 Sep;234:2343–2350. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- NEUFELD E. F., HALL C. W. INHIBITION OF UDP-D-GLUCOSE DEHYDROGENASE BY UDP-D-XYLOSE: A POSSIBLE REGULATORY MECHANISM. Biochem Biophys Res Commun. 1965 May 3;19:456–461. doi: 10.1016/0006-291x(65)90146-4. [DOI] [PubMed] [Google Scholar]
- PALADINI A. C., LELOIR L. F. Studies on uridine-diphosphate-glucose. Biochem J. 1952 Jun;51(3):426–430. doi: 10.1042/bj0510426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSETT T. COOLING DEVICE FOR USE WITH A SONIC OSCILLATOR. Appl Microbiol. 1965 Mar;13:254–256. doi: 10.1128/am.13.2.254-256.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH E. E., MILLS G. T., BERNHEIMER H. P., AUSTRIAN R. The formation of uridine pyrophosphoglucuronic acid from uridine pyrophosphoglucose by extracts of a noncapsulated strain of pneumococcus. Biochim Biophys Acta. 1958 Apr;28(1):211–212. doi: 10.1016/0006-3002(58)90455-4. [DOI] [PubMed] [Google Scholar]
- Schmidt M., Dmochowski A., Wierzbowska B. Galactose and xylose as structural components of vertebrate chondroitin sulfate-protein complexes. Biochim Biophys Acta. 1966 Mar 28;117(1):258–261. doi: 10.1016/0304-4165(66)90174-7. [DOI] [PubMed] [Google Scholar]
- Sutherland I. W., Wilkinson J. F. The composition of lipopolysaccharides of Klebsiella aerogenes and Aerobacter cloacae. Biochim Biophys Acta. 1966 Mar 28;117(1):261–263. doi: 10.1016/0304-4165(66)90175-9. [DOI] [PubMed] [Google Scholar]
- Wardi A. H., Allen W. S., Turner D. L., Stary Z. Isolation of arabinose-containing hyaluronate peptides and xylose-containing chondroitin sulfate peptides from protease-digested brain tissue. Arch Biochem Biophys. 1966 Oct;117(1):44–53. doi: 10.1016/0003-9861(66)90123-8. [DOI] [PubMed] [Google Scholar]


