Abstract
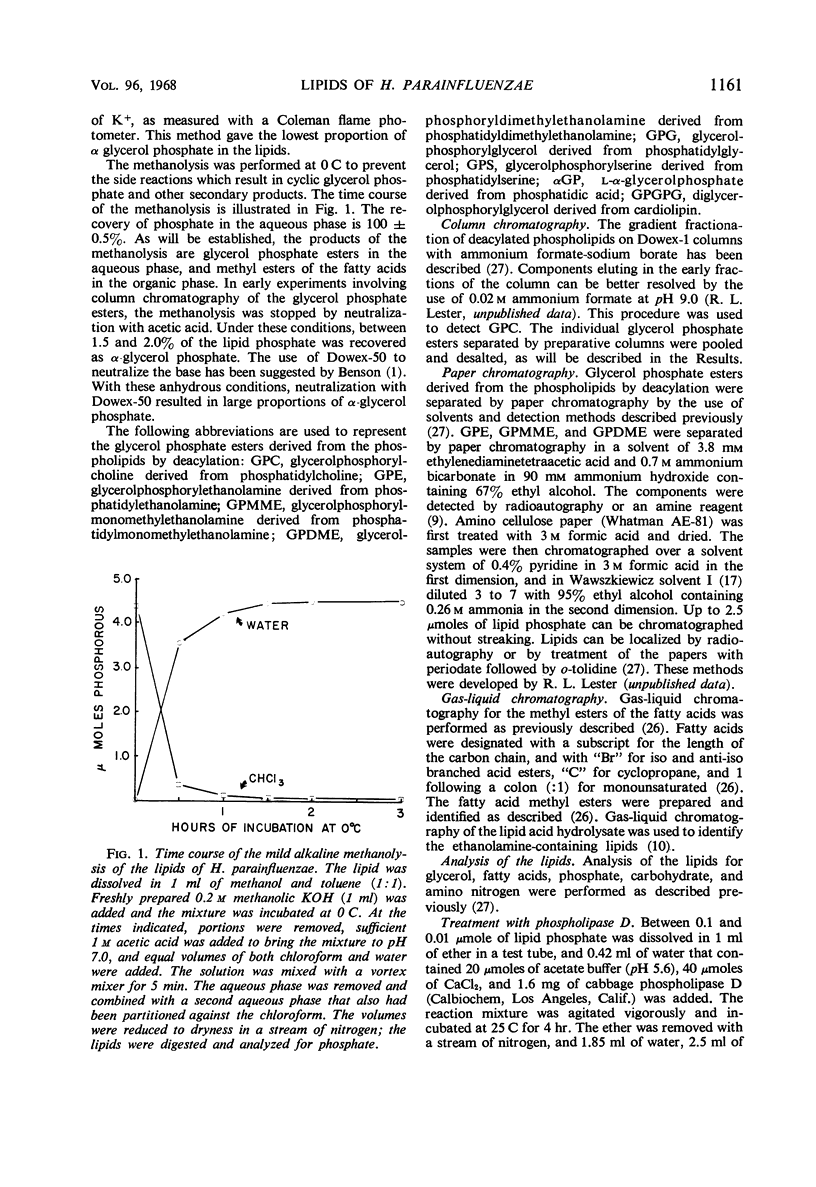
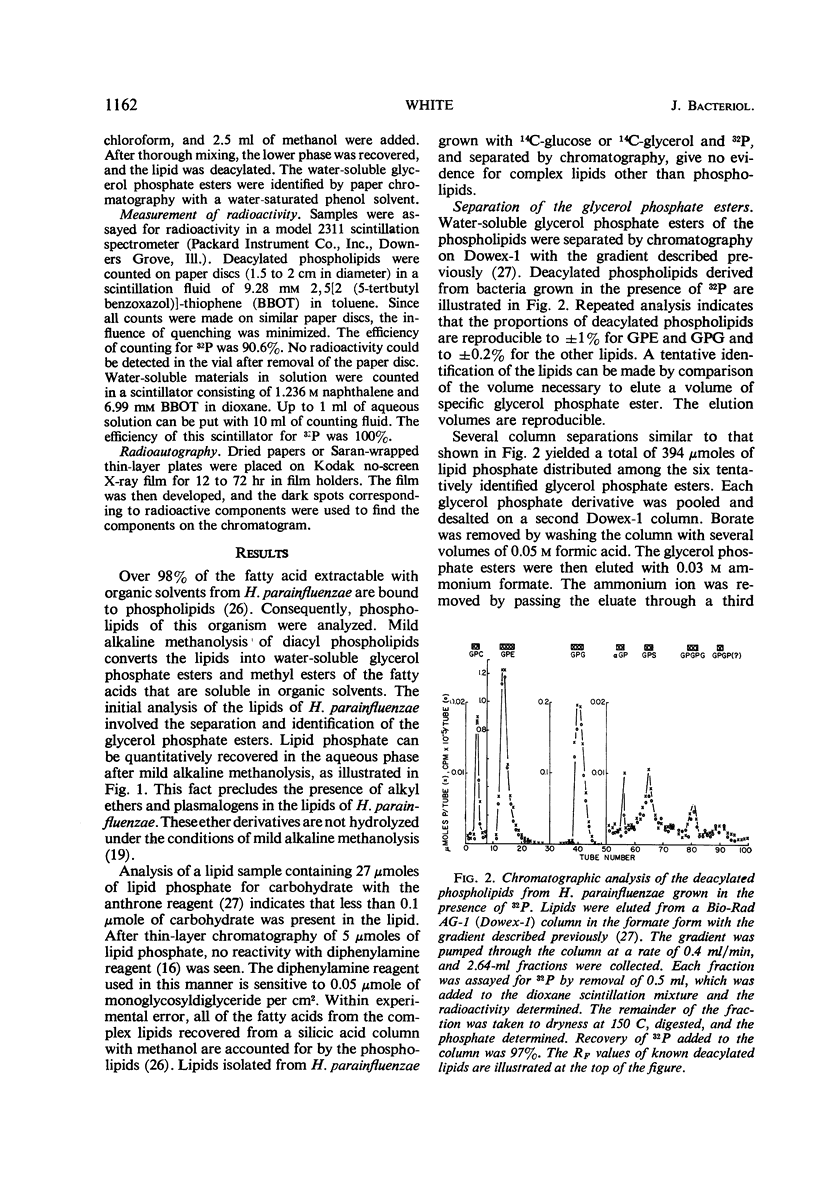
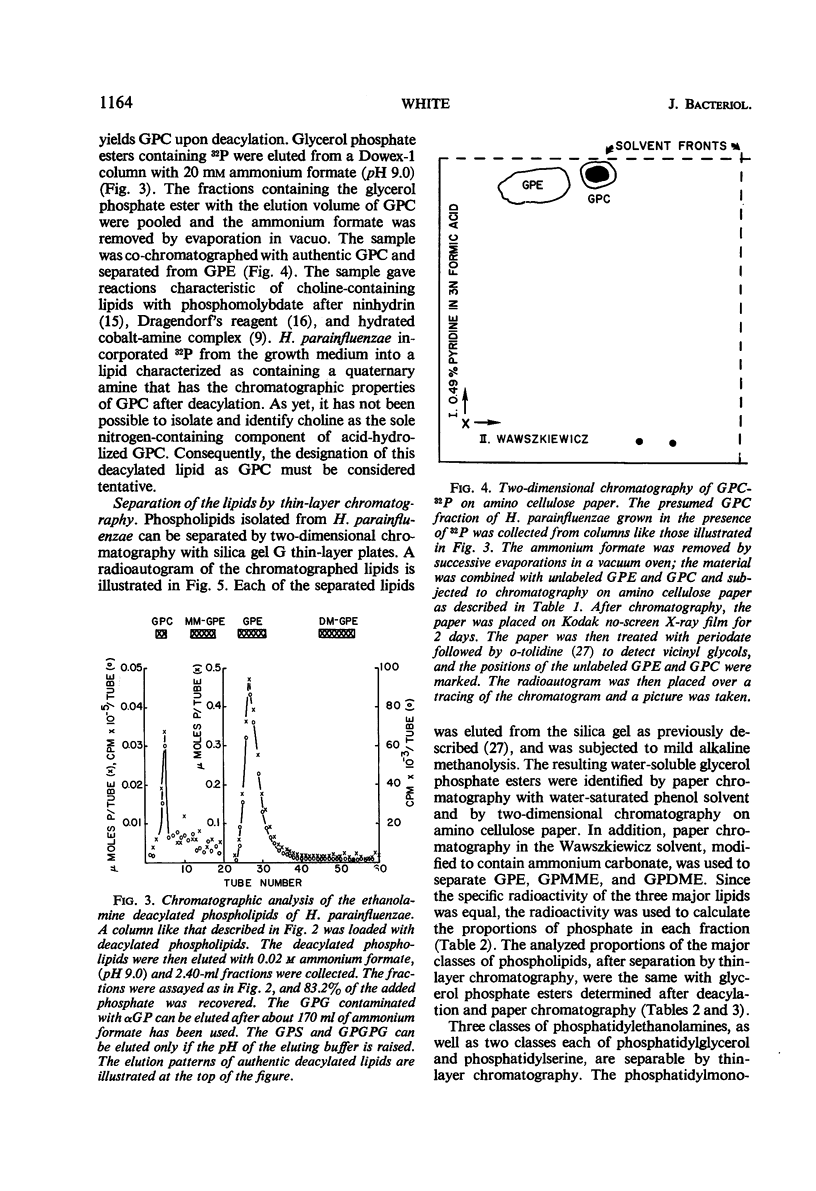
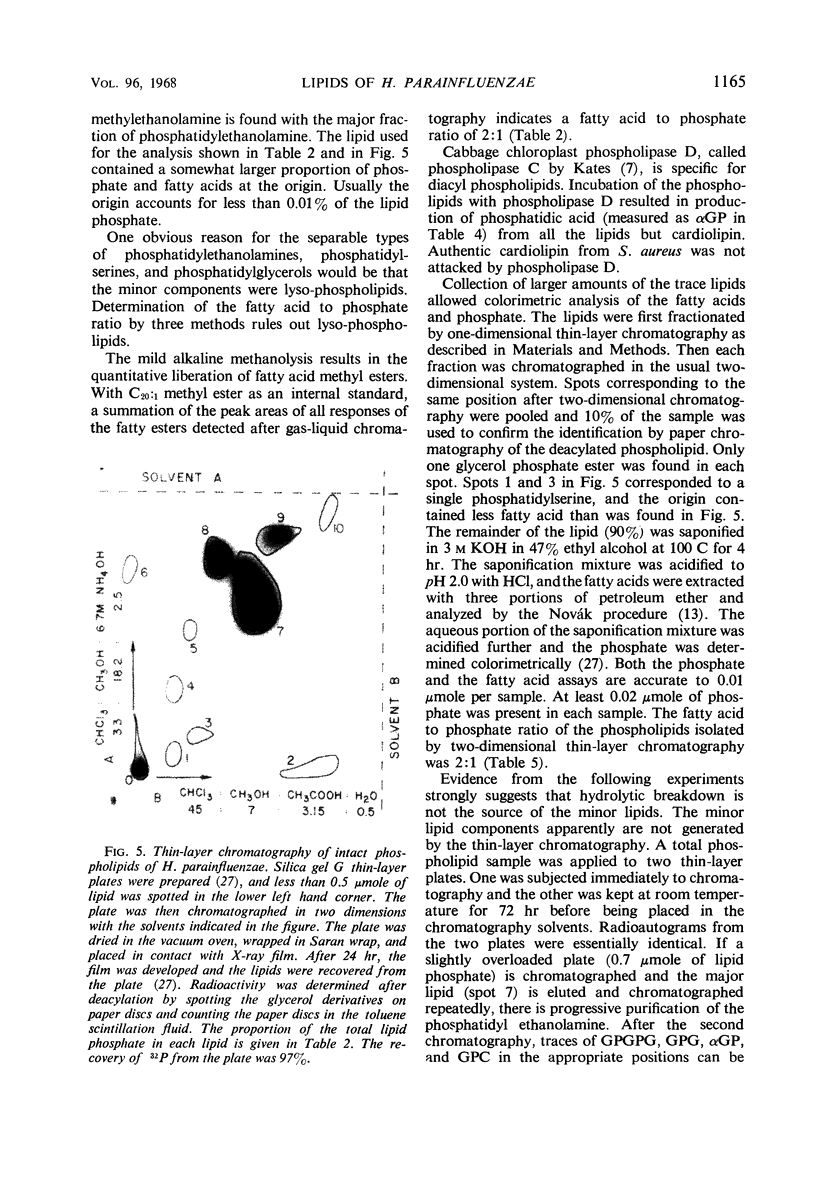
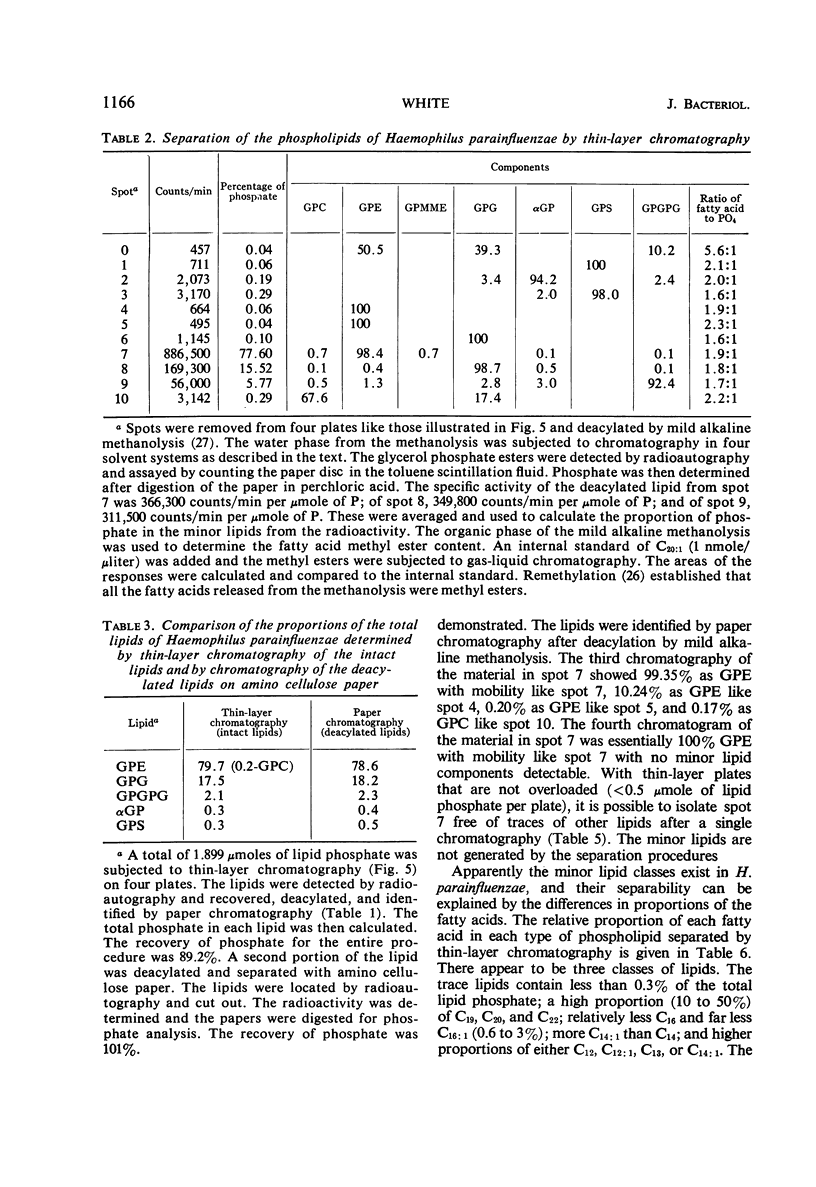
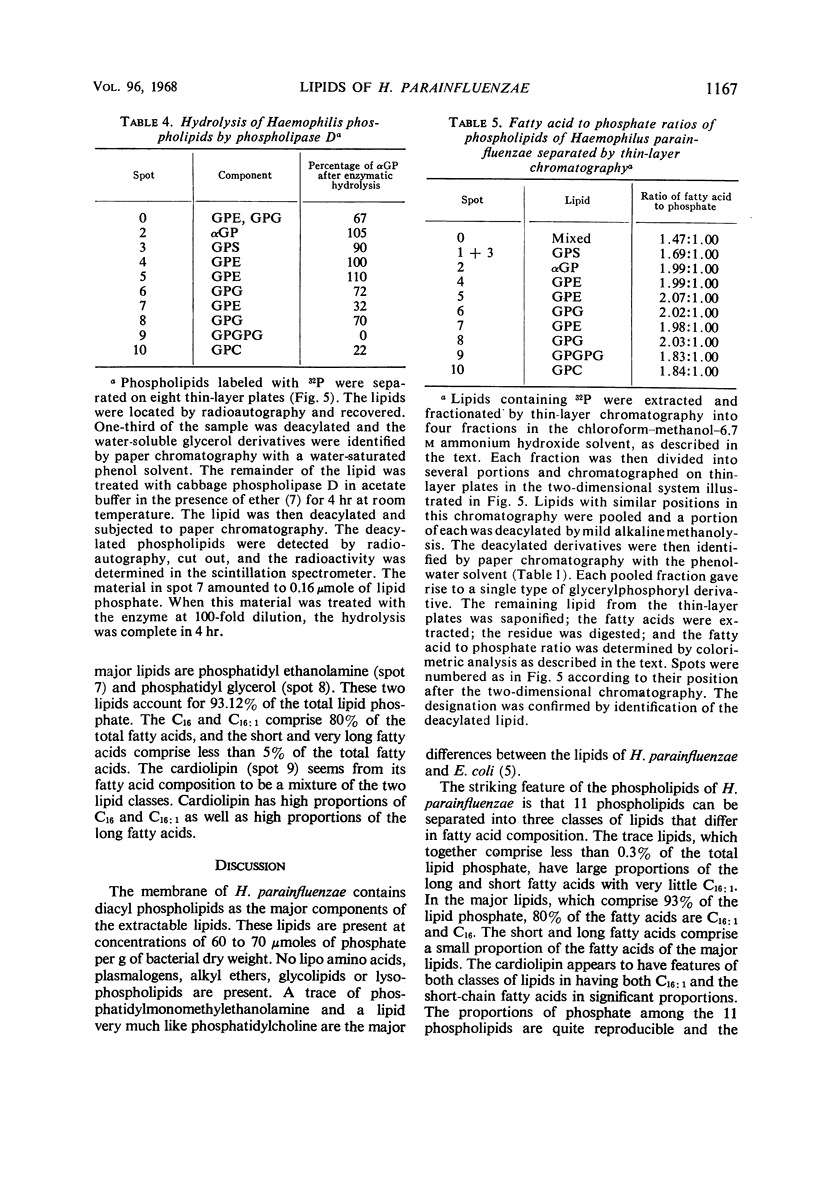
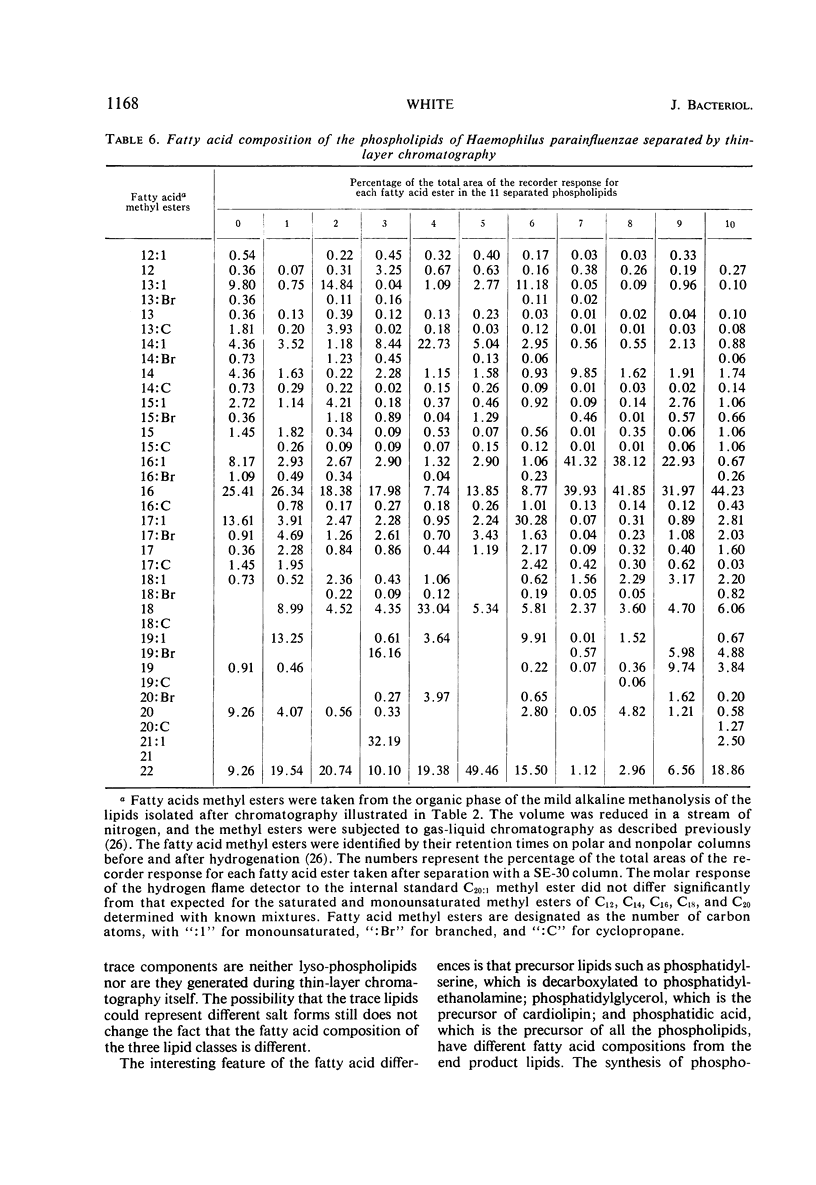
The principal lipids associated with the electron transport membrane of Haemophilus parainfluenzae are phosphatidylethanolamine (78%), phosphatidylmonomethylethanolamine (0.4%), phosphatidylglycerol (18%), phosphatidylcholine (0.4%), phosphatidylserine (0.4%), phosphatidic acid (0.2%), and cardiolipin (3.0%). Phospholipids account for 98.4% of the extractible fatty acids. There are no glycolipids, plasmalogens, alkyl ethers, or lipo amino acid esters in the membrane lipids. Glycerol phosphate esters derived from the phospholipids by mild alkaline methanolysis were identified by their staining reactions, mobility on paper and ion-exchange column chromatography, and by the molar glycerol to phosphate ratios. Eleven diacyl phospholipids can be separated by two-dimensional thin-layer chromatography. Each lipid served as a substrate for phospholipase D, and had a fatty acid to phosphate ratio of 2:1. Each separated diacyl phospholipid was deacylated and the glycerol phosphate ester was identified by paper chromatography in four solvent systems. Of the 11 separated phospholipids, 3 were phosphatidylethanolamines, 2 were phosphatidylserines, and 2 were phosphatidylglycerols. Phosphatidylcholine, cardiolipin, and phosphatidic acid were found at a single location. Phosphatidylmonomethylethanolamine was found with the major phosphatidylethanolamine. Three distinct classes of phospholipids are separable according to their relative fatty acid compositions. (i) The trace lipids consist of two phosphatidylethanolamines, two phosphatidylserines, phosphatidylcholine, phosphatidic acid, and a phosphatidylglycerol. Each lipid represents less than 0.3% of the total lipid phosphate. These lipids are characterized by high proportions of the short (C10 to C14) and long (C19 to C22) fatty acids with practically no palmitoleic acid. (ii) The major phospholipids (93% of the lipid phosphate) are phosphatidylethanolamine, phosphatidylmonomethylethanolamine, and phosphatidylglycerol. These lipids contain a low proportion of the short (<C14) and long (>C19) fatty acids. Palmitic and palmitoleic acids represent over 80% of the total fatty acids. (iii) The fatty acid composition of the cardiolipin is intermediate between the other two classes. Both palmitoleic and the longer fatty acids represent a significant proportion of the total fatty acid.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENSON A. A., MARUO B. Piant phospholipids. I. Identification of the phosphatidyl glycerols. Biochim Biophys Acta. 1958 Jan;27(1):189–195. doi: 10.1016/0006-3002(58)90308-1. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Hagen P. O., Goldfine H., Williams P. J. Phospholipids of bacteria with extensive intracytoplasmic membranes. Science. 1966 Mar 25;151(3717):1543–1544. doi: 10.1126/science.151.3717.1543. [DOI] [PubMed] [Google Scholar]
- KANFER J., KENNEDY E. P. METABOLISM AND FUNCTION OF BACTERIAL LIPIDS. II. BIOSYNTHESIS OF PHOSPHOLIPIDS IN ESCHERICHIA COLI. J Biol Chem. 1964 Jun;239:1720–1726. [PubMed] [Google Scholar]
- KATES M. Chromatographic and radioisotopic investigations of the lipid components of runner bean leaves. Biochim Biophys Acta. 1960 Jul 1;41:315–328. doi: 10.1016/0006-3002(60)90015-9. [DOI] [PubMed] [Google Scholar]
- KATES M. Hydrolysis of glycerolphosphatides by plastid phosphatidase C. Can J Biochem Physiol. 1956 Sep;34(5):967–980. [PubMed] [Google Scholar]
- Kanemasa Y., Akamatsu Y., Nojima S. Composition and turnover of the phospholipids in Escherichia coli. Biochim Biophys Acta. 1967 Oct 2;144(2):382–390. [PubMed] [Google Scholar]
- LANE E. S. THIN-LAYER CHROMATOGRAPHY OF LONG-CHAIN TERTIARY AMINES AND RELATED COMPOUNDS. J Chromatogr. 1965 May;18:426–430. doi: 10.1016/s0021-9673(01)80394-0. [DOI] [PubMed] [Google Scholar]
- Lester R. L., White D. C. Quantitative gas-liquid chromatographic analysis of ethanolamine, monomethyl ethanolamine, and dimethyl ethanolamine from lipids. J Lipid Res. 1967 Nov;8(6):565–568. [PubMed] [Google Scholar]
- NOVAK M. COLORIMETRIC ULTRAMICRO METHOD FOR THE DETERMINATION OF FREE FATTY ACIDS. J Lipid Res. 1965 Jul;6:431–433. [PubMed] [Google Scholar]
- Nichols B. W. Light induced changes in the lipids of Chlorella vulgaris. Biochim Biophys Acta. 1965 Oct 4;106(2):274–279. doi: 10.1016/0005-2760(65)90035-4. [DOI] [PubMed] [Google Scholar]
- Okuyama H., Kankura T., Nojima S. Positional distribution of fatty acids in phospholipids from Mycobacteria. J Biochem. 1967 Jun;61(6):732–737. doi: 10.1093/oxfordjournals.jbchem.a128607. [DOI] [PubMed] [Google Scholar]
- VORBECK M. L., MARINETTI G. V. SEPARATION OF GLYCOSYL DIGLYCERIDES FROM PHOSPHATIDES USING SILICIC ACID COLUMN CHROMATOGRAPHY. J Lipid Res. 1965 Jan;6:3–6. [PubMed] [Google Scholar]
- WELLS M. A., DITTMER J. C. THE USE OF SEPHADEX FOR THE REMOVAL OF NONLIPID CONTAMINANTS FROM LIPID EXTRACTS. Biochemistry. 1963 Nov-Dec;2:1259–1263. doi: 10.1021/bi00906a015. [DOI] [PubMed] [Google Scholar]
- WHITE D. C. Cytochrome and catalase patterns during growth of Haemophilus parainfluenzae. J Bacteriol. 1962 Apr;83:851–859. doi: 10.1128/jb.83.4.851-859.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE D. C. DIFFERENTIAL SYNTHESIS OF FIVE PRIMARY ELECTRON TRANSPORT DEHYDROGENASES IN HEMOPHILUS PARAINFLUENZAE. J Biol Chem. 1964 Jun;239:2055–2060. [PubMed] [Google Scholar]
- WHITE D. C., SMITH L. LOCALIZATION OF THE ENZYMES THAT CATALYZE HYDROGEN AND ELECTRON TRANSPORT IN HEMOPHILUS PARAINFLUENZAE AND THE NATURE OF THE RESPIRATORY CHAIN SYSTEM. J Biol Chem. 1964 Nov;239:3956–3963. [PubMed] [Google Scholar]
- WHITE D. C. SYNTHESIS OF 2-DEMETHYL VITAMIN K2 AND THE CYTOCHROME SYSTEM IN HAEMOPHILUS. J Bacteriol. 1965 Feb;89:299–305. doi: 10.1128/jb.89.2.299-305.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE D. C. THE FUNCTION OF 2-DEMETHYL VITAMIN K2 IN THE ELECTRON TRANSPORT SYSTEM OF HEMOPHILUS PARAINFLUENZAE. J Biol Chem. 1965 Mar;240:1387–1394. [PubMed] [Google Scholar]
- Wells M. A., Dittmer J. C. A microanalytical technique for the quantitative determination of twenty-four classes of brain lipids. Biochemistry. 1966 Nov;5(11):3405–3418. doi: 10.1021/bi00875a004. [DOI] [PubMed] [Google Scholar]
- White D. C., Cox R. H. Indentification and localization of the fatty acids in Haemophilus parainfluenzae. J Bacteriol. 1967 Mar;93(3):1079–1088. doi: 10.1128/jb.93.3.1079-1088.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C. Effect of glucose on the formation of the membrane-bound electron transport system in Haemophilus parainfluenzae. J Bacteriol. 1967 Feb;93(2):567–573. doi: 10.1128/jb.93.2.567-573.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C., Frerman F. E. Extraction, characterization, and cellular localization of the lipids of Staphylococcus aureus. J Bacteriol. 1967 Dec;94(6):1854–1867. doi: 10.1128/jb.94.6.1854-1867.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C., Frerman F. E. Fatty acid composition of the complex lipids of Staphylococcus aureus during the formation of the membrane-bound electron transport system. J Bacteriol. 1968 Jun;95(6):2198–2209. doi: 10.1128/jb.95.6.2198-2209.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C. The obligatory involvement of the electron transport system in the catabolic metabolism of Haemophilus parainfluenzae. Antonie Van Leeuwenhoek. 1966;32(2):139–158. doi: 10.1007/BF02097454. [DOI] [PubMed] [Google Scholar]
- Wood B. J., Nichols B. W., James A. T. The lipids and fatty acid metabolism of photosynthetic bacteria. Biochim Biophys Acta. 1965 Oct 4;106(2):261–273. doi: 10.1016/0005-2760(65)90034-2. [DOI] [PubMed] [Google Scholar]
- Wright E. A., White D. C. Formation of a functional electron transport system during growth of penicillin-induced spheroplasts of Haemophilus parainfluenzae. J Bacteriol. 1966 Mar;91(3):1356–1362. doi: 10.1128/jb.91.3.1356-1362.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]