Abstract
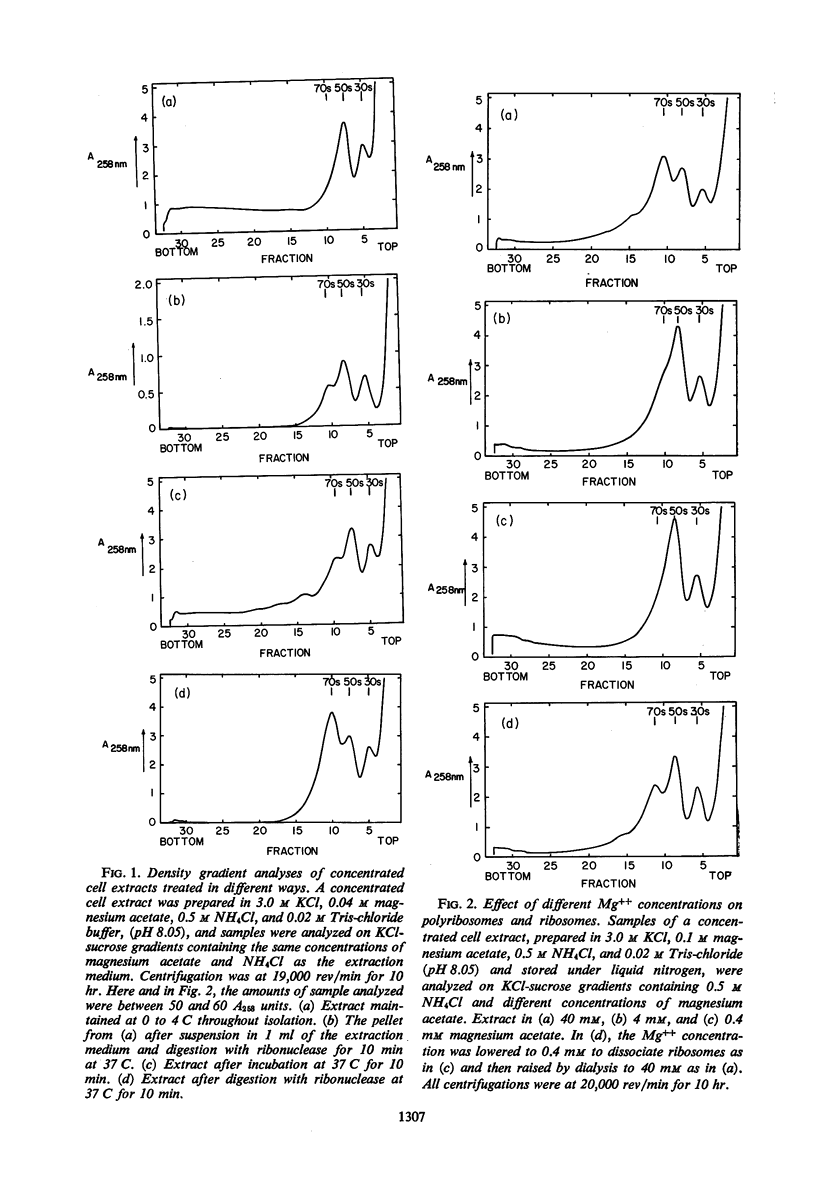
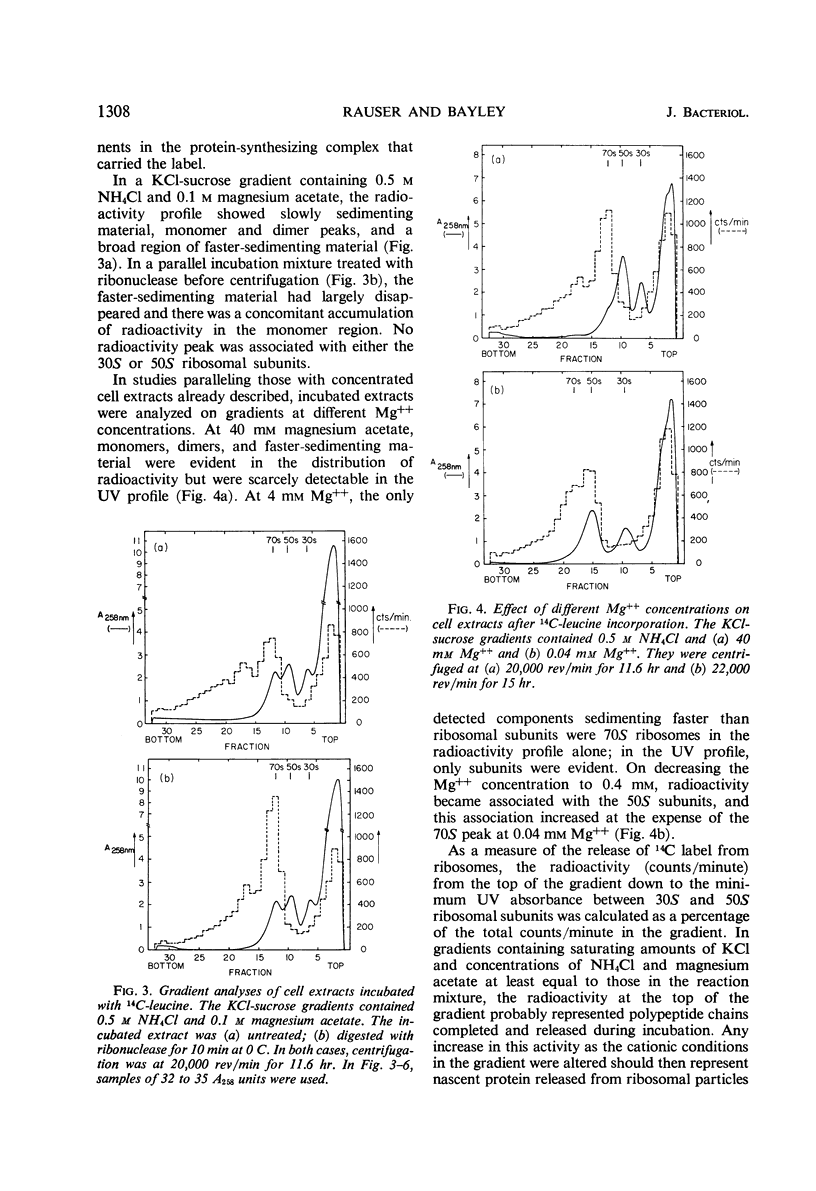
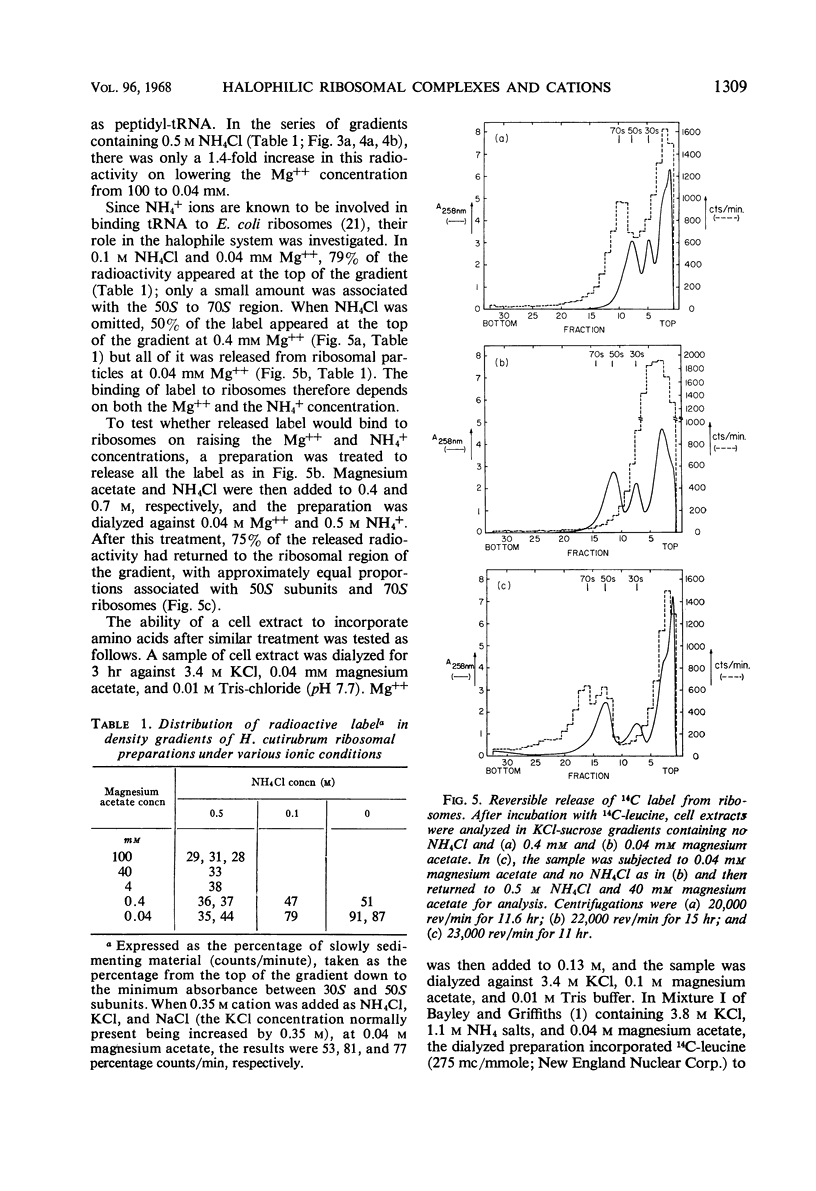
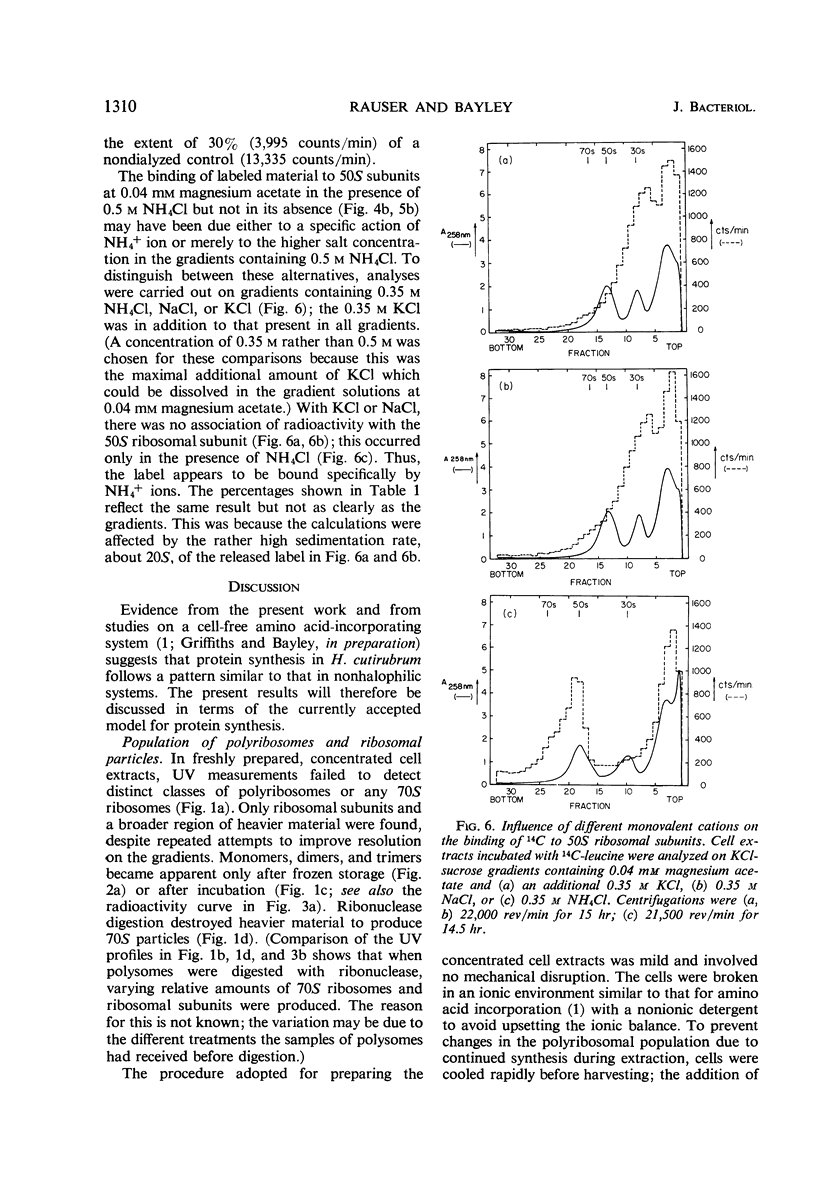
Concentrated extracts of Halobacterium cutirubrum were prepared at 0 C by gently disrupting cells with a nonionic detergent in a medium containing 3.0 m KCl, 0.5 m NH4Cl, and 0.04 m (or more) magnesium acetate and then treating the gelatinous mass with deoxyribonuclease. On KCl-sucrose gradients containing 0.5 m NH4Cl and 0.04 m magnesium acetate, these extracts showed 30S and 50S ribosomal subunits plus a flat profile of faster-sedimenting material up to high S values. Only after frozen storage or brief incubation of the extract were 70S ribosomes and distinct classes of small polyribosomes detected. Digestion with ribonuclease converted faster-sedimenting material to 70S particles. The presence of chloramphenicol during preparation of the extracts did not affect these results. The evidence suggests that ribosomal particles exist in these cells as subunits or as polyribosomes but not as 70S ribosomes. To investigate the function of Mg++ and NH4+ ions in ribosomal complexes from this halophile, concentrated cell extracts and extracts incubated with 14C-leucine were examined on KCl-sucrose gradients containing different concentrations of these ions. Polyribosomes and the bulk of 70S ribosomes dissociated reversibly to subunits at about 0.01 m Mg++, whereas a small fraction of the 70S particles, including those which in vitro incorporated 14C-leucine into nascent protein, dissociated only below 1 mm Mg++. Below this concentration of Mg++, nascent protein remained attached to the 50S subunit even at 0.04 mm Mg++ in the presence of 0.35 to 0.5 m NH4Cl. Nascent protein, presumably as peptidyl-transfer ribonucleic acid, dissociated reversibly from 50S subunits only at 0.04 mm Mg++ and 0.1 m or less NH4+. Thus, the stability of polyribosomes from H. cutirubrum depends specifically on both Mg++ and NH4+ ions.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAYLEY S. T., KUSHNER D. J. THE RIBOSOMES OF THE EXTREMELY HALOPHILIC BACTERIUM, HALOBACTERIUM CUTIRUBRUM. J Mol Biol. 1964 Sep;9:654–669. doi: 10.1016/s0022-2836(64)80173-x. [DOI] [PubMed] [Google Scholar]
- Bayley S. T., Griffiths E. A cell-free amino acid incorporating system from an extremely halophilic bacterium. Biochemistry. 1968 Jun;7(6):2249–2256. doi: 10.1021/bi00846a030. [DOI] [PubMed] [Google Scholar]
- Bayley S. T., Griffiths E. Codon assignments and fidelity of translation in a cell-free protein-synthesizing system from an extremely halophilic bacterium. Can J Biochem. 1968 Aug;46(8):937–944. doi: 10.1139/o68-140. [DOI] [PubMed] [Google Scholar]
- Flessel C. P., Ralph P., Rich A. Polyribosomes of growing bacteria. Science. 1967 Nov 3;158(3801):658–660. doi: 10.1126/science.158.3801.658. [DOI] [PubMed] [Google Scholar]
- GIERER A. Function of aggregated reticulocyte ribosomes in protein synthesis. J Mol Biol. 1963 Feb;6:148–157. doi: 10.1016/s0022-2836(63)80131-x. [DOI] [PubMed] [Google Scholar]
- GILBERT W. Polypeptide synthesis in Escherichia coli. I. Ribosomes and the active complex. J Mol Biol. 1963 May;6:374–388. doi: 10.1016/s0022-2836(63)80050-9. [DOI] [PubMed] [Google Scholar]
- GILBERT W. Polypeptide synthesis in Escherichia coli. II. The polypeptide chain and S-RNA. J Mol Biol. 1963 May;6:389–403. doi: 10.1016/s0022-2836(63)80051-0. [DOI] [PubMed] [Google Scholar]
- Ghosh H. P., Khorana H. G. Studies on polynucleotides, LXXXIV. On the role of ribosomal subunits in protein synthesis. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2455–2461. doi: 10.1073/pnas.58.6.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIHO Y., RICH A. INDUCED ENZYME FORMED ON BACTERIAL POLYRIBOSOMES. Proc Natl Acad Sci U S A. 1964 Jan;51:111–118. doi: 10.1073/pnas.51.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiarotti G., Schlessinger D. Polyribosome metabolism in Escherichia coli. I. Extraction of polyribosomes and ribosomal subunits from fragile, growing Escherichia coli. J Mol Biol. 1966 Sep;20(1):123–143. doi: 10.1016/0022-2836(66)90122-7. [DOI] [PubMed] [Google Scholar]
- Nomura M., Lowry C. V., Guthrie C. The initiation of protein synthesis: joining of the 50S ribosomal subunit to the initiation complex. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1487–1493. doi: 10.1073/pnas.58.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M., Lowry C. V. PHAGE f2 RNA-DIRECTED BINDING OF FORMYLMETHIONYL-TRNA TO RIBOSOMES AND THE ROLE OF 30S RIBOSOMAL SUBUNITS IN INITIATION OF PROTEIN SYNTHESIS. Proc Natl Acad Sci U S A. 1967 Sep;58(3):946–953. doi: 10.1073/pnas.58.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKAMOTO T., TAKANAMI M. INTERACTION OF RIBOSOMES AND NATURAL POLYRIBONUCLEOTIDES. Biochim Biophys Acta. 1963 Oct 15;76:266–274. [PubMed] [Google Scholar]
- Ron E. Z., Kohler R. E., Davis B. D. Polysomes extracted from Escherichia coli by freeze-thaw-lysozyme lysis. Science. 1966 Sep 2;153(3740):1119–1120. doi: 10.1126/science.153.3740.1119. [DOI] [PubMed] [Google Scholar]
- SCHLESSINGER D., GROS F. STRUCTURE AND PROPERTIES OF ACTIVE RIBOSOMES OF ESCHERICHIA COLI. J Mol Biol. 1963 Oct;7:350–359. doi: 10.1016/s0022-2836(63)80029-7. [DOI] [PubMed] [Google Scholar]
- SEHGAL S. N., GIBBONS N. E. Effect of some metal ions on the growth of Halobacterium cutirubrum. Can J Microbiol. 1960 Apr;6:165–169. doi: 10.1139/m60-018. [DOI] [PubMed] [Google Scholar]
- SPYRIDES G. J. THE EFFECT OF UNIVALENT CATIONS ON THE BINDING OF SRNA TO THE TEMPLATE-RIBOSOME COMPLEX. Proc Natl Acad Sci U S A. 1964 Jun;51:1220–1226. doi: 10.1073/pnas.51.6.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAEHELIN T., BRINTON C. C., WETTSTEIN F. O., NOLL H. STRUCTURE AND FUNCTION OF E. COLI ERGOSOMES. Nature. 1963 Aug 31;199:865–870. doi: 10.1038/199865a0. [DOI] [PubMed] [Google Scholar]
- Schlessinger D., Mangiarotti G., Apirion D. The formation and stabilization of 30S and 50S ribosome couples in Escherichia coli. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1782–1789. doi: 10.1073/pnas.58.4.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARNER J. R., KNOPF P. M., RICH A. A multiple ribosomal structure in protein synthesis. Proc Natl Acad Sci U S A. 1963 Jan 15;49:122–129. doi: 10.1073/pnas.49.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WETTSTEIN F. O., STAEHELIN T., NOLL H. Ribosomal aggregate engaged in protein synthesis: characterization of the ergosome. Nature. 1963 Feb 2;197:430–435. doi: 10.1038/197430a0. [DOI] [PubMed] [Google Scholar]


