Abstract
Posttranslational modification of Rab proteins by geranylgeranyltransferase type II requires that they first bind to Rab escort protein (REP). Following prenylation, REP is postulated to accompany the modified GTPase to its specific target membrane. REP binds preferentially to Rab proteins that are in the GDP state, but the specific structural domains involved in this interaction have not been defined. In p21 Ras, the α2 helix of the Switch 2 domain undergoes a major conformational change upon GTP hydrolysis. Therefore, we hypothesized that the corresponding region in Rab1B might play a key role in the interaction with REP. Introduction of amino acid substitutions (I73N, Y78D, and A81D) into the putative α2 helix of Myc-tagged Rab1B prevented prenylation of the recombinant protein in cell-free assays, whereas mutations in the α3 and α4 helices did not. Additionally, upon transient expression in transfected HEK-293 cells, the Myc-Rab1B α2 helix mutants were not efficiently prenylated as determined by incorporation of [3H]mevalonate. Metabolic labeling studies using [32P]orthophosphate indicated that the poor prenylation of the Rab1B α2 helix mutants was not directly correlated with major disruptions in guanine nucleotide binding or intrinsic GTPase activity. Finally, gel filtration analysis of cytosolic fractions from 293 cells that were coexpressing T7 epitope-tagged REP with various Myc-Rab1B constructs revealed that mutations in the α2 helix of Rab1B prevented the association of nascent (i.e., nonprenylated) Rab1B with REP. These data indicate that the Switch 2 domain of Rab1B is a key structural determinant for REP interaction and that nucleotide-dependent conformational changes in this region are largely responsible for the selective interaction of REP with the GDP-bound form of the Rab substrate.
INTRODUCTION
Small Ras-related GTP-binding proteins encoded by the rab genes participate in the regulation of vesicular transport within specific segments of the exocytic and endocytic pathways in mammalian cells. More than 30 different Rab proteins have been identified, and many of these have been localized to discrete subcellular compartments (Simons and Zerial, 1993; Pfeffer, 1994). Although the exact role of Rab proteins in the vesicular transport machinery has not been completely defined, a widely accepted model envisions that each Rab protein cycles on and off distinct donor and acceptor membranes in conjunction with changes in its guanine nucleotide state (Novick and Brennwald, 1993; Nuoffer and Balch, 1994). According to this view, the cycle begins with the inactive GDP-bound form of the Rab protein residing in a cytoplasmic complex with a carrier protein termed Rab GDP-dissociation inhibitor (GDI). GDI delivers the Rab protein to a budding transport vesicle, whereupon GDI is released through the action of a GDI displacement factor (Dirac-Svejestrup et al., 1997) and exchange of GTP for GDP is stimulated by a Rab-specific guanine nucleotide exchange factor (Horiuchi et al., 1995; Wada et al., 1997). The activated Rab protein remains associated with the transport vesicle and facilitates docking and fusion with the correct acceptor compartment, possibly through interaction with the SNARE complex (Sogaard et al., 1994). GTP hydrolysis occurs in concert with the latter events, returning the Rab protein to the inactive GDP-bound state. Finally, the GDP-bound Rab protein is removed from the acceptor membrane by GDI and becomes available for another round of vesicle transport.
Posttranslational modification of Rab carboxyl-terminal cysteines by one or two 20-carbon geranylgeranyl groups (i.e, prenylation) is critical for at least two aspects of the foregoing functional cycle: association of Rab proteins with cellular membranes (Khosravi-Far et al., 1991; Overmeyer and Maltese, 1992) and formation of a stable complex with GDI (Musha et al., 1992; Soldati et al., 1993; Wilson et al., 1996a). The prenylation of Rab proteins is catalyzed by geranylgeranyltransferase type II (GGTaseII), which can modify Rab proteins ending with a variety of carboxyl-terminal cysteine motifs (e.g., XCXC, XXCC, CCXX, where C = cysteine and X = any amino acid) (Farnsworth et al., 1994). GGTaseII was originally identified as “component B” of the Rab geranylgeranyltransferase complex (Seabra et al., 1992b). The enzyme is a heterodimer, consisting of α and β subunits (Armstrong et al., 1993). In this respect GGTaseII resembles farnesyltransferase (FTase) and geranylgeranyltransferase type I (GGTaseI), which modify different members of the Ras superfamily that end with CAAX sequence motifs (C = cysteine, A = aliphatic residue, X = any amino acid), e.g., Ras, Rac, Rap, and Rho (Casey and Seabra, 1996). However, early studies revealed several unique features of the Rab prenylation mechanism. Specifically, although the presence of a carboxyl-terminal CAAX sequence element is sufficient for prenylation of most Ras-related substrates by FTase and GGTaseI (Casey et al., 1991; Goldstein et al., 1991; Reiss et al., 1991; Yokoyama et al., 1991; Yokoyama and Gelb, 1993; Zhang et al., 1994), the modification of Rab proteins by GGTaseII is sensitive to alterations in structural domains remote from the carboxyl-terminal prenylation site (Moores et al., 1991; Kinsella and Maltese, 1992; Khosravi-Far et al., 1992; Wilson and Maltese, 1993; Sanford et al., 1993; Beranger et al., 1994). Most notably, prenylation of Rab substrates requires that they reside in a particular guanine nucleotide state, i.e., the GDP conformation is preferred (Sanford et al., 1993; Schiedel et al., 1995; Wilson et al., 1996b). An explanation for the unique structural requirements for Rab prenylation has come with the discovery that, unlike FTase and GGTaseI, the catalytic αβ dimer of GGTaseII is unable to modify monomeric GTPase substrates. Instead, the Rab protein must first bind to a carrier protein termed Rab escort protein (REP, originally called “component A”) to form a viable substrate complex (Seabra et al., 1992a; Andres et al., 1993). Indeed, a recent study has established that the nucleotide sensitivity of the Rab prenylation reaction is directly related to the preferential interaction of REP with Rab proteins in the GDP state (Seabra, 1996). Upon completion of the prenylation reaction, REP remains stably associated with geranylgeranylated Rab proteins in vitro, suggesting that REP may serve as a molecular chaperone which delivers newly modified Rab proteins to their appropriate membrane target sites in vivo (Alexandrov et al., 1994; Shen and Seabra, 1996). Consistent with this role, the two known mammalian REPs contain several domains that are similar to regions of Rab GDI (Schalk et al., 1996).
The specific structural elements involved in the interaction of nascent Rab proteins with REP have not been fully defined, although previous studies have shown that the efficiency of Rab prenylation in cell-free assays is substantially reduced by alterations in three distinct regions: 1) the extreme amino-terminal domain (Wilson and Maltese, 1993; Sanford et al., 1995); 2) the region analogous to the Ras effector-domain (loop-2/β2) (Wilson and Maltese, 1993); and 3) the region corresponding to loop-3/β3 in the Ras structure (Beranger et al., 1994). The basis for the reduced prenylation of these altered proteins is not known, although a recent study utilizing the yeast two-hybrid assay has suggested that the amino-terminal and effector regions of Rab3A may interact with the β-subunit of GGTaseII (Johannes et al., 1996). The preferential binding of REP to Rab proteins in the GDP state suggests that this association involves Rab structural elements that undergo major conformational changes when the nucleotide binding site is occupied by GDP versus GTP. In H-Ras two specific domains termed Switch 1 (amino acids 32–40) and Switch 2(amino acids 60–76) fit this description (Krengel et al., 1990; Pai et al., 1990; Tong et al., 1991). Both regions play important roles in the interaction of Ras with downstream effectors (Polakis and McCormick, 1993; Moodie et al., 1995). However, the α2 helix of the Switch 2 region appears to be particularly important for the association of Ras-GDP with guanine nucleotide exchange factors (Crechet et al., 1996; Quilliam et al., 1996). These observations prompted us to examine the role of the putative Switch 2 domain of Rab1B in REP-dependent posttranslational modification by GGTaseII. The results described in this report indicate that the region of Rab1B analogous to the α2 helix of Ras is a key structural determinant for interaction with REP.
MATERIALS AND METHODS
Mutagenesis of Rab1B
The cDNAs encoding Myc-Rab1B(wt), Myc-Rab1B(ΔCC), which lacks the C-terminal cysteines, and Myc-Rab1B(Q67L) were generated as described previously (Wilson et al., 1996b). As noted earlier (Wilson and Maltese, 1993), the Rab1B(wt) cDNA sequence used in our studies differs from that originally reported by Vielh et al. (1989) insofar as it encodes isoleucine rather than valine at position 73. New point mutations were introduced into the Rab1B cDNA by means of the polymerase chain reaction utilizing Taq DNA polymerase (Perkin Elmer-Cetus, Norwalk, CT) (I73N, Y78D, A81D, and A110D) or Deep Vent polymerase (New England Biolabs, Beverly, MA) (L103R, K137E, and G144N). All constructs were subsequently modified to encode a 5′ Myc-epitope tag sequence and subcloned into pCMV5neo (Krupinski et al., 1992) for mammalian expression studies or pET17b (Novagen, Madison, WI) for expression in Escherichia coli. The sequences of all constructs were verified by the dideoxy chain termination technique using Sequenase 2.0 (United States Biochemical Corp., Cleveland, OH).
Geranylgeranylation of Recombinant Myc-Rab1B
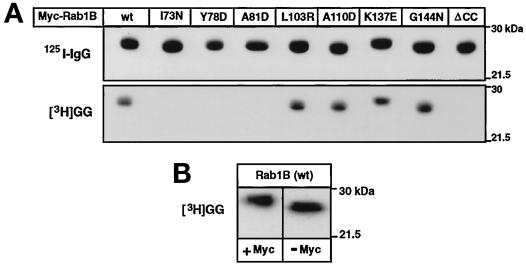
Rab proteins were expressed in E. coli as previously described and the relative amounts of Myc-Rab1B(wt), Myc-Rab1B(I73N), Myc-Rab1B(Y78D), Myc-Rab1B(A81D), Myc-Rab1B(L103R), Myc-Rab1B(A110D), Myc-Rab1B(K137E), Myc-Rab1B(G144N), and Myc-Rab1B(ΔCC) in each lysate were determined by immunoblot analysis using a primary antibody against the Myc epitope (EQKLISEEDL) and 125I-labeled goat anti-mouse IgG secondary antibody (Wilson et al., 1996a). Bound 125I-labeled IgG was quantified by phosphorimager analysis. For cell-free prenylation of the recombinant proteins, aliquots of E. coli lysate containing comparable amounts (20–30 pmol) of each Myc-Rab1B protein were added to 50-μl reaction mixtures containing 50 mM HEPES (pH 7.4), 5 mM MgCl2, 1 mM dithiothreitol, 10 μM GDP, 0.2 mM Nonidet P-40, and 10 μl of a rat brain ammonium sulfate fraction enriched in Rab geranylgeranyl transferase activity (Seabra et al., 1992b). Prenylation reactions were initiated by the addition of 1 μCi of [3H]geranylgeranyl pyrophosphate (15 Ci/mmol, American Radiochemical Corp., St. Louis, MO) and terminated after 1 h at 37°C by addition of SDS-sample buffer (Laemmli, 1970). Each reaction mixture was divided into two equal portions that were subjected to SDS-PAGE on separate gels. The first gel was dried and incorporation of [3H]geranylgeranyl (GG moiety) into Myc-Rab1B was determined by fluorography (Kinsella and Maltese, 1992). Proteins in the second gel were transferred to polyvinylidene difluoride (PVDF) membrane and the relative amount of Myc-Rab1B was determined by immunoblot analysis as described earlier. Results were quantified by densitometric analysis with a Molecular Dynamics personal densitometer and ImageQuant software.
Prenylation of Myc-Rab1B Constructs in Cultured Cells
HEK-293 cells were obtained from the American Type Culture Collection (Bethesda, MD) and grown in DMEM supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum (FBS). Cells were plated in 100-mm dishes at a density of 3.2 × 103 cells/cm2 on the day before transfection. A calcium phosphate precipitation technique (Gorman et al., 1990) was used to transfect parallel cultures with 40 μg of each of the following vectors: pCMVrab1B(wt), pCMVrab1B(Q67L), pCMVrab1B(I73N), pCMVrab1B(Y78D), pCMVrab1B(A81D), pCMVrab1B(A110D), pCMVrab1B(L103R), pCMVrab1B(K137L), pCMVrab1B(G144N), or pCMVrab1B(ΔCC). The transfected cells were incubated for 18 h in medium containing 200 μCi/ml [3H]mevalonic acid lactone (MVA; approximately 3.4 Ci/mmol) and 10 μM lovastatin (provided by A. Alberts, Merck Sharp and Dohme Research Laboratories, Rahway, NJ). Detailed procedures for cell lysis and measurement of the incorporation of [3H]MVA into immunoprecipitated Myc-tagged Rab proteins have been described previously (Wilson et al., 1996b).
Assessment of the Guanine Nucleotide State of Myc-Rab1B Expressed in Cultured Cells
The guanine nucleotide content of various Myc-Rab1B constructs expressed in 293 cells was determined by a modification of the procedure described by Brondyk et al. (1993). On the day before transfection, cells were plated in 60-mm dishes at a density of 1.8 × 104 cells/cm2. Separate cultures were transfected with 10 μg pCMVrab1B(wt), pCMVrab1B(I73N), pCMVrab1B(Y78D), pCMVrab1B(A81D), pCMVrab1B(A110D), or pCMVrab1B(Q67L). During the transfection, the cells were maintained in DMEM with 2% (vol/vol) heat-inactivated FBS and 10 μM lovastatin. Three hours after addition of the DNA, the cells were shocked with 15% (vol/vol) glycerol in PBS for 30 s and then fed with fresh DMEM containing 10% FBS and 10 μM lovastatin. One day after transfection, each culture was incubated for 5 h with 100 μCi/ml [32P]orthophosphate (9000 Ci/mmol, New England Nuclear) in phosphate-free DMEM (Life Technologies, Gaithersburg, MD) supplemented with 10% heat-inactivated FBS and 10 μM lovastatin. The cells were washed three times with Hanks’ balanced salt solution and disrupted in 200 μl ice-cold lysis buffer consisting of 20 mM HEPES (pH 7.3), 20 mM MgCl2, 150 mM NaCl, 0.75% vol/vol Nonidet P-40, complete mini-EDTA-free protease inhibitors (Boehringer Mannheim, Indianapolis, IN). All subsequent steps were carried out at 4°C. Particulate material was removed by centrifugation at 10,000 × g for 2 min and the epitope-tagged Rab1B proteins were immunoprecipitated with Myc monoclonal antibody for 1 h. To reduce the free guanine nucleotides, 100 μl of 10% (wt/vol) activated charcoal in phosphate-buffered saline were added to each sample (Gibbs et al., 1990). The charcoal had been previously incubated with 10 mg/ml bovine serum albumin and washed three times with phosphate-buffered saline. After a 30-min incubation, the charcoal was removed by centrifugation at 10,000 × g for 2 min, and immune complexes were collected by incubation for 1 h with protein A-Sepharose beads coupled to goat anti-mouse IgG. The beads were washed five times with lysis buffer and 32P-labeled guanine nucleotides were eluted by incubation for 20 min at 68°C in 20 μl of elution buffer (20 mM HEPES, pH 7.3, 25 mM EDTA, 2.0% wt/vol SDS, 0.5 mM GDP, 0.5 mM GTP). A 5-μl aliquot of each eluate was subjected to TLC on polyethyleneimine cellulose plates (Sigma, St. Louis, MO) developed in 0.75 M KH2PO4 (pH 3.4). Radioactivity in the GTP and GDP spots was quantified with a Molecular Dynamics phosphorimaging system. In calculating GDP:GTP ratios, the phosphorimager units of GDP were multiplied by 1.5 to correct for moles of phosphate/mole guanosine in GDP versus GTP. Since the intrinsic GTPase activities of the various Myc-Rab1B constructs at 4°C were unknown, no attempt was made to correct for GTP hydrolysis that might have occurred during the immunoprecipitation procedure. The relative amount of Myc-Rab1B in each sample was estimated by subjecting 10 μl of the eluate to SDS-PAGE and immunoblot analysis using the anti-Myc antibody and the ECL (Amersham, Arlington Heights, IL) detection system.
Association of T7-REP1 and Myc-Rab1B in Cultured Cells
The cDNA encoding rat REP-1 (Andres et al., 1993) was provided by Joseph Goldstein and Miguel Seabra (University of Texas Southwestern Medical Center, Dallas, TX). A HindIII–BamHI fragment containing the REP-1 coding sequence was excised from pBlueScript and inserted into a modified version of pCMV5 (Andersson et al., 1989) that had been engineered to encode an in-frame T7 epitope tag (amino acid sequence, MASMTGGQQMG) upstream of the HindIII site. The resulting construct is termed pCMVREP1.
HEK-293 cells were plated in 100-mm dishes at 2.8 × 103 cells/cm2 the day before transfection. Cells were cotransfected with 15 μg of pCMVREP1 combined with 20 μg of pCMVrab1B(wt), pCMVrab1B(I73N), pCMVrab1B(Y78D), pCMVrab1B(A81D), pCMVrab1B(A110D), or pCMVrab1B(Q67L) and cultures were maintained in medium containing 10 μM lovastatin throughout the posttransfection period. Two days after transfection, cells were washed three times with Hanks’ balanced salt solution, harvested, then homogenized in buffer A (50 mM HEPES, pH 7.4, 5 mM MgCl2, 1 mM dithiothreitol, and complete mini-EDTA-free protease inhibitor cocktail). Nuclei and unbroken cells were removed by centrifugation at 400 × g for 10 min. The supernatant solution was centrifuged at 100,000 × g for 30 min, and the resulting cytosolic fraction was applied to an FPLC Superose-12 gel filtration column (Pharmacia, Pistcataway, NJ) that had been equilibrated in buffer A at a flow rate of 0.5 ml/min and calibrated with appropriate standard proteins (Sigma). The column was eluted with the same buffer and 0.2-ml fractions were collected on ice. Aliquots (100 μl) were removed from each fraction and the proteins were resolved by SDS-PAGE and transferred to a PVDF membrane. The upper half of the blot was incubated with a monoclonal antibody against the T7 epitope (Novagen) to detect the presence of T7-REP in the column fractions, whereas the lower half of the blot was incubated with the monoclonal antibody against the Myc epitope to detect Myc-Rab1B. 125I-labeled goat anti-mouse IgG was applied as the secondary antibody and bound IgG was quantified with a Molecular Dynamics phosphorimager.
Triton X-114 Partitioning of Rab Proteins
Column fractions containing immunodetectable Myc-Rab1B protein that coeluted with T7-REP at approximately 150 kDa were combined to form pool-1, whereas fractions containing Myc-Rab1B that eluted as a monomer at the position of the 29-kDa marker were combined to form pool-2. Triton X-114 was added to each pool to a final concentration of 1% (vol/vol). The samples were kept on ice for 10 min and then warmed to 37°C for 2 min. The aqueous and detergent phases were separated by centrifugation in a microfuge at top speed for 2 min. Proteins from each phase were collected by precipitation with trichloroacetic acid, solubilized in SDS sample buffer, resolved by SDS-PAGE, and transferred to a PVDF membrane. The relative amounts of Myc-Rab1B in the aqueous and detergent fractions was then determined by immunoblot analysis using the anti-Myc antibody and 125I-labeled IgG for detection.
RESULTS
Effects of Mutations in the Switch 2 Region of Rab1B on Geranylgeranylation of the Recombinant Protein
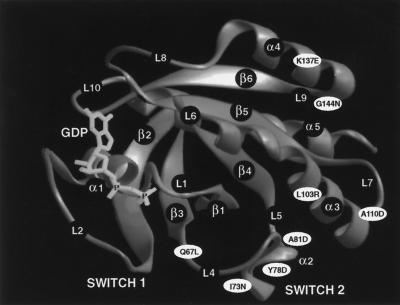
The three-dimensional (3-D) structure of H-Ras has been determined and the domains that undergo guanine nucleotide-dependent conformational changes have been mapped (Krengel et al. 1990; Pai et al. 1990; Tong et al. 1991; Polakis and McCormick, 1993). Most of the general structural features in Ras are conserved in other low molecular weight GTP-binding proteins (Bourne et al. 1991; Valencia et al. 1991). Therefore, the Ras structure has been widely used as a prototype to aid in the selection of sites for mutagenesis in structure-function studies of Rab proteins (Burstein et al., 1992; Tisdale et al., 1992; Li and Stahl, 1993; Stenmark et al., 1994). To assess the role of the Switch 2 domain of Rab1B in the interaction with REP/GGTaseII, we created three different amino acid substitutions in the region predicted to correspond to the α2 helix of Ras: I73N, Y78D, and A81D. For purposes of comparison, mutations were also introduced into regions corresponding to the α3 helix (L103R, A110D) and the α4 helix (K137E, G144N). In Figure 1 these Rab1B amino acid substitutions are superimposed on the Swiss 3-D image of H-Ras (GDP-bound form) to illustrate their approximate locations. To facilitate immunoprecipitation analyses of Rab1B proteins expressed in intact cells, all of the rab1B cDNA constructs were engineered to encode a Myc epitope tag at the amino terminus of the protein. The addition of such tag sequences to Ras-related proteins, including several members of the Rab family, does not alter their subcellular localization (Adamson et al., 1992; Brondyk et al., 1993; Chen et al., 1993; Beranger et al., 1994). Moreover, consistent with previous studies of Rab6 (Schiedel et al., 1995), we have observed that the presence of the Myc tag does not interfere with the geranylgeranylation of recombinant Rab1B (Figure 2B).
Figure 1.
Approximate locations of amino acid substitutions introduced into Rab1B. The GIF image (S3D00503) of the 3-D structure of p21 H-Ras (GDP-bound form) was downloaded from the Swiss Prot 3-D database. Approximate positions of the various amino acid substitutions in Rab1B were deduced by sequence alignments as described by Valencia et al. (1991).
Figure 2.
Geranylgeranylation of recombinant Myc-Rab1B. (A) Aliquots of E. coli lysate containing 20–30 pmol of each of the indicated recombinant Myc-tagged Rab1B proteins were geranylgeranylated in cell-free reactions as described in MATERIALS AND METHODS. The top portion shows an autoradiograph of the 125I-labeled immunoblot (18-h exposure) containing half of each prenylation reaction, confirming that comparable amounts of Myc-Rab1B were present in the assays. The bottom portion shows the corresponding fluorograph (24-h exposure) of the other half of the reaction used for determination of [3H]GG incorporation. The 3H:125I ratios determined for each Rab1B construct are summarized in Table 1. (B) Aliquots of E. coli lysate containing approximately 10 pmol of recombinant Rab1B expressed with or without the amino-terminal Myc epitope tag were geranylgeranylated in parallel reactions and the proteins were subjected to SDS-PAGE and fluorography (72-h exposure).
The Rab1B mutants described above were initially screened for their ability to undergo geranylgeranylation in cell-free assays. When equivalent amounts of recombinant Myc-tagged protein were compared in parallel assays, there was no detectable incorporation of [3H]GG into the three α2 helix mutants, whereas geranylgeranylation of the α3 and α4 helix mutants was similar to wild-type Myc-Rab1B (Figure 2A and Table 1).
Table 1.
Summary of the effects of various point mutations on Rab1B prenylation and REP interaction
| Rab1B Protein | Prenylation In vitroa | Prenylation In vivob | Protein-bound GDP/GTPc | REP association In vivod |
|---|---|---|---|---|
| wt | 100 | 100 | 18.0, 12.0 | + |
| Q67L | N.D.e | 111 | 0.8 | + |
| I73N | <1 | 7 | 1.0 | − |
| Y78D | 2 | <1 | 5.0 | − |
| A81D | <1 | 11 | 9.5 | − |
| L103R | 104 | 183 | N.D.f | N.D. |
| A110D | 124 | 232 | 2.9 | N.D. |
| K137E | 112 | 63 | N.D. | N.D. |
| G144N | 120 | 118 | N.D. | N.D. |
Ratio of units [3H]GG incorporated/units bound 125I-labeled IgG, expressed as percentage of the wild-type value (from Figure 2A).
Ratio of units [3H]MVA incorporated/units bound 125I-labeled IgG, expressed as percentage of the matching wild-type value (from Figure 3).
Ratio of 32P-labeled guanine nucleotides eluted from immunoprecipitated proteins (from Figure 4). Where proteins were analyzed in separate experiments, values are aligned in columns under the matching wild-type controls. Ratios are the means of two separate determinations from parallel cultures, although only one autoradiogram from each pair is shown in Figure 4.
Ability of overexpressed nonprenylated protein to associate with overexpressed REP in transfected cells (from Figure 7).
Not determined in the present study, but in a previous study (Wilson et al., 1996b) the Q67L mutant was prenylated in vitro as efficiently as wild-type Rab1B.
N.D., not determined.
Effects of Mutations in the Switch 2 Region on Prenylation of Rab1B in HEK-293 Cells
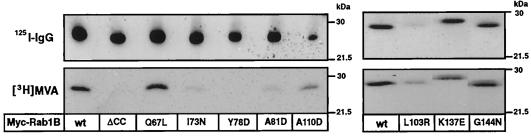
The results of cell-free geranylgeranyltransferase assays may not always accurately reflect the ability of Rab proteins to undergo prenylation in mammalian cells (Wilson et al., 1996ab), where the conformation and/or guanine nucleotide state of the nascent polypeptide may be influenced by undefined molecular chaperones, nucleotide exchange factors, or GTPase-activating proteins. Therefore, the Rab1B α2 helix mutants were further evaluated as GGTaseII substrates by transiently overexpressing them in HEK-293 cells and measuring the incorporation of the isoprenoid precursor, [3H]MVA, into the immunoprecipitated Myc-tagged proteins. As shown in Figure 3 and Table 1, prenylation of the I73N and A81D mutants was markedly reduced compared with Myc-Rab1B(wt), and there was no detectable incorporation of [3H]MVA into Myc-Rab1B(Y78D). In contrast, incorporation of [3H]MVA into Myc-Rab1B proteins with nonconservative substitutions in the α3 helix (A110D, L103R) or the α4 helix (K137E, G144N) was readily detected by this metabolic labeling assay. In the cases of the A110D and L103R mutants, there was a noticeable reduction in the amount of Myc-Rab1B recovered in the immunoprecipitates due to weaker expression of these proteins in the transfected cells. However, the [3H]MVA incorporation per unit of immunodetectable protein was actually increased in comparison to wild-type Rab1B (see Table 1). The basis for this increase is presently unclear. In agreement with our previous observations (Wilson et al., 1996b), we also found that Myc-Rab1B(Q67L), which bears an amino acid substitution in loop 4 near the α2 helix (see Figure 1) was efficiently prenylated in 293 cells, despite the fact that the mutation reduces the intrinsic GTPase activity of the protein.
Figure 3.
Prenylation of Myc-Rab1B proteins expressed in HEK-293 cells. Shown are the results of two separate metabolic labeling studies in which various Rab1B mutants were compared with the wild-type protein. Cultures were transfected with each of the indicated Myc-Rab1B constructs. Immediately after transfection, cells were incubated with [3H]MVA for 18 h. The Myc-tagged proteins were immunoprecipitated as described in MATERIALS AND METHODS. In the upper panels, one-tenth of each immunoprecipitate was subjected to SDS-PAGE and immunoblot analysis was performed using a primary antibody against Rab1B and goat anti-rabbit 125I-labeled IgG as the secondary antibody. In the lower panels, the remainder of each immunoprecipitate was subjected to SDS-PAGE and fluorography to visualize the prenylated proteins. The 3H:125I ratios were determined by densitometry and compared with the matching wt values as summarized in Table 1.
Rab1B Switch 2 Mutants Expressed in 293 Cells Bind and Hydrolyze GTP
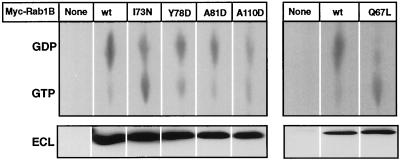
Previous studies have established that mutations that reduce the affinity of Rab proteins for guanine nucleotides render these proteins poor substrates for GGTaseII (Sanford et al., 1993), presumably because they cannot assume the GDP state required for optimal interaction with REP (Seabra, 1996). To determine whether the mutations introduced into the Switch 2 region of Rab1B might cause general protein misfolding with consequent loss of nucleotide binding, 293 cells expressing wild-type or mutant Myc-Rab1B constructs were metabolically labeled with [32P]orthophosphate, and guanine nucleotides were eluted from the expressed proteins after they were immunoprecipitated with an antibody against the Myc epitope. To ensure that the observations would be representative of the nonprenylated Myc-Rab1B substrate pool, the transfected cells were treated with 10 μM lovastatin to block isoprenoid biosynthesis and prevent protein prenylation before and during the labeling period. This approach is limited in two respects: 1) The stoichiometry of nucleotide binding is difficult to calculate because the specific radioactivities of the guanine nucleotide pools are not known and the chemiluminescent immunoblot signals cannot be readily translated into moles of Myc-Rab1B protein. 2) The proportion of Myc-Rab1B in the GDP state in the intact cells may be overestimated, since no correction was made for GTP hydrolysis that might have occurred during the immunoprecipitation procedure. Nevertheless, as shown in Figure 4, general comparisons of the 32P-labeled nucleotides recovered on the TLC plates with the corresponding ECL signals for the immunoprecipitated proteins indicated that overall nucleotide binding was not markedly deficient in any of the Myc-Rab1B mutants compared with Myc-Rab1B(wt). There were some clear variations in the GDP:GTP ratios among the proteins (Table 1), suggesting possible differences in GTP hydrolysis. For example, although the Y78D and A81D mutants contained a five to ninefold excess of GDP over GTP, their GDP:GTP ratios were somewhat lower than that determined for Myc-Rab1B(wt). In the case of the I73N mutant this tendency was more pronounced, with the GDP:GTP ratio approaching the value determined for the Q67L mutant. However, it should be noted that even in the I73N and Q67L mutants 45–50% of the total radiolabeled nucleotide was GDP, implying that both proteins retained a significant capacity for GTP hydrolysis.
Figure 4.
Guanine nucleotide composition of Myc-Rab1B constructs expressed in HEK-293 cells. In two separate experiments (left and right panels), 293 cells were labeled for 5 h with [32P]orthophosphate, beginning 24 h after transfection with the indicated Myc-Rab1B constructs (see MATERIALS AND METHODS). Myc-tagged proteins were immunoprecipitated and 50% of each sample was subjected to immunoblot analysis with antibody to Rab1B using chemiluminescent detection (ECL, lower panel). 32P-labeled guanine nucleotides in the immunoprecipitates were detected by subjecting 25% of each sample to TLC (upper panel). Radioactivity was quantified by scanning with a phosphorimager and GDP:GTP ratios were determined. The results are summarized in Table 1.
Effects of Switch 2 Mutations on the Interaction Between Epitope-tagged Rab1B and REP in 293 Cells
We next wished to determine whether the deficient prenylation of the Rab1B α2 helix mutants in cultured cells (Figure 3) was due to a decreased ability of these proteins to associate with REP. Several studies have shown that in mammalian cells Rab proteins reside predominantly in membranes or in cytosolic complexes with GDI (Regazzi et al., 1992; Soldati et al., 1993; Peter et al. 1994; Yang et al., 1994). Complexes between nascent Rabs and REP are not readily detected, presumably because these are formed only transiently during the prenylation reaction and normally exist at very low concentrations. However, Alexandrov et al. (1994) have shown that when the prenylation machinery is saturated by Rab overexpression, nonprenylated Rab substrates accumulate to the extent that it becomes possible to capture the nascent Rab–REP complex. Thus, we decided to pursue a strategy wherein each of the Myc-Rab1B constructs would be transiently coexpressed in 293 cells along with T7 epitope-tagged REP-1. Since we were particularly interested in assessing the ability of the nonprenylated Myc-Rab1B proteins to form an initial complex with REP (i.e., the requisite substrate for modification by GGTaseII), all 293 cell cultures were maintained in medium containing 10 μM lovastatin to block isoprenoid synthesis during and after the transfection procedure. We first attempted to isolate T7-REP/Myc-Rab1B complexes by coimmunoprecipitation with antibodies against the epitope tags. However, in our hands this approach proved to be impractical because the detergent concentrations required to obtain clean immunoprecipitates were in a range expected to dissociate the initial REP–Rab complex. As an alternative, we prepared detergent-free cytosolic fractions from the transfected cells and subjected these preparations to gel filtration chromatography using antibodies against the T7 and Myc epitopes to monitor the elution of the expressed T7-REP and Myc-Rab1B proteins.
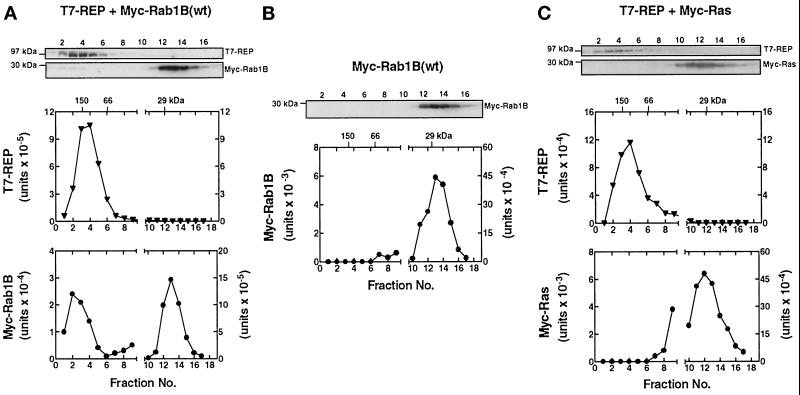
As illustrated in Figure 5A, when Myc-Rab1B(wt) was coexpressed with T7-REP, the epitope-tagged REP was eluted as a broad peak at approximately 150,000 kDa, consistent with the previously reported properties of recombinant REP and REP–Rab complexes (Seabra, 1996; Shen and Seabra, 1996). Most of the expressed Myc-Rab1B(wt) was eluted at the position of the 29-kDa marker, as expected for a nonprenylated Rab monomer. However, a small percentage (2–5%) of the total Myc-Rab1B was consistently found in the high molecular weight fractions containing the early portions of the T7-REP peak. Two lines of evidence indicated that this represented a specific complex between Myc-Rab1B and T7-REP. First, overexpression of Myc-Rab1B alone (Figure 5B) did not give rise to a detectable Myc signal in the high molecular weight fractions. Second, when the same experiment was performed with Myc-Ras instead of Myc-Rab1B (Figure 5C), no Myc-tagged protein was detected in the fractions containing T7-REP, in agreement with the reported inability of Ras to associate with REP (Andres et al., 1993).
Figure 5.
Gel filtration assay detects a complex between nascent Myc-Rab1B and T7-REP in HEK-293 cells. Parallel cultures were cotransfected with plasmids encoding T7-REP and Myc-Rab1B (A), Myc-Rab1B alone (B), or T7-REP and Myc-Ras (C), and the cells were maintained in medium containing 10 μM lovastatin for 48 h. Cell lysates were prepared and subjected to FPLC on a Superose-12 column as described in MATERIALS AND METHODS. Individual fractions were subjected to SDS-PAGE and immunoblot analysis using monoclonal antibodies against the T7 and Myc epitopes of the overexpressed proteins. The graphs show the results obtained when bound 125I-labeled IgG was quantified by phosphorimager analysis. Peak elution positions of the marker proteins, alcohol dehydrogenase (150 kDa), bovine serum albumin (66 kDa), and carbonic anhydrase (29 kDa), are indicated at the top. Early fractions corresponding to the exclusion volume and markers above 200 kDa did not contain Myc- or T7-immunoreactive proteins and are not shown. It should be noted that in the Myc-Rab1B and Myc-Ras panels, the scale of the y-axis on the left side is expanded one order of magnitude compared with the right side.
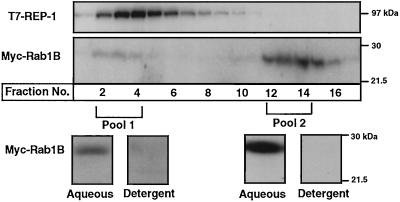
In the foregoing studies we hypothesized that any Myc-Rab1B associated with T7-REP in the lovastatin-treated cells would be in the nonprenylated form. To test this hypothesis directly, the pools of Myc-Rab1B that cofractionated either with T7-REP or with the 29-kDa marker were subjected to a standard Triton X-114 phase partitioning assay, with the expectation that any geranylgeranylated Rabs would be extracted into the detergent phase (Beranger et al., 1994). The results clearly demonstrate that the Myc-Rab1B collected in both the monomeric pool and the T7-REP-associated pool behaved as nonprenylated protein, remaining almost entirely in the aqueous phase (Figure 6).
Figure 6.
Triton X-114 partitioning of monomeric and T7-REP-associated Myc-Rab1B pools. 293 cells were cotransfected with plasmids encoding Myc-Rab1B(wt) and T7-REP and maintained in medium containing 10 μM lovastatin for 48 h. Cytosol was fractionated on a Superose-12 column (upper panels) and fractions containing Myc-Rab1B that eluted with T7-REP (pool 1) or at the position of the 29-kDa marker (pool 2) were combined as indicated. Both pools were subjected to the Triton X-114 phase partitioning assay described in MATERIALS AND METHODS. The proteins in the resulting aqueous and detergent phases were run on SDS gels and immunoblotted with the anti-Myc antibody (bottom panels).
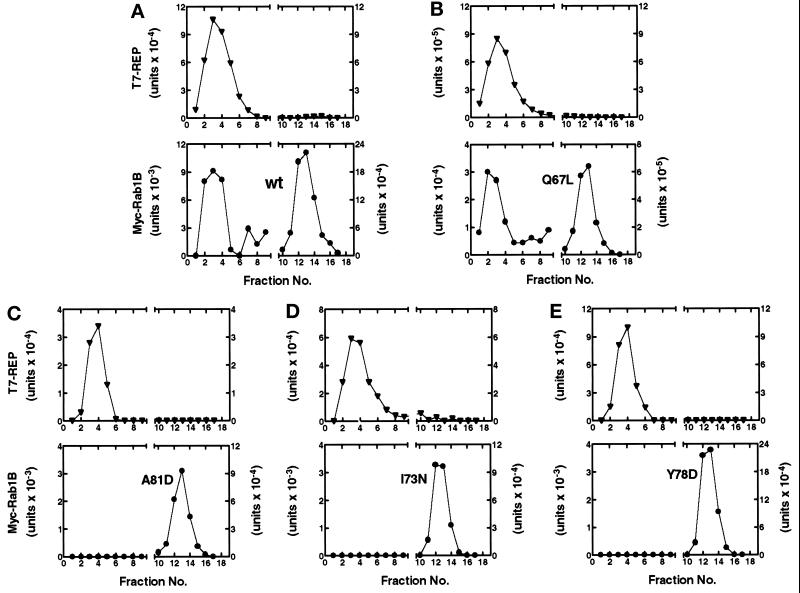
Figure 7 (C–E) shows the results obtained when the coexpression assay was used to evaluate the potential interactions between T7-REP and the three Myc-Rab1B Switch 2 mutants (A81D, I73N, and Y78D) that were previously found to be poorly labeled by [3H]MVA in 293 cells. In each case, we failed to detect any Myc-Rab1B in the fractions containing T7-REP eluted from the gel filtration column. By comparison, a peak of Myc-Rab1B that comigrated with T7-REP was readily detected in cells expressing the Q67L mutant (Figure 7B), in agreement with the demonstrated ability of this protein to undergo prenylation (Figure 3). Although there was inevitably some variability in the levels of total T7-REP and Myc-Rab1B expression in these studies, the ratios of T7-REP:Myc-Rab1B were generally similar in each experiment. Moreover, in the case of the wild-type protein, we were able to detect a pool of Myc-Rab1B that cofractionated with T7-REP over a wide range of Myc-Rab1B expression levels (e.g., compare Figures 5A and 7A). Thus, differences in protein expression levels could not account for the complete absence of T7-REP-associated Myc-Rab1B in the cases of the I73N, A81D, and Y78D mutants. In light of the data in Figure 4 which suggest that these mutants are capable of assuming the GDP-bound conformation required for interaction with REP, these observations indicate that the introduction of nonconservative amino acid substitutions into the α2 helix of Rab1B impedes the physical interaction of REP with the Switch 2 domain of the GDP-bound Rab protein.
Figure 7.
Gel filtration analysis of prenylation-deficient Myc-Rab1B constructs coexpressed with T7-REP in 293 cells. Cultures coexpressing each of the indicated Myc-Rab1B constructs and T7-REP were maintained for 48 h in medium containing 10 μM lovastatin. Cytosolic proteins were fractionated by gel filtration and the Myc- and T7- tagged proteins were detected by immunoblot analysis as described in the legend to Figure 5.
DISCUSSION
Based on the Ras model, the conformation of the Switch 2 region of Rab1B is predicted to be substantially different when the protein is in the GDP state versus the GTP state (Krengel et al., 1990; Pai et al., 1990; Tong et al., 1991). The present studies define the putative α2 helix of the Switch 2 region in Rab1B as a major structural determinant for association with REP in intact cells. Consistent with this finding, the sequences of the amino acids that form the loop2/α2 region are highly conserved among members of the Rab subgroup compared with other proteins in the Ras superfamily (Valencia et al., 1991).
The data indicate that the striking impairment of geranylgeranylation of Rab1B caused by the I73N, Y78D, and A81D α2 helix substitutions is due primarily to disruption of the intermolecular interaction between Rab1B and REP rather than indirect effects of these mutations on the nucleotide state of the GTPase. None of the α2 helix mutants showed a substantial decrease in the ability to bind guanine nucleotides in intact cells (Figure 4). Moreover, while two of these mutants (I73N, Y78D) appeared to have reduced GTPase activity relative to Rab1B(wt), it is unlikely that this accounts for the defective interaction with REP. This conclusion is based on comparisons of the guanine nucleotide analyses to the prenylation data and REP interaction studies (summarized in Table 1). For example, although there were marked variations in the 32P GDP:GTP ratios among the α2 helix mutants (ranging from a near-wild type value of 9.5 for A81D to a low of 1.0 for I73N), the steady-state prenylation of these proteins, determined by mevalonate labeling, was uniformly reduced by 90–100%. This point is further emphasized when the results obtained for the α2 helix mutants are compared with results for the GTPase-deficient Q67L mutant (Table 1). Specifically, although the latter protein had the lowest 32P GDP:GTP ratio determined for any of the mutant Rab1B constructs analyzed, the prenylation of Rab1B(Q67L) was indistinguishable from Rab1B(wt) (Figure 3; Wilson et al., 1996b). On the surface, this finding might seem to conflict with previous studies showing that the GDP-bound form of the Rab protein is the preferred substrate for prenylation (Sanford et al., 1993; Schiedel et al., 1995; Wilson et al., 1996b) and REP interaction (Seabra, 1996). However, it is important to note that when Rab proteins are overexpressed in cultured cells, a significant amount of nonprenylated protein accumulates in the cytosol, indicating that the Rab geranylgeranyltransferase machinery may be overloaded under such conditions (Alexandrov et al., 1994; Wilson et al., 1996a). Thus, although the relative amount of expressed Myc-Rab1B(Q67L) residing in the GDP state appears to be relatively small when compared with the other Rab1B constructs, the amount of available GDP-bound Q67L substrate (approximately 50% of the total expressed protein) is probably sufficient to saturate REP and drive the GGTaseII reaction when the protein is overexpressed in 293 cells. The same argument would apply to the A110D mutant which, despite a lower-than-normal 32P GDP:GTP ratio, is prenylated efficiently in 293 cells. In light of these observations, it is difficult to envision a mechanism whereby the poor prenylation of the Y78D and A81D Switch 2 mutants (both of which had substantially higher 32P GDP:GTP ratios than Q67L or A110D) could be explained on the basis of an inability to attain the GDP state.
Although the present study highlights the Switch 2 domain of Rab1B as being critical for association with REP, it is probable that additional structural elements of the Rab protein also contribute to this interaction. The other major domain predicted to undergo a significant nucleotide-dependent conformational change in the Rab proteins is the Switch 1 region, which includes the effector domain (loop 2 and the proximal portion of β2 in the Ras model). We previously showed that amino acid substitutions (D44N, I41N) in the effector domain severely reduced the ability of Rab1B to undergo prenylation in reticulocyte lysates (Wilson and Maltese, 1993). When the D44N mutant was overexpressed in 293 cells, its prenylation was reduced by approximately 50% compared with Rab1B(wt) (Wilson et al., 1996a). Although this finding was not as dramatic as the near elimination of prenylation observed with the Switch 2 mutants, it suggests that the affinity of Rab1B for REP may have been weakened, but not entirely lost, by perturbation of the Switch 1 effector domain. Thus, it is entirely possible that both the Switch 1 and Switch 2 regions cooperate to form a complex 3-D structure that is recognized by REP. Additional evidence to support this concept comes from an early study of chimeric proteins by Khosravi-Far et al. (1992) in which a 139-residue carboxyl-terminal segment of Rab1B (containing the Switch 2 region, but not the intact Rab effector domain) failed to undergo prenylation when grafted to the amino-terminal portion of Ras. Finally, in addition to the nucleotide-sensitive Switch 1 and Switch 2 regions, we and others have shown that structural alterations in the amino-terminal (Wilson and Maltese, 1993; Sanford et al., 1995) and carboxyl-terminal (Kinsella and Maltese, 1991; Cremers et al., 1994) variable regions of Rab proteins can have a profound influence on prenylation efficiency. However, it remains to be determined whether these effects are directly related to decreased affinity for REP or changes in the interaction of the REP–Rab complex with the catalytic αβ subunits of GGTaseII.
Although most members of the Rab family can be geranylgeranylated with similar efficiency in the presence of either REP-1 or REP-2 (Cremers et al., 1994), an interesting exception was reported by Seabra et al. (1995), who noted that Rab27 has a significantly reduced affinity for REP-2 compared with REP-1. The gene encoding REP-1 is known to be defective in the retinal degenerative disease choroideremia (Cremers et al., 1990), and Rab27 is localized in the specific cell layers that are affected in this disease (Seabra et al., 1995). Taken together, these findings have suggested that the reduced ability of REP-2 to support prenylation of Rab27 in the absence of REP-1, and a consequent disruption of undefined Rab27-mediated transport events, could underlie the pathophysiology of choroideremia. We have performed CLUSTALW sequence alignments between Rab1B and Rab27 to determine whether there might be any significant differences in the domains comprising the Switch 2 region (i.e., residues 64–83 in Rab1B, which align with the Ras loop 4/α2 helix). The results indicated a high degree of similarity (55% identity) between the Switch 2 domains of Rab1B and Rab27. However, Rab27 contains two separate 5-amino acid inserts that occur outside the Switch 2 domain at positions 46 and 56 in Rab1B. These inserts are predicted to occur in regions corresponding to β2 and β3 in Ras (see Figure 1) and are absent in other Rab proteins (Valencia et al., 1991). Since the β2 and β3 regions anchor the Switch 1 and Switch 2 domains, it is conceivable that the presence of the unique sequence elements in Rab27 might alter the manner in which the two key guanine nucleotide-sensitive Switch domains are juxtaposed, thus accounting for the novel selectivity of Rab27 with respect to the two forms of REP.
As mentioned in the INTRODUCTION, the Rab GDIs (e.g., GDIα and GDIβ) are functionally similar to REP insofar as they form cytosolic complexes specifically with the GDP-bound form of the Rab protein and are able to deliver prenylated Rabs to intracellular membranes (Araki et al., 1990; Dirac-Svejestrup et al., 1994; Peter et al., 1994; Ullrich, et al., 1994). Moreover, several structural features appear to be highly conserved between GDI and REP (Schalk et al., 1996). In particular, mutagenesis studies have implicated two GDI/REP “sequence-conserved regions” in the domain I portion of GDI as being important for Rab binding (Schalk et al., 1996). We have previously shown that introduction of the D44N substitution into the Switch 1 effector domain essentially eliminates the ability of Rab1B to bind to GDI in vitro and in intact cells (Wilson et al., 1996a). Given the importance of the Switch 2 region for REP binding demonstrated in the present study, it is reasonable to speculate that the Switch 2 region may also play an important role in the interaction between GDP-bound Rab proteins and the domain I structure of GDI. Direct experimental verification of this idea is complicated by the fact that Rab proteins must be geranylgeranylated for optimal interaction with GDI (Musha et al., 1992; Soldati et al., 1993; Wilson et al., 1996a) and the Switch 2 mutants cannot be efficiently modified by REP/GGTaseII. However, previous studies have suggested that it may be possible to circumvent the REP requirement for prenylation by engineering the Rab carboxyl-terminal cysteine motif to conform to the typical CAAX box found on other Ras-related proteins that are modified in a REP-independent manner by FTase or GGTaseI (Khosravi-Far et al., 1992). Application of this approach to the Rab1B Switch 2 mutants may therefore permit a direct assessment of their ability to associate with GDI. Moreover, if adequate REP-independent prenylation of the Switch 2 mutants can be obtained in intact cells, it may be possible to use these and similar REP-binding-deficient Rab constructs to examine the roles of REP and the Rab Switch 2 domain in the targeting of nascent Rab proteins to their specific intracellular destinations.
ACKNOWLEDGEMENT
This work was supported by National Institutes of Health grant CA34569 to W.A.M.
REFERENCES
- Adamson P, Paterson HF, Hall A. Intracellular localization of P21rho proteins. J Cell Biol. 1992;119:617–627. doi: 10.1083/jcb.119.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrov K, Horiuchi H, Steele-Mortimer O, Seabra MC, Zerial M. Rab escort protein-1 is a multifunctional protein that accompanies newly prenylated rab proteins to their target membranes. EMBO J. 1994;13:5262–5273. doi: 10.1002/j.1460-2075.1994.tb06860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson S, Davis DL, Dahlback H, Jornvall H, Russell DW. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J Biol Chem. 1989;264:8222–8229. [PubMed] [Google Scholar]
- Andres DA, Seabra MC, Brown MS, Armstrong SA, Smeland TE, Cremers FPM, Goldstein JL. cDNA cloning of component A of rab geranylgeranyl transferase and demonstration of its role as a rab escort protein. Cell. 1993;73:1091–1099. doi: 10.1016/0092-8674(93)90639-8. [DOI] [PubMed] [Google Scholar]
- Araki S, Kikuchi A, Hata Y, Isomura M, Takai Y. Regulation of reversible binding of smg p25A, a ras p21-like GTP- binding protein, to synaptic plasma membranes and vesicles by its specific regulatory protein, GDP dissociation inhibitor. J Biol Chem. 1990;265:13007–13015. [PubMed] [Google Scholar]
- Armstrong SA, Seabra MC, Sudhof TC, Goldstein JL, Brown MS. cDNA cloning and expression of the alpha and beta subunits of rat Rab geranylgeranyl transferase. J Biol Chem. 1993;268:12221–12229. [PubMed] [Google Scholar]
- Beranger F, Cadwallader K, Profiri E, Powers S, Evans T, de Gunzberg J, Hancock JF. Determination of structural requirements for the interaction of Rab6 with RabGDI and Rab geranylgeranyltransferase. J Biol Chem. 1994;269:13637–13643. [PubMed] [Google Scholar]
- Bourne HR, Sanders DA, McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991;349:117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- Brondyk WH, McKiernan CJ, Burstein ES, Macara IG. Mutants of rab3A analogous to oncogenic ras mutants. J Biol Chem. 1993;268:9410–9415. [PubMed] [Google Scholar]
- Burstein E, Brondyk WH, Macara IG. Amino acid residues in the Ras-like GTPase Rab3A that specify sensitivity to factors that regulate the GTP/GDP cycling of Rab3A. J Biol Chem. 1992;267:22715–22718. [PubMed] [Google Scholar]
- Casey PJ, Seabra MC. Protein prenyltransferases. J Biol Chem. 1996;271:5289–5292. doi: 10.1074/jbc.271.10.5289. [DOI] [PubMed] [Google Scholar]
- Casey PJ, Thissen JA, Moomaw JF. Enzymatic modification of proteins with a geranylgeranyl isoprenoid. Proc Natl Acad Sci USA. 1991;88:8631–8635. doi: 10.1073/pnas.88.19.8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YT, Holcomb C, Moor HPH. Expression and localization of two low molecular weight GTP-binding proteins, Rab8 and Rab10, by epitope tag. Proc Natl Acad Sci USA. 1993;90:6508–6512. doi: 10.1073/pnas.90.14.6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crechet J-B, Bernardi A, Parmeggiani A. Distal switch II region of Ras2p is required for interaction with guanine nucleotide exchange factor. J Biol Chem. 1996;271:17234–17240. doi: 10.1074/jbc.271.29.17234. [DOI] [PubMed] [Google Scholar]
- Cremers FPM, Armstrong SA, Seabra MC, Brown MS, Goldstein JL. REP-2, a Rab escort protein encoded by the choroideremia-like gene. J Biol Chem. 1994;269:2111–2117. [PubMed] [Google Scholar]
- Cremers FPM, van de Pol DJR, van Kerkhoff LPM, Wieringa B, Ropers H-H. Cloning of a gene that is rearranged in patients with choroideraemia. Nature. 1990;347:674–677. doi: 10.1038/347674a0. [DOI] [PubMed] [Google Scholar]
- Dirac-Svejestrup AB, Sumizawa T, Pfeffer SR. Identification of a GDI displacement factor that releases endosomal Rab GTPases from Rab-GDI. EMBO J. 1997;16:465–472. doi: 10.1093/emboj/16.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirac-Svejestrup AB, Soldati T, Shapiro AD, Pfeffer SR. Rab-GDI presents functional Rab9 to the intracellular transport machinery and contributes selectivity to Rab9 membrane recruitment. J Biol Chem. 1994;269:15427–15430. [PubMed] [Google Scholar]
- Farnsworth CC, Seabra M, Ericsson LH, Gelb MH, Glomset JA. Rab geranylgeranyl transferase catalyzes the geranylgeranylation of adjacent cysteines in the small GTPases Rab1A, Rab3A and Rab5A. Proc Natl Acad Sci USA. 1994;91:11963–11967. doi: 10.1073/pnas.91.25.11963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs JB, Marshall MS, Scolnick EM, Dixon RAF, Vogel US. Modulation of guanine nucleotides bound to Ras in NIH3T3 cells by oncogenes, growth factors, and the GTPase activating protein (GAP) J Biol Chem. 1990;265:20437–20442. [PubMed] [Google Scholar]
- Goldstein JL, Brown MS, Stradley S, Reiss Y, Gierasch L. Nonfarnesylated tetrapeptide inhibitors of protein farnesyltransferase. J Biol Chem. 1991;266:15575–15578. [PubMed] [Google Scholar]
- Gorman CM, Gies DR, McCray G. Transient production of proteins using an adenovirus transformed cell line. DNA and Protein Engineering Techniques. 1990;2:3–10. [Google Scholar]
- Horiuchi H, Giner A, Hoflack B, Zerial M. A GDP/GTP exchange-stimulatory activity for the Rab5-RabGDI complex on clathrin-coated vesicles from bovine brain. J Biol Chem. 1995;270:11257–11262. doi: 10.1074/jbc.270.19.11257. [DOI] [PubMed] [Google Scholar]
- Johannes L, Perez F, Laran-Chich M-P, Henry J-P, Darchen F. Characterization of the interaction of the monomeric GTP-binding protein Rab3a with geranylgeranyl transferase II. Eur J Biochem. 1996;239:362–368. doi: 10.1111/j.1432-1033.1996.0362u.x. [DOI] [PubMed] [Google Scholar]
- Khosravi-Far R, Clark GJ, Abe K, Cox AD, McLain T, Lutz RJ, Sinensky M, Der CJ. Ras (CXXX) and rab (CC/CXC) prenylation signal sequences are unique and functionally distinct. J Biol Chem. 1992;267:24363–24368. [PubMed] [Google Scholar]
- Khosravi-Far R, Lutz RJ, Cox AD, Conroy L, Bourne JR, Sinensky M, Balch WE, Buss JE, Der CJ. Isoprenoid modification of rab proteins terminating in CC or CXC motifs. Proc Natl Acad Sci USA. 1991;88:6264–6268. doi: 10.1073/pnas.88.14.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella BT, Maltese WA. rab GTP-binding proteins implicated in vesicular transport are isoprenylated in vitro at cysteines within a novel carboxyl-terminal motif. J Biol Chem. 1991;266:8540–8544. [PubMed] [Google Scholar]
- Kinsella BT, Maltese WA. rab GTP-binding proteins with three different carboxyl-terminal cysteine motifs are modified in vivo by 20-carbon isoprenoids. J Biol Chem. 1992;267:3940–3945. [PubMed] [Google Scholar]
- Krengel U, Schlichting I, Scherer A, Schumann R, Frech M, John J, Kabsch W, Pai EF, Wittinghofer A. Three-dimensional structures of H-ras p21 mutants: molecular basis for their inability to function as signal switch molecules. Cell. 1990;62:539–548. doi: 10.1016/0092-8674(90)90018-a. [DOI] [PubMed] [Google Scholar]
- Krupinski J, Lehman TC, Frankenfield CD, Zwaagstra JC, Watson PA. Molecular diversity in the adenylylcyclase family. J Biol Chem. 1992;267:24858–24862. [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li G, Stahl PD. Structure-function relationship of the small GTPase rab5. J Biol Chem. 1993;268:24475–24480. [PubMed] [Google Scholar]
- Moodie SA, Paris M, Villafranca E, Kirshmeier P, Willumsen BM, Wolfman A. Different structural requirements within the switch II region of the Ras protein for interactions with specific downstream targets. Oncogene. 1995;11:447–454. [PubMed] [Google Scholar]
- Moores SL, Schaber MD, Mosser SD, Rands E, O’Hara MB, Garsky VM, Marshall MS, Pompliano DL, Gibbs JB. Sequence dependence of protein isoprenylation. J Biol Chem. 1991;266:14603–14610. [PubMed] [Google Scholar]
- Musha T, Kawata M, Takai Y. The geranylgeranyl moiety but not the methyl moiety of the smg25A/rab3A protein is essential for the interactions with membrane and its inhibitory GDP/GTP exchange protein. J Biol Chem. 1992;267:9821–9825. [PubMed] [Google Scholar]
- Novick P, Brennwald P. Friends and Family: The role of the rab GTPases in vesicular traffic. Cell. 1993;75:597–601. doi: 10.1016/0092-8674(93)90478-9. [DOI] [PubMed] [Google Scholar]
- Nuoffer C, Balch WE. GTPases: multifunctional molecular switches regulating vesicular traffic. Annu Rev Biochem. 1994;63:949–990. doi: 10.1146/annurev.bi.63.070194.004505. [DOI] [PubMed] [Google Scholar]
- Overmeyer JH, Maltese WA. Isoprenoid requirement for intracellular transport and processing of murine leukemia virus envelope protein. J Biol Chem. 1992;267:22686–22692. [PubMed] [Google Scholar]
- Pai EF, Krengel U, Petsko GA, Goody RS, Kansch W, Wittinghofer A. Refined crystal structure of the triphosphate conformation of H-ras p21 at 1.35A resolution: implications for the mechanism of GTP hydrolysis. EMBO J. 1990;9:2351–2359. doi: 10.1002/j.1460-2075.1990.tb07409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter F, Nuoffer C, Pind SN, Balch WE. Guanine nucleotide dissociation inhibitor is essential for Rab1 function in budding from the endoplasmic reticulum and transport through the Golgi stack. J Cell Biol. 1994;126:1393–1406. doi: 10.1083/jcb.126.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer SR. Rab GTPases: master regulators of membrane trafficking. Curr Opin Cell Biol. 1994;6:522–526. doi: 10.1016/0955-0674(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Polakis P, McCormick F. Structural requirements for the interaction of p21ras with GAP, exchange factors, and its biological effector target. J Biol Chem. 1993;268:9157–9160. [PubMed] [Google Scholar]
- Quilliam LA, Hisaka MM, Zhong S, Lowry A, Mosteller RD, Han J, Drugan JK, Broek D, Campbell SL, Der CJ. Involvement of the switch 2 domain of Ras in its interaction with guanine nucleotide exchange factors. J Biol Chem. 1996;271:11076–11082. doi: 10.1074/jbc.271.19.11076. [DOI] [PubMed] [Google Scholar]
- Regazzi R, Kikuchi A, Takai Y, Wollheim CB. The small GTP-binding proteins in the cytosol of insulin-secreting cells are complexed to GDP dissociation inhibitor proteins. J Biol Chem. 1992;267:17512–17519. [PubMed] [Google Scholar]
- Reiss Y, Stradley SJ, Gierasch LM, Brown MS, Goldstein JL. Sequence requirement for peptide recognition by rat brain p21ras protein farnesyltransferase. Proc Natl Acad Sci USA. 1991;88:732–736. doi: 10.1073/pnas.88.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford JC, Pan Y, Wessling-Resnick M. Prenylation of Rab5 is dependent on guanine nucleotide binding. J Biol Chem. 1993;268:23773–23776. [PubMed] [Google Scholar]
- Sanford JC, Pan Y, Wessling-Resnick M. Properties of Rab5 N-terminal domain dictate prenylation of C-terminal cysteines. Mol Biol Cell. 1995;6:71–85. doi: 10.1091/mbc.6.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalk I, Zeng K, Wu S-K, Stura E, Matteson J, Huang M, Tandon A, Wilson IA, Balch WE. Structure and mutational analysis of Rab GDP-dissociation inhibitor. Nature. 1996;381:42–48. doi: 10.1038/381042a0. [DOI] [PubMed] [Google Scholar]
- Schiedel AC, Barnekow A, Mayer T. Nucleotide induced conformation determines posttranslational isoprenylation of the ras related rab6 protein in insect cells. FEBS Lett. 1995;376:113–119. doi: 10.1016/0014-5793(95)01258-0. [DOI] [PubMed] [Google Scholar]
- Seabra MC. Nucleotide dependence of Rab geranylgeranylation. J Biol Chem. 1996;271:14398–14404. doi: 10.1074/jbc.271.24.14398. [DOI] [PubMed] [Google Scholar]
- Seabra MC, Brown MS, Slaughter, Sudhof TC, Goldstein JL. Purification of component A of Rab geranylgeranyl transferase: possible identity with the choroideremia gene product. Cell. 1992a;70:1049–1057. doi: 10.1016/0092-8674(92)90253-9. [DOI] [PubMed] [Google Scholar]
- Seabra MC, Goldstein JL, Sudhof TC, Brown MS. Rab geranylgeranyl transferase, a multisubunit enzyme that prenylates GTP-binding proteins terminating in csy-x-cys or cys-cys. J Biol Chem. 1992b;267:14497–14503. [PubMed] [Google Scholar]
- Seabra MC, Ho YK, Anant JS. Deficient geranylgeranylation of Ram/Rab27 in choroideremia. J Biol Chem. 1995;270:24420–24427. doi: 10.1074/jbc.270.41.24420. [DOI] [PubMed] [Google Scholar]
- Shen F, Seabra MC. Mechanism of digeranylgeranylation of Rab proteins: formation of a complex between monogeranylgeranyl-Rab and Rab escort protein. J Biol Chem. 1996;271:3692–3698. doi: 10.1074/jbc.271.7.3692. [DOI] [PubMed] [Google Scholar]
- Simons K, Zerial M. Rab proteins and the road maps for intracellular transport. Neuron. 1993;11:789–799. doi: 10.1016/0896-6273(93)90109-5. [DOI] [PubMed] [Google Scholar]
- Sogaard M, Tani K, Ye RR, Geromanos S, Tempst P, Kirchhausen T, Rothman JE, Sollner T. A Rab protein is required for the assembly of SNARE complexes in the docking of transport vesicles. Cell. 1994;78:937–948. doi: 10.1016/0092-8674(94)90270-4. [DOI] [PubMed] [Google Scholar]
- Soldati T, Riederer MA, Pfeffer SR. Rab GDI: a solubilizing and recycling factor for rab9 protein. Mol Biol Cell. 1993;4:425–434. doi: 10.1091/mbc.4.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H, Valencia A, Martinez O, Ullrich O, Goud B, Zerial M. Distinct structural elements of Rab5 define its functional specificity. EMBO J. 1994;13:575–583. doi: 10.1002/j.1460-2075.1994.tb06295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdale EJ, Bourne JR, Khosravi-Far R, Der CJ, Balch WE. GTP-binding mutants of rab1 and rab2 are potent inhibitors of vesicular transport from the endoplasmic reticulum to the Golgi complex. J Cell Biol. 1992;119:749–761. doi: 10.1083/jcb.119.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong L, de Vos AM, Milburn MV, Kim SH. Crystal structures at 2.2 A resolution of the catalytic domains of normal ras protein and an oncogenic mutant complexed with GDP. J Mol Biol. 1991;217:503–516. doi: 10.1016/0022-2836(91)90753-s. [DOI] [PubMed] [Google Scholar]
- Ullrich O, Horiuchi H, Bucci C, Zerial M. Membrane association of Rab5 mediated by GDP-dissociation inhibitor and accompanied by GDP/GTP exchange. Nature. 1994;368:157–160. doi: 10.1038/368157a0. [DOI] [PubMed] [Google Scholar]
- Valencia A, Chardin P, Wittinghofer A, Sander C. The ras protein family: evolutionary tree and role of conserved amino acids. Biochemistry. 1991;30:4637–4648. doi: 10.1021/bi00233a001. [DOI] [PubMed] [Google Scholar]
- Vielh E, Touchot N, Zahraoui A, Tavitian A. Nucleotide sequence of a rat cDNA: Rab1B, encoding a Rab1-YPT related protein. Nucleic Acids Res. 1989;17:1770. doi: 10.1093/nar/17.4.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada M, Nakanishi H, Satoh A, Hirano H, Obaishi H, Matsuura Y, Takai Y. Isolation and characterization of a GDP/GTP exchange protein specific for the Rab3 subfamily small G proteins. J Biol Chem. 1997;272:3875–3878. doi: 10.1074/jbc.272.7.3875. [DOI] [PubMed] [Google Scholar]
- Wilson AL, Erdman RA, Maltese WA. Association of Rab1B with GDP-dissociation inhibitor (GDI) is required for recycling but not initial membrane targeting of the Rab protein. J Biol Chem. 1996a;271:10932–10940. doi: 10.1074/jbc.271.18.10932. [DOI] [PubMed] [Google Scholar]
- Wilson AL, Maltese WA. Isoprenylation of rab1B is impaired by mutations in its effector domain. J Biol Chem. 1993;268:14561–14564. [PubMed] [Google Scholar]
- Wilson AL, Sheridan KM, Erdman RA, Maltese WA. Prenylation of a Rab1B mutant with altered GTPase activity is impaired in cell-free systems but not in intact mammalian cells. Biochem J. 1996b;318:1007–1014. doi: 10.1042/bj3181007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Slepnev VI, Goud B. Rab proteins form in vivo complexes with two isoforms of the GDP-dissociation inhibitor protein (GDI) J Biol Chem. 1994;269:31891–31899. [PubMed] [Google Scholar]
- Yokoyama K, Gelb MH. Purification of a mammalian protein geranylgeranyltransferase. J Biol Chem. 1993;268:4055–4060. [PubMed] [Google Scholar]
- Yokoyama K, Goodwin GW, Ghomaschi F, Glomset JA, Gelb MH. A protein geranylgeranyltransferase from bovine brain: implications for protein prenylation specificity. Proc Natl Acad Sci USA. 1991;88:5302–5306. doi: 10.1073/pnas.88.12.5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang FL, Diehl RE, Kohl NE, Gibbs JB, Giros B, Casey PJ, Omer CA. cDNA cloning and expression of rat and human protein geranylgeranyltransferase type-1. J Biol Chem. 1994;269:3175–3180. [PubMed] [Google Scholar]