Abstract
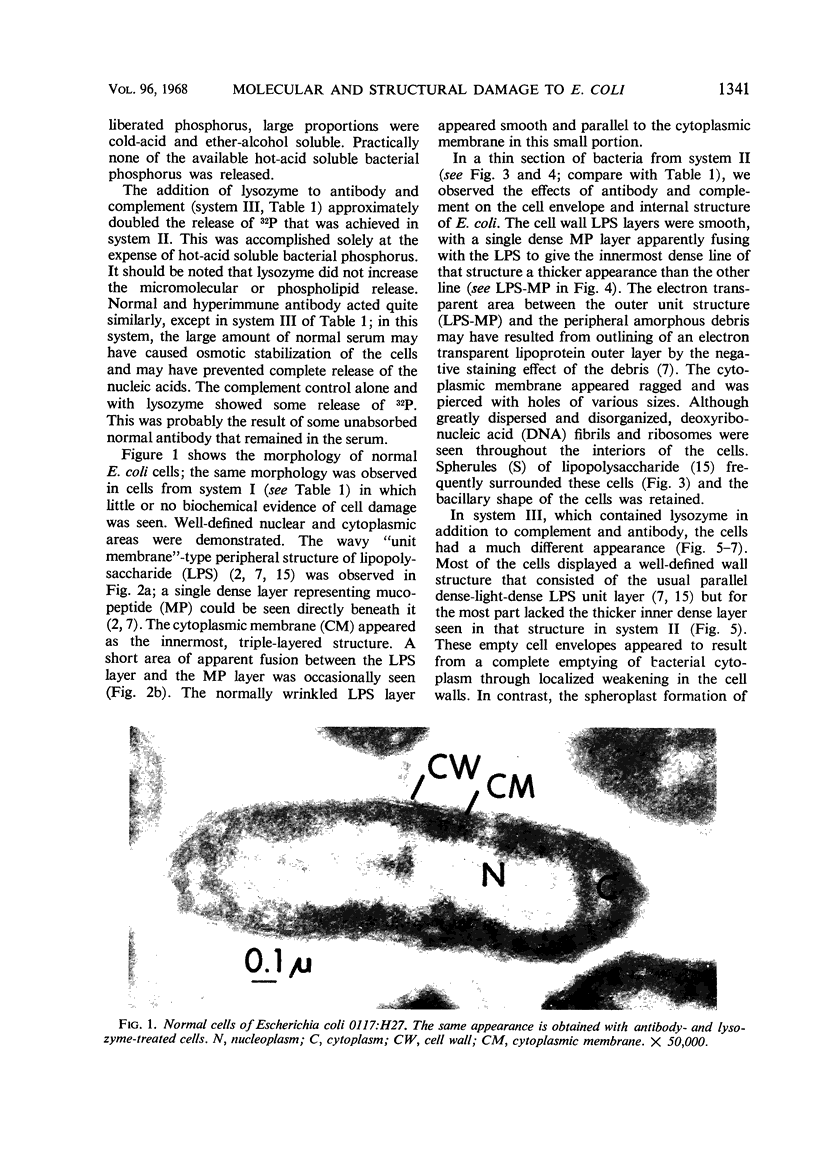
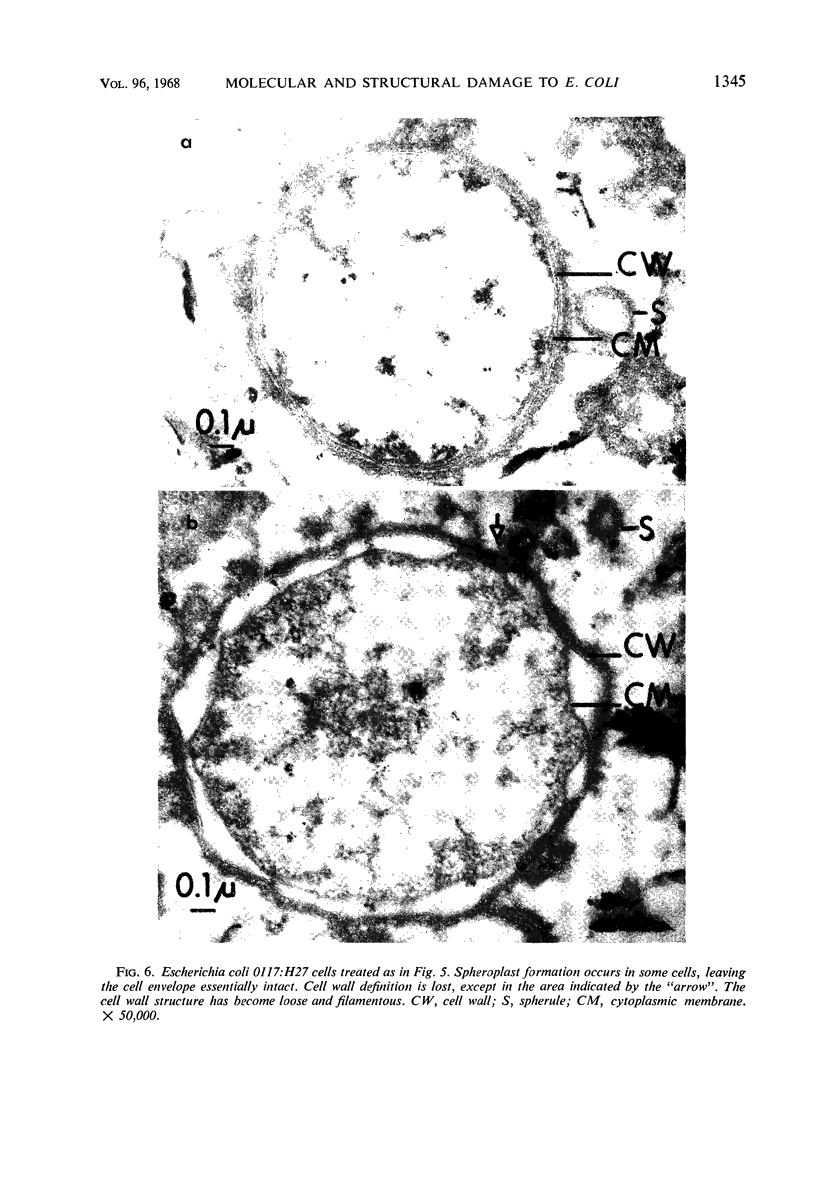
The antibacterial action of antibody (normal and hyperimmune), complement, and lysozyme has been studied by correlating the ultrastructural and biochemical changes that they cause in smooth Escherichia coli. Both normal and hyperimmune antibody, in the absence of lysozyme, produced complement-dependent release, into the suspending medium, of 63 to 72% of the 32P-labeled phospholipid and 74 to 85% of the small molecular bacterial constituents. Macromolecular nucleic acid labeled with 32P was not released. By phase microscopy, these cells appeared as bacilli but their ultrastructure showed general swelling, with smoothing of the normally wrinkled outer cell wall layers. Cytoplasmic membranes were damaged and the internal cell structure was disorganized. Membranous spherules, apparently from the outermost putatively lipopolysaccharide cell layer, were released into the medium. When lysozyme was added to antibody and complement, 32P-labeled macromolecular constituents were released from the cells. Damage to ultrastructure then included loss of cell wall rigidity, cell wall breakage, and some spheroplast formation. Characteristic fibrillar fragmentation was seen in cell wall mucopeptide layers. The relationships between antibody-complement dependent release of bacterial phospholipid, loss of selective cell permeability, and increase in sensitivity to lysozyme are discussed.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer M. E. Response of Cell Walls of Escherichia coli to a Sudden Reduction of the Environmental Osmotic Pressure. J Bacteriol. 1967 Mar;93(3):1104–1112. doi: 10.1128/jb.93.3.1104-1112.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bladen H. A., Gewurz H., Mergenhagen S. E. Interactions of the complement system with the surface and endotoxic lipopolysaccharide of Veillonella alcalescens. J Exp Med. 1967 May 1;125(5):767–786. doi: 10.1084/jem.125.5.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIDSON J. N., SMELLIE R. M. S. Phosphorus compounds in the cell. III. The incorporation of radioactive phosphorus into the ribonucleotide fraction of liver tissue. Biochem J. 1952 Dec;52(4):599–606. doi: 10.1042/bj0520599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn A. A., Milne C. M. A kinetic study of the bacteriolytic and bactericidal action of human serum. Immunology. 1967 Jun;12(6):639–653. [PMC free article] [PubMed] [Google Scholar]
- MURRAY R. G., STEED P., ELSON H. E. THE LOCATION OF THE MUCOPEPTIDE IN SECTIONS OF THE CELL WALL OF ESCHERICHIA COLI AND OTHER GRAM-NEGATIVE BACTERIA. Can J Microbiol. 1965 Jun;11:547–560. doi: 10.1139/m65-072. [DOI] [PubMed] [Google Scholar]
- MUSCHEL L. H., TREFFERS H. P. Quantitative studies on the bactericidal actions of serum and complement. I. A rapid photometric growth assay for bactericidal activity. J Immunol. 1956 Jan;76(1):1–10. [PubMed] [Google Scholar]
- Rosse W. F., Dourmashkin R., Humphrey J. H. Immune lysis of normal human and paroxysmal nocturnal hemoglobinuria (PNH) red blood cells. 3. The membrane defects caused by complement lysis. J Exp Med. 1966 Jun 1;123(6):969–984. doi: 10.1084/jem.123.6.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitznagel J. K. Normal serum cytotoxicity for P32-labeled smooth Enterobacteriaceae. 3. Isolation of a gammag normal antibody and characterization of other serum factors causing P32 loss. J Bacteriol. 1966 Jan;91(1):401–408. doi: 10.1128/jb.91.1.401-408.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitznagel J. K. Normal serum cytotoxicity for P32-labeled smooth Enterobacteriaceae. II. Fate of macromolecular and lipid phosphorus of damaged cells. J Bacteriol. 1966 Jan;91(1):148–152. doi: 10.1128/jb.91.1.148-152.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitznagel J. K., Wilson L. A. Normal serum cytotoxicity for P32-labeled smooth Enterobacteriaceae. I. Loss of label, death, and ultrastructural damage. J Bacteriol. 1966 Jan;91(1):393–400. doi: 10.1128/jb.91.1.393-400.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Work E. The chemistry and morphology of surface structures of lysine-limited Escherichia coli. Folia Microbiol (Praha) 1967;12(3):220–226. doi: 10.1007/BF02868735. [DOI] [PubMed] [Google Scholar]










