Abstract
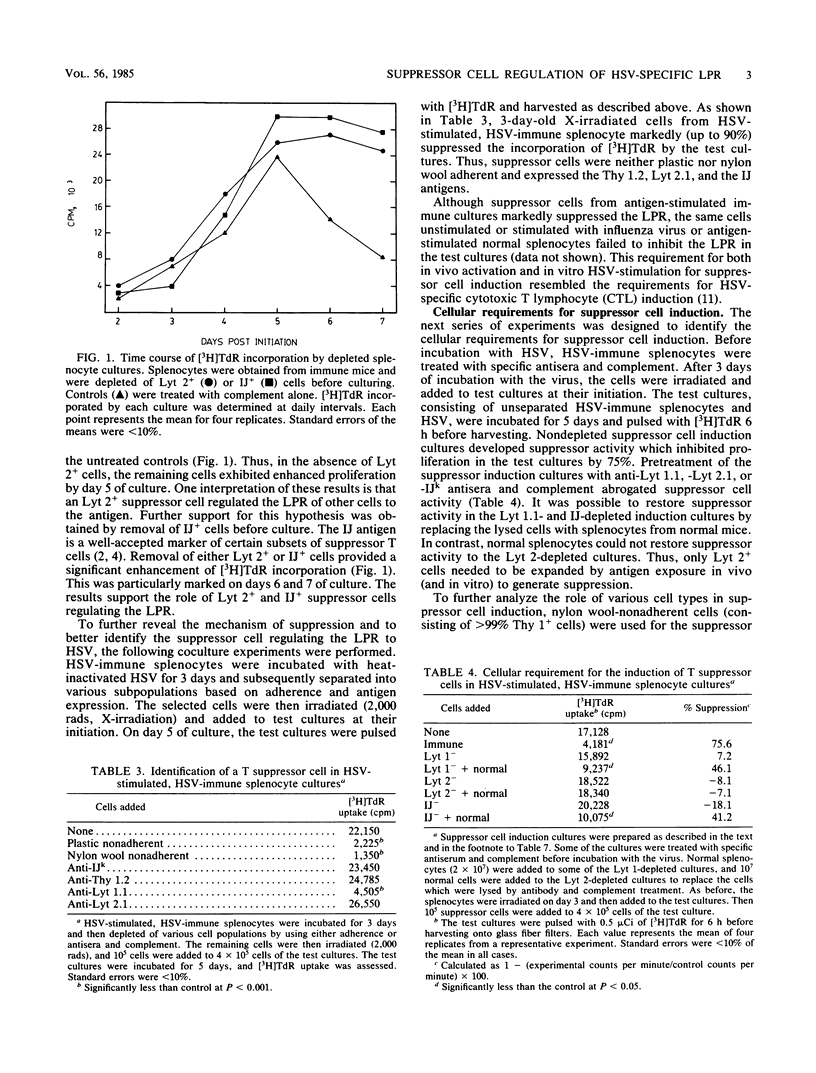
We investigated the regulation of the herpes simplex virus (HSV)-specific lymphoproliferative response (LPR) by suppressor cells. The chief cell types in HSV-immune splenocytes proliferating in response to the antigen were Lyt 1+ and Lyt 2+ T cells, which accounted for approximately 60 and 40% of the response, respectively. Because the total responsiveness of splenocytes was enhanced after depletion of Lyt 2+ cells, the LPR was assumed to be subject to regulation by an Lyt 2+ suppressor cell. This was shown to be the case with an experimental design in which suppressor cell activity was induced in one culture, the cells were irradiated, and the effects on LPR were measured in a test antigen-stimulated culture. The cell responsible for suppression was shown to be Lyt 2+ IJ+, and the actual suppressor effect was not antigen specific. Cellular requirements for the generation of suppression were also investigated. The three distinct cell types that appeared to be required were Lyt 2+ and Lyt 1+ T cells and an IJ+ antigen-presenting cell. Of the three cell types, only the Lyt 2+ cell needed to be from HSV-immune animals. The implications of our model system for the better understanding of the role of immunity in herpesvirus pathogenesis are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benacerraf B., Germain R. N. A single major pathway of T-lymphocyte interactions in antigen-specific immune suppression. Scand J Immunol. 1981;13(1):1–10. doi: 10.1111/j.1365-3083.1981.tb00104.x. [DOI] [PubMed] [Google Scholar]
- Dorf M. E., Benacerraf B. Suppressor cells and immunoregulation. Annu Rev Immunol. 1984;2:127–157. doi: 10.1146/annurev.iy.02.040184.001015. [DOI] [PubMed] [Google Scholar]
- Fitch F. W., Engers H. D., Cerottini J. C., Bruner K. T. Generation of cytotoxic T lymphocytes in vitro. VII. Suppressive effect of irradiated MLC cells on CTL response. J Immunol. 1976 Mar;116(3):716–723. [PubMed] [Google Scholar]
- Guerne P. A., Piguet P. F., Vassalli P. Production of interleukin 2, interleukin 3, and interferon by mouse T lymphocyte clones of Lyt-2+ and -2- phenotype. J Immunol. 1984 Apr;132(4):1869–1871. [PubMed] [Google Scholar]
- Gullberg M., Larsson E. L. Studies on induction and effector functions of concanavalin A-induced suppressor cells that limit TCGF production. J Immunol. 1982 Feb;128(2):746–750. [PubMed] [Google Scholar]
- Iwasaka T., Sheridan J. F., Aurelian L. Immunity to herpes simplex virus type 2: recurrent lesions are associated with the induction of suppressor cells and soluble suppressor factors. Infect Immun. 1983 Dec;42(3):955–964. doi: 10.1128/iai.42.3.955-964.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer M., Koszinowski U. T cell-specific suppressor factor(s) with regulatory influence on interleukin 2 production and function. J Immunol. 1982 Feb;128(2):784–790. [PubMed] [Google Scholar]
- Larsen H. S., Feng M. F., Horohov D. W., Moore R. N., Rouse B. T. Role of T-lymphocyte subsets in recovery from herpes simplex virus infection. J Virol. 1984 Apr;50(1):56–59. doi: 10.1128/jvi.50.1.56-59.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawman M. J., Rouse B. T., Courtney R. J., Walker R. D. Cell-mediated immunity against herpes simplex induction of cytotoxic T lymphocytes. Infect Immun. 1980 Jan;27(1):133–139. doi: 10.1128/iai.27.1.133-139.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. A., Stutman O. Use of positively selected Lyt-2+ mouse splenocytes to examine interleukin-2 secretion in responses to alloantigens and to TNP-modified syngeneic cells. Cell Immunol. 1982 Mar 15;68(1):114–127. doi: 10.1016/0008-8749(82)90094-6. [DOI] [PubMed] [Google Scholar]
- Schmid D. S., Rouse B. T. Cellular interactions in the cytotoxic T lymphocyte response to herpes simplex virus antigens: differential antigen activation requirements for the helper T lymphocyte and cytotoxic T lymphocyte precursors. J Immunol. 1983 Jul;131(1):479–484. [PubMed] [Google Scholar]
- Sheridan J. F., Donnenberg A. D., Aurelian L., Elpern D. J. Immunity to herpes simplex virus type 2. IV. Impaired lymphokine production during recrudescence correlates with an imbalance in T lymphocyte subsets. J Immunol. 1982 Jul;129(1):326–331. [PubMed] [Google Scholar]
- Shillitoe E. J., Wilton J. M., Lehner T. Sequential changes in cell-mediated immune responses to herpes simplex virus after recurrent herpetic infection in humans. Infect Immun. 1977 Oct;18(1):130–137. doi: 10.1128/iai.18.1.130-137.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. A. T-cell growth factor. Immunol Rev. 1980;51:337–357. doi: 10.1111/j.1600-065x.1980.tb00327.x. [DOI] [PubMed] [Google Scholar]
- Zembala M. A., Asherson G. L., James B. M., Stein V. E., Watkins M. C. Anti-haptene T suppressor factor acts through an I-J+, Ly1-2+, T acceptor cell that releases a nonspecific inhibitor of the transfer of contact sensitivity when exposed to antigen. J Immunol. 1982 Nov;129(5):1823–1829. [PubMed] [Google Scholar]


