Abstract
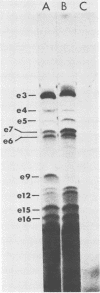
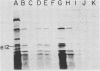
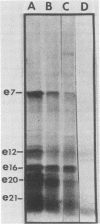
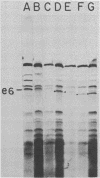
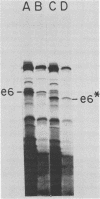
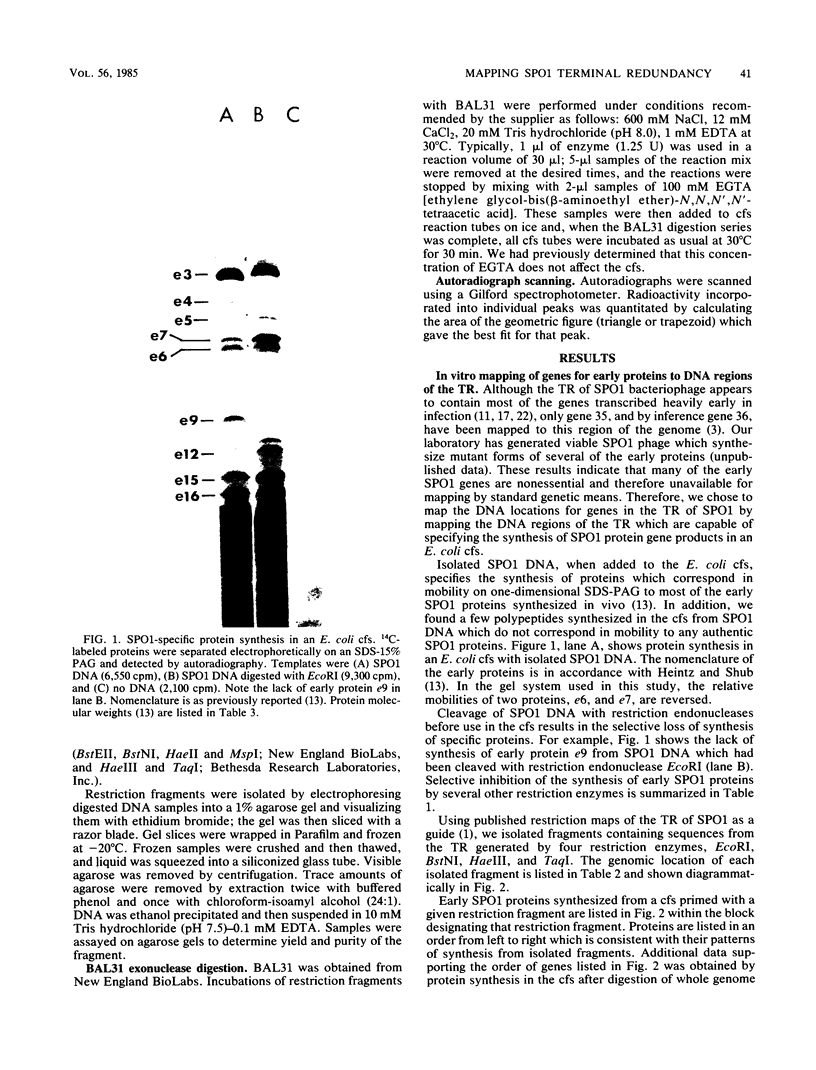
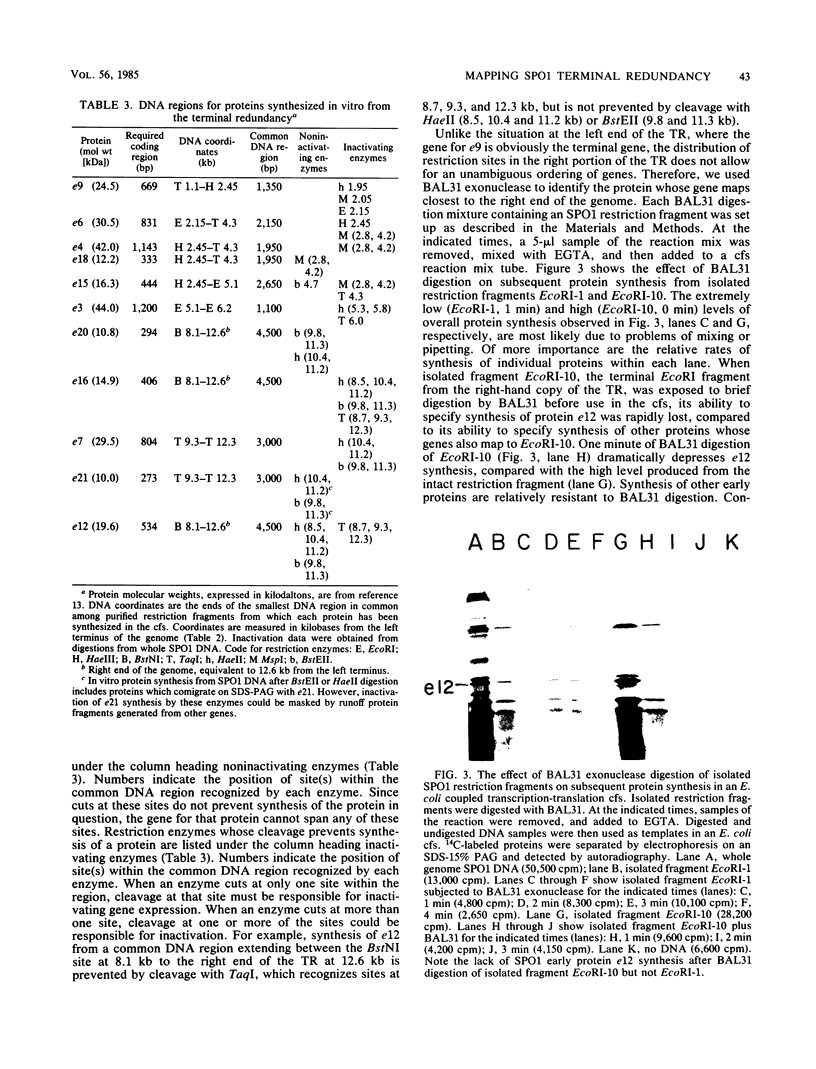
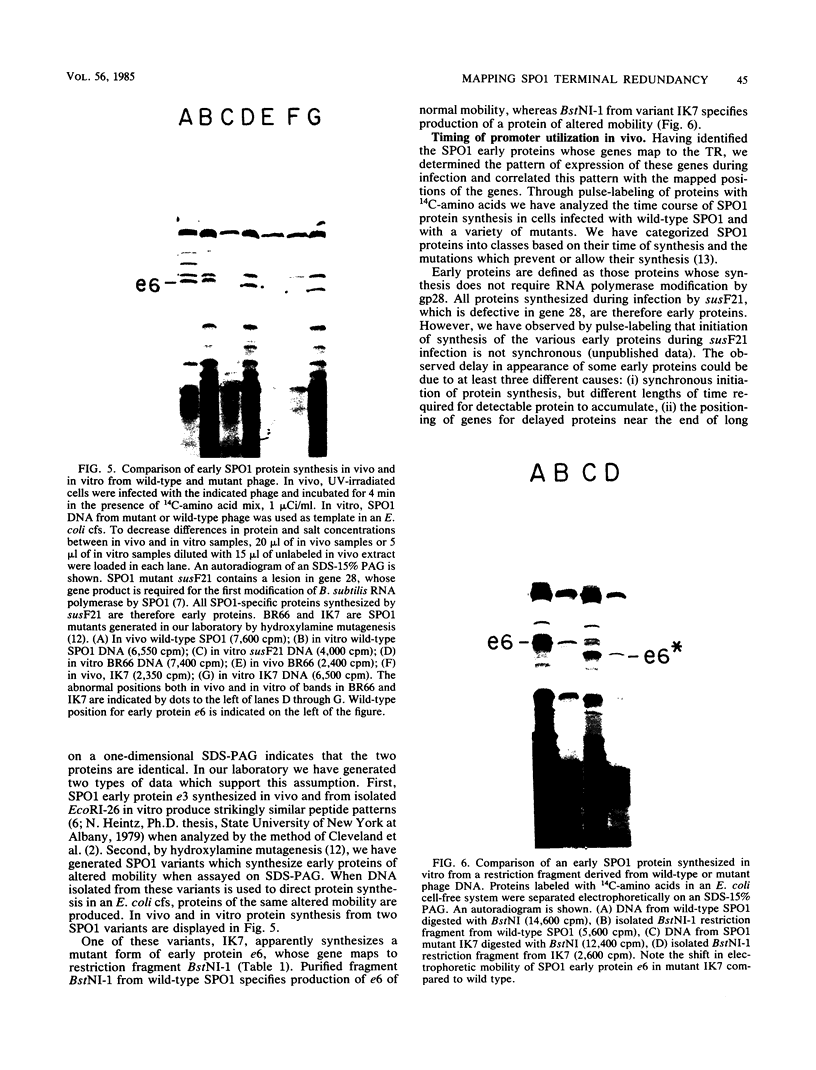
Although most early transcription from SPO1, a lytic DNA bacteriophage of Bacillus subtilis, is specified by the 12.6-kilobase region of the terminal redundancy, early genes from this region have not been identified by standard genetic means. We mapped genes to DNA regions of the SPO1 terminal redundancy by analyzing in vitro protein synthesis from isolated SPO1 restriction fragments in an Escherichia coli-coupled transcription-translation cell-free system. DNA from the terminal redundancy directs the synthesis in vitro of eleven proteins, e3, e4, e6, e7, e9, e12, e15, e16, e18, e20, and e21, which correspond in mobility on sodium dodecyl sulfate-polyacrylamide gels with authentic SPO1 early proteins. From their mapped positions on the DNA, genes were positioned downstream from most, but not all, of the twelve early promoter regions identified in vitro in the terminal redundancy. The temporal patterns of early protein synthesis in vivo suggest a differential turning on and off of early promoters in the terminal redundancy. Both in vivo and in vitro evidence suggests the existence of previously unidentified early promoter regions upstream from the genes for e6 and e4 as well as a middle promoter region upstream from the gene for e16.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chelm B. K., Romeo J. M., Brennan S. M., Geiduschek E. P. A transcriptional map of the bacteriophage SPO1 genome. III. A region of early and middle promoters (the gene 28 region). Virology. 1981 Jul 30;112(2):572–588. doi: 10.1016/0042-6822(81)90303-2. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cregg J. M., Stewart C. R. EcoRI cleavage of DNA from Bacillus subtilis phage SPO1. Virology. 1978 Apr;85(2):601–605. doi: 10.1016/0042-6822(78)90464-6. [DOI] [PubMed] [Google Scholar]
- Cregg J. M., Stewart C. R. Terminal redundancy of "high frequency of recombination" markers of Bacillus subtilis phage SPO1. Virology. 1978 May 15;86(2):530–541. doi: 10.1016/0042-6822(78)90091-0. [DOI] [PubMed] [Google Scholar]
- Davison B. L., Murray C. L., Rabinowitz J. C. Specificity of promoter site utilization in vitro by bacterial RNA polymerases on Bacillus phage phi 29 DNA. Transcription mapping with exonuclease III. J Biol Chem. 1980 Sep 25;255(18):8819–8830. [PubMed] [Google Scholar]
- Fischer S. G. Peptide mapping in gels. Methods Enzymol. 1983;100:424–430. doi: 10.1016/0076-6879(83)00071-3. [DOI] [PubMed] [Google Scholar]
- Fox T. D., Losick R., Pero J. Regulatory gene 28 of bacteriophage SPO1 codes for a phage-induced subunit of RNA polymerase. J Mol Biol. 1976 Mar 5;101(3):427–433. doi: 10.1016/0022-2836(76)90157-1. [DOI] [PubMed] [Google Scholar]
- Gage L. P., Geiduschek E. P. RNA synthesis during bacteriophage SPO1 development: six classes of SPO1 RNA. J Mol Biol. 1971 Apr 28;57(2):279–297. doi: 10.1016/0022-2836(71)90346-9. [DOI] [PubMed] [Google Scholar]
- Greene J. R., Chelm B. K., Geiduschek E. P. SP01 gene 27 is required for viral late transcription. J Virol. 1982 Feb;41(2):715–720. doi: 10.1128/jvi.41.2.715-720.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D. H., Tessman I. T4 mutants unable to induce deoxycytidylate deaminase activity. Virology. 1966 Jun;29(2):339–345. doi: 10.1016/0042-6822(66)90041-9. [DOI] [PubMed] [Google Scholar]
- Heintz N., Shub D. A. Transcriptional regulation of bacteriophage SPO1 protein synthesis in vivo and in vitro. J Virol. 1982 Jun;42(3):951–962. doi: 10.1128/jvi.42.3.951-962.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G., Talkington C., Pero J. Nucleotide sequence of a promoter recognized by Bacillus subtilis RNA polymerase. Mol Gen Genet. 1980;180(1):57–65. doi: 10.1007/BF00267352. [DOI] [PubMed] [Google Scholar]
- Moran C. P., Jr, Lang N., LeGrice S. F., Lee G., Stephens M., Sonenshein A. L., Pero J., Losick R. Nucleotide sequences that signal the initiation of transcription and translation in Bacillus subtilis. Mol Gen Genet. 1982;186(3):339–346. doi: 10.1007/BF00729452. [DOI] [PubMed] [Google Scholar]
- Okubo S., Yanagida T., Fujita D. J., Olsson-Wilhelm B. M. The genetics of bacteriophage SPO1. Biken J. 1972 Jun;15(2):81–97. [PubMed] [Google Scholar]
- Pero J., Hannett N. M., Talkington C. Restriction cleavage map of SP01 DNA: general location of early, middle, and late genes. J Virol. 1979 Jul;31(1):156–171. doi: 10.1128/jvi.31.1.156-171.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polisky B., Greene P., Garfin D. E., McCarthy B. J., Goodman H. M., Boyer H. W. Specificity of substrate recognition by the EcoRI restriction endonuclease. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3310–3314. doi: 10.1073/pnas.72.9.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shub D. A. Bacteriophage SPO1 DNA- and RNA-directed protein synthesis in vitro: comparison with in vivo control. Mol Gen Genet. 1975;137(2):171–180. doi: 10.1007/BF00341683. [DOI] [PubMed] [Google Scholar]
- Shub D. A., Swanton M., Smith D. H. The nature of transcription selectivity of bacteriophage SPO1-modified RNA polymerase. Mol Gen Genet. 1979 May 4;172(2):193–197. doi: 10.1007/BF00268282. [DOI] [PubMed] [Google Scholar]
- Talkington C., Pero J. Restriction fragment analysis of the temporal program of bacteriophage SPO1 transcription and its control by phage-modified RNA polymerases. Virology. 1977 Dec;83(2):365–379. doi: 10.1016/0042-6822(77)90181-7. [DOI] [PubMed] [Google Scholar]
- Wiggs J. L., Bush J. W., Chamberlin M. J. Utilization of promoter and terminator sites on bacteriophage T7 DNA by RNA polymerases from a variety of bacterial orders. Cell. 1979 Jan;16(1):97–109. doi: 10.1016/0092-8674(79)90191-0. [DOI] [PubMed] [Google Scholar]