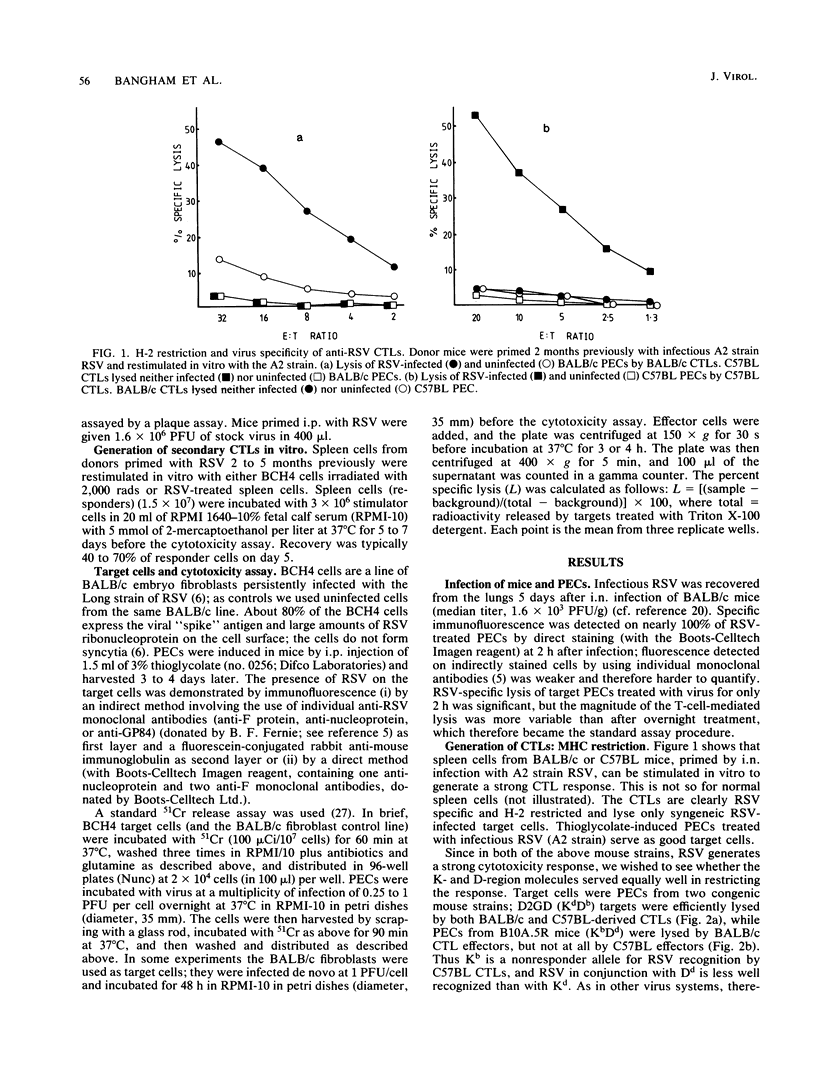
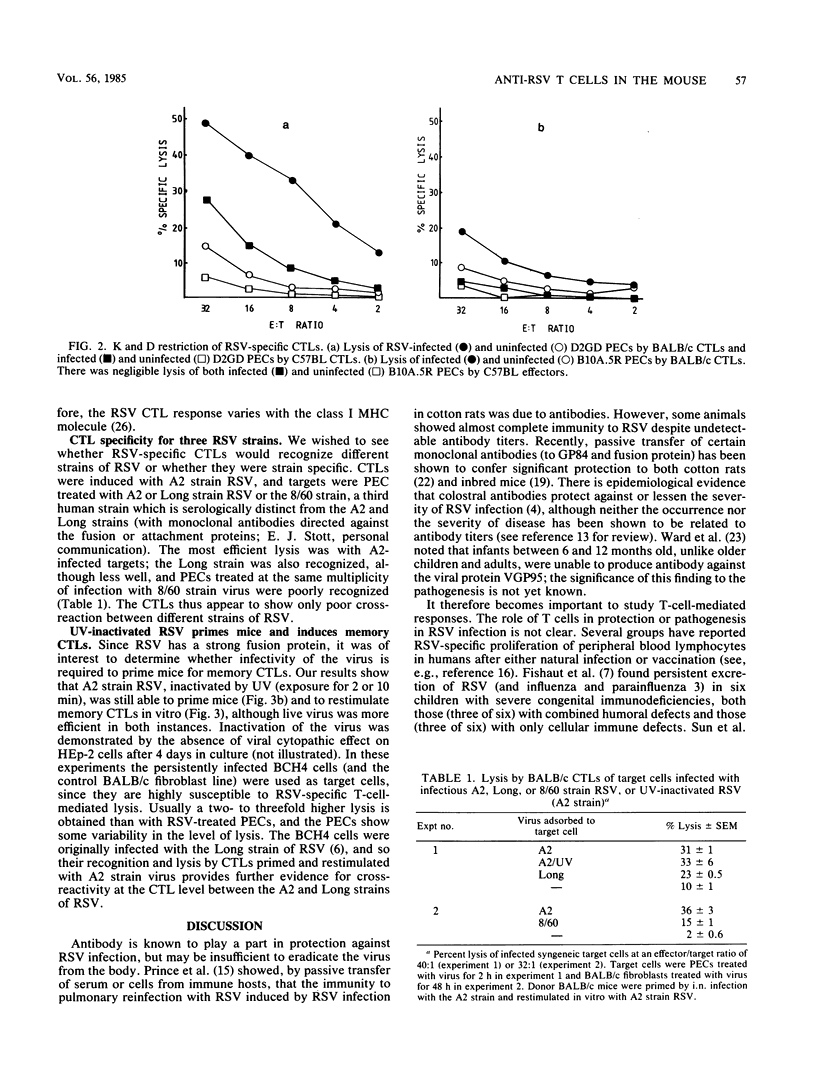
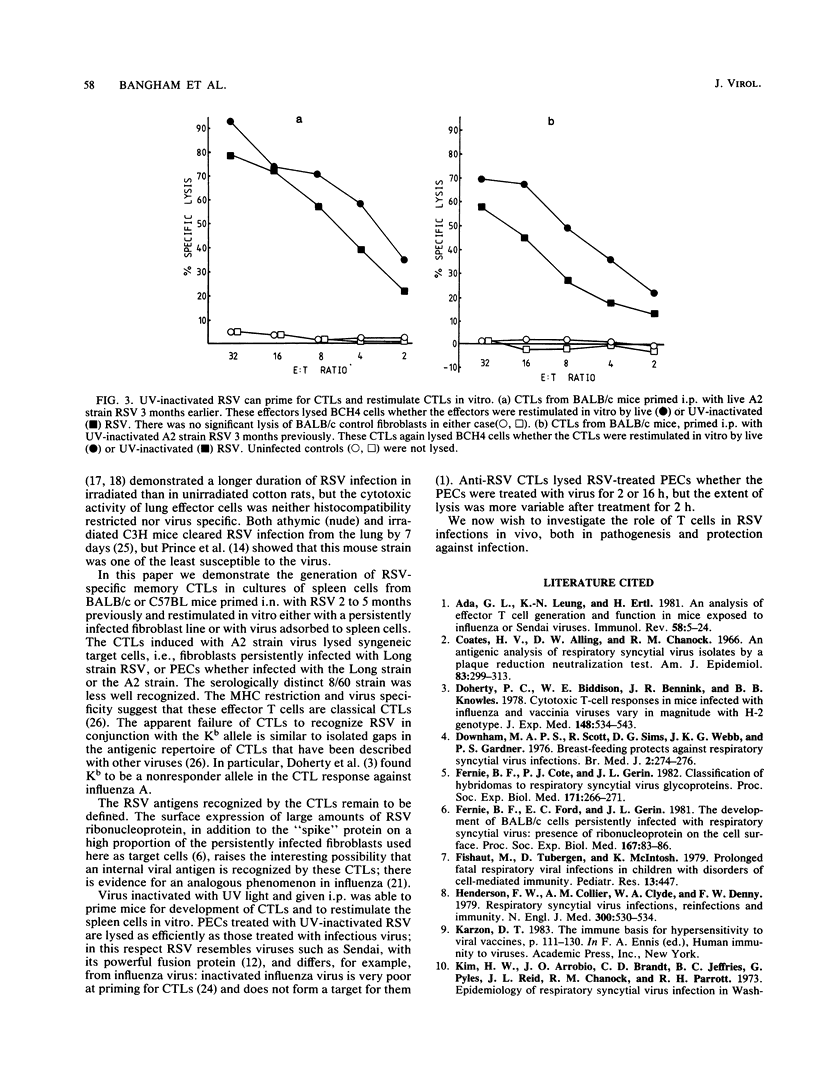
Abstract
The role of the humoral and cellular arms of the immune response in protection against respiratory syncytial virus (RSV) infection and in the pathogenesis of the severe forms of this disease is poorly understood. The recent demonstration that some inbred mouse strains can be infected with RSV has opened the way to a detailed investigation of RSV immunity. We report here the finding of major histocompatibility complex-restricted, RSV-specific memory cytotoxic T cells in the spleens of BALB/c and C57BL mice after intranasal infection; these T cells recognize the Long, A2, and 8/60 (human) strains of RSV. Both K and D locus major histocompatibility complex alleles can restrict the cytotoxic response; however, in the two haplotypes tested, Dd is a low-responder allele and Kb is a nonresponder allele for RSV. UV-inactivated RSV (when given intraperitoneally) can prime mice for development of cytotoxic T cell memory, restimulate cytotoxic T cell cultures in vitro, and form a target for the cytotoxic cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ada G. L., Leung K. N., Ertl H. An analysis of effector T cell generation and function in mice exposed to influenza A or Sendai viruses. Immunol Rev. 1981;58:5–24. doi: 10.1111/j.1600-065x.1981.tb00347.x. [DOI] [PubMed] [Google Scholar]
- Coates H. V., Alling D. W., Chanock R. M. An antigenic analysis of respiratory syncytial virus isolates by a plaque reduction neutralization test. Am J Epidemiol. 1966 Mar;83(2):299–313. doi: 10.1093/oxfordjournals.aje.a120586. [DOI] [PubMed] [Google Scholar]
- Doherty P. C., Biddison W. E., Bennink J. R., Knowles B. B. Cytotoxic T-cell responses in mice infected with influenza and vaccinia viruses vary in magnitude with H-2 genotype. J Exp Med. 1978 Aug 1;148(2):534–543. doi: 10.1084/jem.148.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downham M. A., Scott R., Sims D. G., Webb J. K., Gardner P. S. Breast-feeding protects against respiratory syncytial virus infections. Br Med J. 1976 Jul 31;2(6030):274–276. doi: 10.1136/bmj.2.6030.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie B. F., Cote P. J., Jr, Gerin J. L. Classification of hybridomas to respiratory syncytial virus glycoproteins. Proc Soc Exp Biol Med. 1982 Dec;171(3):266–271. doi: 10.3181/00379727-171-41509. [DOI] [PubMed] [Google Scholar]
- Fernie B. F., Ford E. C., Gerin J. L. The development of Balb/c cells persistently infected with respiratory syncytial virus: presence of ribonucleoprotein on the cell surface. Proc Soc Exp Biol Med. 1981 May;167(1):83–86. doi: 10.3181/00379727-167-41129. [DOI] [PubMed] [Google Scholar]
- Henderson F. W., Collier A. M., Clyde W. A., Jr, Denny F. W. Respiratory-syncytial-virus infections, reinfections and immunity. A prospective, longitudinal study in young children. N Engl J Med. 1979 Mar 8;300(10):530–534. doi: 10.1056/NEJM197903083001004. [DOI] [PubMed] [Google Scholar]
- Kim H. W., Arrobio J. O., Brandt C. D., Jeffries B. C., Pyles G., Reid J. L., Chanock R. M., Parrott R. H. Epidemiology of respiratory syncytial virus infection in Washington, D.C. I. Importance of the virus in different respiratory tract disease syndromes and temporal distribution of infection. Am J Epidemiol. 1973 Sep;98(3):216–225. doi: 10.1093/oxfordjournals.aje.a121550. [DOI] [PubMed] [Google Scholar]
- Kim H. W., Canchola J. G., Brandt C. D., Pyles G., Chanock R. M., Jensen K., Parrott R. H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969 Apr;89(4):422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- Koszinowski U., Gething M. J., Waterfield M. T-cell cytotoxicity in the absence of viral protein synthesis in target cells. Nature. 1977 May 12;267(5607):160–163. doi: 10.1038/267160a0. [DOI] [PubMed] [Google Scholar]
- McIntosh K., Fishaut J. M. Immunopathologic mechanisms in lower respiratory tract disease of infants due to respiratory syncytial virus. Prog Med Virol. 1980;26:94–118. [PubMed] [Google Scholar]
- Prince G. A., Horswood R. L., Berndt J., Suffin S. C., Chanock R. M. Respiratory syncytial virus infection in inbred mice. Infect Immun. 1979 Nov;26(2):764–766. doi: 10.1128/iai.26.2.764-766.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince G. A., Horswood R. L., Camargo E., Koenig D., Chanock R. M. Mechanisms of immunity to respiratory syncytial virus in cotton rats. Infect Immun. 1983 Oct;42(1):81–87. doi: 10.1128/iai.42.1.81-87.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R., Pullan C. R., Scott M., McQuillin J. Cell-mediated immunity in respiratory syncytial virus disease. J Med Virol. 1984;13(1):105–114. doi: 10.1002/jmv.1890130112. [DOI] [PubMed] [Google Scholar]
- Sun C. S., Wyde P. R., Knight V. Correlation of cytotoxic activity in lungs to recovery of normal and gamma-irradiated cotton rats from respiratory syncytial virus infection. Am Rev Respir Dis. 1983 Oct;128(4):668–672. doi: 10.1164/arrd.1983.128.4.668. [DOI] [PubMed] [Google Scholar]
- Sun C. S., Wyde P. R., Wilson S. Z., Knight V. Cell-mediated cytotoxic responses in lungs of cotton rats infected with respiratory syncytial virus. Am Rev Respir Dis. 1983 Apr;127(4):460–464. doi: 10.1164/arrd.1983.127.4.460. [DOI] [PubMed] [Google Scholar]
- Taylor G., Stott E. J., Bew M., Fernie B. F., Cote P. J., Collins A. P., Hughes M., Jebbett J. Monoclonal antibodies protect against respiratory syncytial virus infection in mice. Immunology. 1984 May;52(1):137–142. [PMC free article] [PubMed] [Google Scholar]
- Taylor G., Stott E. J., Hughes M., Collins A. P. Respiratory syncytial virus infection in mice. Infect Immun. 1984 Feb;43(2):649–655. doi: 10.1128/iai.43.2.649-655.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend A. R., Skehel J. J. Influenza A specific cytotoxic T-cell clones that do not recognize viral glycoproteins. Nature. 1982 Dec 16;300(5893):655–657. doi: 10.1038/300655a0. [DOI] [PubMed] [Google Scholar]
- Walsh E. E., Schlesinger J. J., Brandriss M. W. Protection from respiratory syncytial virus infection in cotton rats by passive transfer of monoclonal antibodies. Infect Immun. 1984 Feb;43(2):756–758. doi: 10.1128/iai.43.2.756-758.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward K. A., Lambden P. R., Ogilvie M. M., Watt P. J. Antibodies to respiratory syncytial virus polypeptides and their significance in human infection. J Gen Virol. 1983 Sep;64(Pt 9):1867–1876. doi: 10.1099/0022-1317-64-9-1867. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Askonas B. A. Cross-protection and cross-reactive cytotoxic T cells induced by influenza virus vaccines in mice. Eur J Immunol. 1980 May;10(5):396–401. doi: 10.1002/eji.1830100515. [DOI] [PubMed] [Google Scholar]
- Wyde P. R., Sun C. S., Knight V. Replication of respiratory syncytial virus in lungs of immunodeficient mice. J Reticuloendothel Soc. 1983 Aug;34(2):125–129. [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]
- Zweerink H. J., Askonas B. A., Millican D., Courtneidge S. A., Skehel J. J. Cytotoxic T cells to type A influenza virus; viral hemagglutinin induces A-strain specificity while infected cells confer cross-reactive cytotoxicity. Eur J Immunol. 1977 Sep;7(9):630–635. doi: 10.1002/eji.1830070910. [DOI] [PubMed] [Google Scholar]


