Abstract
The endothelial-derived G-protein–coupled receptor EDG-1 is a high-affinity receptor for the bioactive lipid mediator sphingosine-1-phosphate (SPP). In the present study, we constructed the EDG-1–green fluorescent protein (GFP) chimera to examine the dynamics and subcellular localization of SPP–EDG-1 interaction. SPP binds to EDG-1–GFP and transduces intracellular signals in a manner indistinguishable from that seen with the wild-type receptor. Human embryonic kidney 293 cells stably transfected with the EDG-1–GFP cDNA expressed the receptor primarily on the plasma membrane. Exogenous SPP treatment, in a dose-dependent manner, induced receptor translocation to perinuclear vesicles with a τ1/2 of ∼15 min. The EDG-1–GFP–containing vesicles are distinct from mitochondria but colocalize in part with endocytic vesicles and lysosomes. Neither the low-affinity agonist lysophosphatidic acid nor other sphingolipids, ceramide, ceramide-1-phosphate, or sphingosylphosphorylcholine, influenced receptor trafficking. Receptor internalization was completely inhibited by truncation of the C terminus. After SPP washout, EDG-1–GFP recycles back to the plasma membrane with a τ1/2 of ∼30 min. We conclude that the high-affinity ligand SPP specifically induces the reversible trafficking of EDG-1 via the endosomal pathway and that the C-terminal intracellular domain of the receptor is critical for this process.
INTRODUCTION
The bioactive sphingolipid mediator sphingosine-1-phosphate (SPP) is synthesized by sphingosine kinase-catalyzed phosphorylation of sphingosine (Buehrer and Bell, 1993). It is produced by many cells, including platelets (Yatomi et al., 1995, 1997), fibroblasts (Olivera and Spiegel, 1993), and neuron-like pheochromocytoma-12 cells (Edsall et al., 1997). SPP elicits a variety of biological responses, such as fibroblast proliferation (Zhang et al., 1991), neurite retraction (Postma et al., 1996), calcium signaling (Ghosh et al., 1990), regulation of apoptosis (Cuvillier et al., 1996; Hung and Chuang, 1996), morphogenetic differentiation (Lee et al., 1998b), inhibition of cell motility (Sadahira et al., 1992; Bornfeldt et al., 1995), induction of activator protein-1 transcription factor activity (Su et al., 1994), regulation of G-protein–dependent cAMP levels (Zhang et al., 1991; Bornfeldt et al., 1995), and mitogen-activated protein (MAP) kinase activity (Wu et al., 1995). Many of the effects of SPP are mediated by plasma membrane receptors that are coupled to G-proteins (reviewed in Hla et al., 1999). For example, SPP induces the calcium transients, which are inhibited by pertussis toxin (Van Koppen et al., 1996). However, some effects of SPP may occur via the intracellular action of this mediator on novel, although unknown, intracellular targets (reviewed in Spiegel and Merrill, 1996). Several lines of evidence support this concept; first, dimethylsphingosine, which inhibits endogenous synthesis of SPP, blocked apoptosis in monocytic cell lines (Cuvillier et al., 1996) and platelet-derived growth factor–induced mitogenesis in Swiss 3T3 fibroblasts (Olivera and Spiegel, 1993). Second, SPP increased intracellular calcium currents (Ghosh et al., 1990; Mattie et al., 1994). Third, Fc receptor activation of calcium transients was also inhibited by dimethylsphingosine (Choi et al., 1996), and fourth, G-protein–coupled receptor activation of intracellular calcium currents was attenuated by sphingosine kinase inhibitors (Heringdorf et al., 1998). These data raise the possibility that SPP may have dual actions; it interacts with receptors on the plasma membrane as well as with intracellular targets (Van Brocklyn et al., 1998).
Recently, we demonstrated that the endothelial cell-derived G-protein–coupled receptor (GPR) EDG-1 is a high-affinity SPP receptor (Lee et al., 1998b). EDG-1 was originally cloned as an immediate early gene induced during phorbol myristic acetate-induced differentiation of endothelial cells (Hla and Maciag, 1990). Recently, it was cloned independently as a shear stress-induced gene in endothelial cells (Takada et al., 1997). The transcript for EDG-1 is widely expressed in vivo and is developmentally regulated (Liu and Hla, 1997). SPP binds to EDG-1 GPR with a Kd of ∼8 nM, and low concentrations of SPP stimulated EDG-1–dependent signaling events, indicating that EDG-1 is a high-affinity SPP receptor (Lee et al., 1998b). Although EDG-1 binds to SPP as a high-affinity ligand, it appears to regulate only a subset of SPP actions. We recently provided evidence that SPP–EDG-1 signaling activates Gi-dependent MAP kinase activation as well as the inhibition of forskolin-activated cAMP formation (Lee et al., 1998b; Van Brocklyn et al., 1998). Similar findings were also observed in other cell systems (Zondag et al., 1998). In addition, Rho-dependent signaling pathways are activated, resulting in the up-regulation of P- and E-cadherin levels, adherens junction assembly, and morphogenesis (Lee et al., 1998b). However, the following signaling pathways were not regulated by EDG-1 in human embryonic kidney (HEK)293 fibroblast-like cells: intracellular Ca2+ transients, phospholipase D activation, and focal adhesion kinase phosphorylation (Van Brocklyn et al., 1998). However, in Chinese hamster ovary cells, SPP activation of EDG-1 results in Gi/Go-dependent phospholipase C/calcium signaling (Okamoto et al., 1998). Moreover, we also showed that lysophosphatidic acid (LPA), which is structurally similar to SPP, directly bound to EDG-1 and induced EDG-1–dependent signals (Lee et al., 1998a). However, LPA binding to EDG-1 was of much lower affinity (Kd of ∼2.3 μM), and higher concentrations of LPA were required to induce EDG-1–dependent signals, suggesting that it is a low-affinity ligand for EDG-1. Both SPP and LPA are secreted by platelets upon activation (Yatomi et al., 1995; Moolenaar et al., 1997), suggesting that EDG-1 signaling in the endothelium may be important in platelet–endothelial cell interactions. Recently, An and Goetzl reported that GPRs of the EDG-1 family, EDG-3 and EDG-5 (also known as H218), were activated by low doses of SPP in heterologous overexpression systems (An et al., 1997). These data suggest that EDG-1 and the related receptors EDG-3 and EDG-5 are high-affinity SPP receptors that mediate the pleiotropic actions of this bioactive lipid mediator.
The aim of this study was to define the subcellular localization of EDG-1 after ligand activation. We prepared an EDG-1–green fluorescent protein (GFP) chimera and investigated the pathways and dynamics of receptor trafficking.
MATERIALS AND METHODS
Materials
pEGFP-N1 that contains the enhanced GFP cDNA under the control of the cytomegalovirus promoter and neomycin-resistance gene was obtained from Clontech (Palo Alto, CA). Lipids (SPP, sphingosylphosphorylcholine, and LPA) were purchased from Biomol (Plymouth Meeting, PA). The radioimmunoassay kit for cAMP was from Amersham (Arlington Heights, IL).
Construction of EDG-1–GFP Chimera and Transfection
Human EDG-1 cDNA (Hla and Maciag, 1990) excluding the C-terminal termination codon was amplified from the cDNA using the primers AGA TCT CGA GCC ACC ATG GGG CCC ACC AGC GTC CCG and ACC GGT GGA TCC CCG GAA GAA GAG TTG ACG TTT CC and was subcloned into a TA cloning vector (Invitrogen, San Diego, CA). The EDG-1 fragment was subcloned into the multiple cloning site of the pEGFP-N1 plasmid using XhoI and BamHI sites, resulting in the fusion of the GFP polypeptide in the extreme C terminus of the EDG-1 receptor. The resultant clone was sequenced completely, and no mutations were found. C-terminal deletions were done using PCR as indicated. Cloning and sequencing of the deletion clones were done as described above. HEK293 cells were transfected with pEDG-1–GFP, the deletion clones, and the parental pEGFP-N1 plasmid using the lipid Superfect reagent (Qiagen, Hilden, Germany) and following the manufacturer’s instructions. Transfected cells were selected in G418 (0.5 mg/ml) (Life Technologies, Gaithersburg, MD), and individual clones were isolated after observation in a Zeiss (Thornwood, NY) TV100 fluorescence microscope. At least two independent clones for each construct were used in all experiments with identical results.
Immunoblot Analysis
Stably transfected HEK293 cells were extracted with the radioimmunoprecipitation buffer (150 mM NaCl, 1% Nonidet P-40, 0.5% deoxycholic acid, 0.1% SDS, 50 mM Tris·HCl, pH 8.0), separated on 10% SDS-PAGE, immunoblotted with the polyclonal anti-GFP antibody (Molecular Probes, Eugene, OR), and visualized with the ECL chemiluminescence detection kit (Amersham).
[32P]SPP Binding Assays
For the SPP binding assay, cells were grown to confluence, and binding assays were conducted at 4°C with [32P]SPP as described previously (Lee et al., 1998b).
MAP Kinase Assay
Cos-1 cells were cotransfected with 0–1 μg of EDG-1–GFP or GFP plasmid and 0.1 μg of tagged extracellular signal-regulated kinase (ERK)-2 plasmid as described previously (Lee et al., 1998b). The amount of DNA used for transfection was normalized with vector DNA. Thirty hours later, cells were made quiescent in 0.5% fetal bovine serum (FBS) and DMEM for 16 h. As indicated, some cells were pretreated with pertussis toxin (400 ng/ml) for 3 h before ligand stimulation. After stimulation with 50 nM SPP for 2 min, cell lysates were prepared and immunoprecipitated with the anti-HA monoclonal antibody. The expression of HA–ERK-2 polypeptide was quantitated by immunoblot analysis and was found to be equal. The ERK-2 activity of the immune complexes was assayed.
Immunofluorescence and Confocal Microscopy
GFP fluorescence of live or fixed (10% formalin in PBS) cells was visualized in a Zeiss TV100 inverted microscope using the FITC filter (488 nm) and 63× oil immersion objective lens. Confocal microscopy was conducted on a Zeiss CLSM410 laser-scanning confocal microscope at the Center for Biomedical Imaging at the University of Connecticut Health Center (Farmington, CT). GFP fluorescence was excited using a 488-nm argon/krypton laser, and emitted fluorescence was detected with a 515- to 540-nm bandpass filter. For Lysotracker red and tetramethylrhodamine ethyl ester (TMRE) dyes and Texas red–labeled transferrin (Molecular Probes), a 568-nm argon/krypton laser was used for excitation, and fluorescence was detected with a 590-nm filter. For real-time images, confocal microscopy was done using the Noran (Middleton, WI) confocal microscope with an attached heated stage and an environmental chamber. Confocal images were digitized and quantitated using the Ratio-View software developed by the Center for Biomedical Imaging at the University of Connecticut Health Center (http://www2.uchc.edu/htbit/home pages/software.html). Z-series scans of cells were obtained from six independent fields, and plasma membrane fluorescence at the equatorial plane was quantified.
RESULTS
Construction and Characterization of the EDG-1–GFP Polypeptide
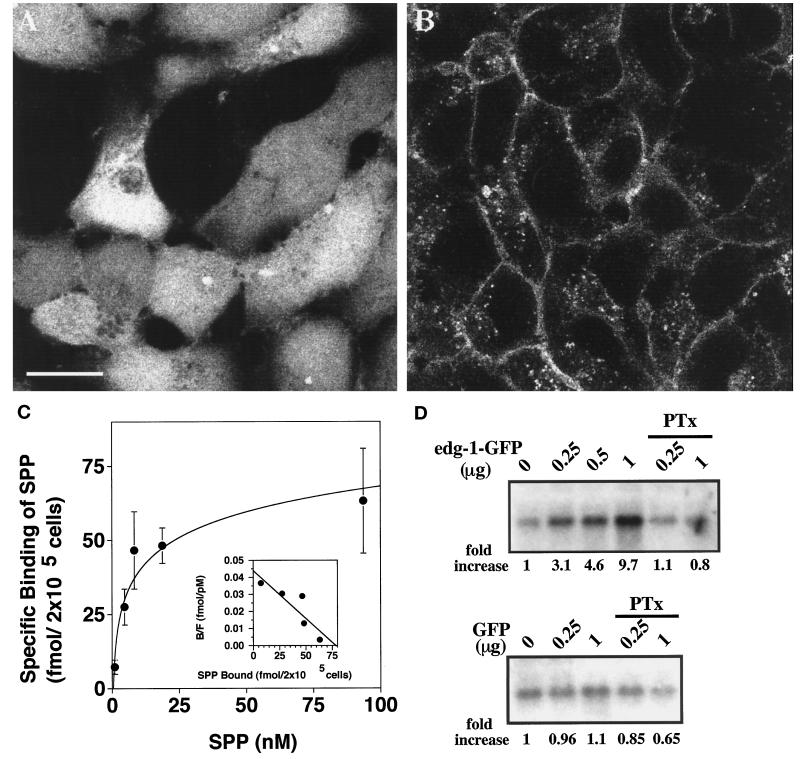
The enhanced GFP construct was fused in-frame at the C terminus of the human EDG-1 polypeptide. The production of fusion protein was determined by immunoblot analysis of HEK293 cell stable transfectants (see below). Subcellular localization of the EDG-1–GFP receptor was determined by confocal fluorescence microscopy. As shown in Figure 1, A and B, the GFP polypeptide was localized primarily in the cytosol. In contrast, the EDG-1–GFP receptor was expressed predominantly on the plasma membrane. However, a significant fraction of EDG-1–GFP fluorescence was also observed in punctate, intracellular vesicles. This pattern of receptor localization is similar to that of wild-type EDG-1 in transfected fibroblasts and endothelial cells, as determined by indirect immunofluorescence analysis (Lee et al., 1998a) (our unpublished observations). The functionality of the EDG-1–GFP construct was assayed by binding and signaling experiments. As shown in Figure 1C, [32P]SPP bound to HEK-293–EDG-1–GFP cells specifically and with high affinity (Kd = 7.4 nM, Bmax = 77 fmol per 200,000 cells). The binding characteristics of EDG-1–GFP are almost identical to that of EDG-1 (Lee et al., 1998b). Cotransfection of EDG-1–GFP with the HA–ERK-2 construct into Cos-1 cells, followed by immune complex MAP kinase assay, indicated that SPP signals via the EDG-1–GFP construct to activate MAP kinase activity (Figure 1D). As anticipated, GFP transfection did not influence MAP kinase activity. EDG-1–GFP–induced MAP kinase activity was suppressed by pertussis toxin pretreatment. These data suggest that the EDG-1–GFP construct functions similarly to the wild-type EDG-1 receptor to bind SPP and induce intracellular signaling events.
Figure 1.
Characterization of the EDG-1–GFP fusion protein. (A and B) Subcellular localization of GFP and EDG-1–GFP polypeptides is shown. HEK293 cells stably transfected with GFP (A) or EDG-1–GFP (B) were visualized by confocal fluorescence microscopy. Note that the GFP polypeptide is primarily cytosolic, whereas the EDG-1–GFP polypeptide is localized on the plasma membrane and intracellular vesicles. Bar, 10 μm. (C) EDG-1–GFP binds to SPP. HEK293 cells stably transfected with the EDG-1–GFP construct were used in whole-cell binding assays with [32P]SPP as described (Lee et al., 1998b). Data represent the mean of triplicate determinations of specific binding: (total − nonspecific binding) in 2 × 105 cells. Inset, Scatchard analysis of the binding isotherm indicates a Kd of 7.4 nM and a Bmax of 77 fmol per 2 × 105 cells. (D) EDG-1–GFP–dependent MAP kinase activation by SPP is shown. Cos-1 cells were transiently cotransfected with different amounts of EDG-1–GFP or GFP plasmids and a fixed amount of HA–ERK-2 plasmid. Total DNA input was kept constant with vector DNA. Thirty hours after transfection, cells were starved for 16 h and then stimulated with 50 nM SPP for 2 min. Cell lysates were prepared, and ERK-2 activity was measured using the immune complex kinase assay as described. Autoradiographs of the phosphorylated substrate, myelin basic protein, are shown. Quantitative data derived from densitometric scans are shown below the autoradiographs (fold increase). Some cultures received pertussis toxin (PTx) (400 ng/ml) for 3 h before SPP stimulation.
Effect of Exogenous SPP on Subcellular Localization of EDG-1–GFP
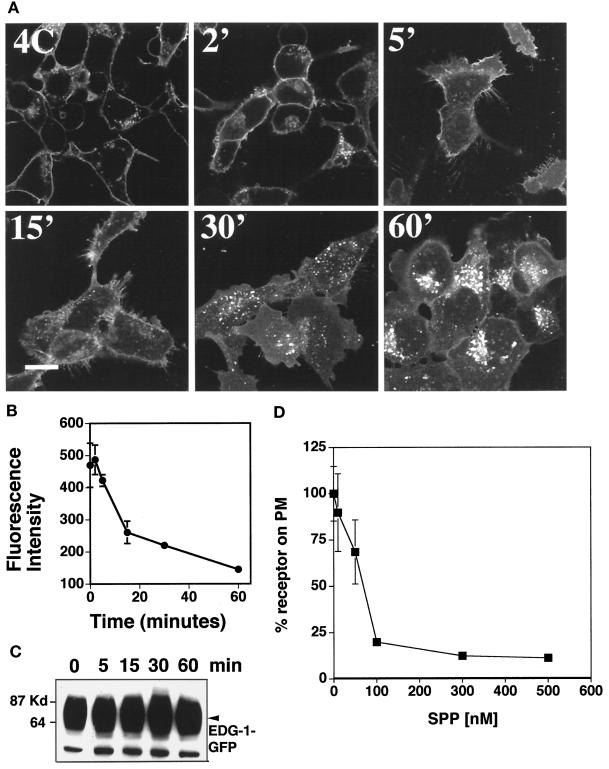
EDG-1–GFP was expressed primarily on the plasma membrane when cells were incubated with charcoal-stripped FBS (CFBS), which is deficient in serum-borne lipids. Exogenous SPP treatment (100 nM) at 37°C induced an initial transient increase in plasma membrane fluorescence followed by a decreased cell surface localization and increased vesicular localization of EDG-1–GFP within 15–60 min (Figure 2A). Quantitative analysis of EDG-1–GFP internalization was conducted by image analysis of fluorescence intensity on the plasma membrane. As shown in Figure 2B, SPP treatment induced rapid internalization of EDG-1–GFP with a τ1/2 of ∼15 min. In contrast, SPP treatment at 4°C did not induce receptor internalization. The vesicles are rounded, punctate, and localized in the perinuclear region. Hypertrophy of a subpopulation of vesicles was observed at later times. Ligand-induced EDG-1–GFP vesicular localization was not blocked by treatment with brefeldin A, which disrupts the ER–Golgi biosynthetic pathway (our unpublished observations), or cycloheximide (see below), which blocks protein synthesis. Immunoblot analysis of cell extracts with the anti-GFP antibody indicates the total amount if the EDG-1–GFP receptor did not change after SPP treatment (Figure 2C). This indicates that SPP induces the trafficking of the EDG-1 receptor and not receptor degradation. The dose–response study of EDG-1–GFP internalization is shown (Figure 2D); 100 nM SPP induced complete receptor internalization. Lower concentrations (10–50 nM) were much less effective.
Figure 2.
Exogenous SPP-induced internalization of EDG-1–GFP. (A) Confocal fluorescence microscopy. HEK293 cells stably expressing the EDG-1–GFP polypeptide were preincubated in CFBS for 2 d and treated with 100 nM SPP at 4°C for 15 min (4C). Cells were then shifted to 37°C for various time periods (2–60 min), fixed, and imaged in a confocal fluorescence microscope. Bar, 10 μm. (B) Quantitative analysis of EDG-1–GFP localization on the plasma membrane. Digitized fluorescence intensity on the plasma membrane after SPP treatment was quantitated as described and analyzed from six randomly selected fields. (C) Immunoblot analysis. HEK293 cells stably expressing the EDG-1–GFP polypeptide were starved in CFBS for 2 d and stimulated with 100 nM SPP for various times, cell extracts were prepared, and immunoblot analysis with the anti-GFP antibody was conducted as described. (D) Dose–response of SPP-induced internalization of EDG-1–GFP. HEK293 cells stably expressing the EDG-1–GFP polypeptide were starved in CFBS for 2 d, treated with the indicated concentrations of SPP at 37°C for 30 min, fixed, and imaged in a confocal fluorescence microscope. Digitized fluorescence intensity on the plasma membrane after SPP treatment was quantitated as described and analyzed from six randomly selected fields. Percent fluorescence intensity on the plasma membrane (PM) is shown.
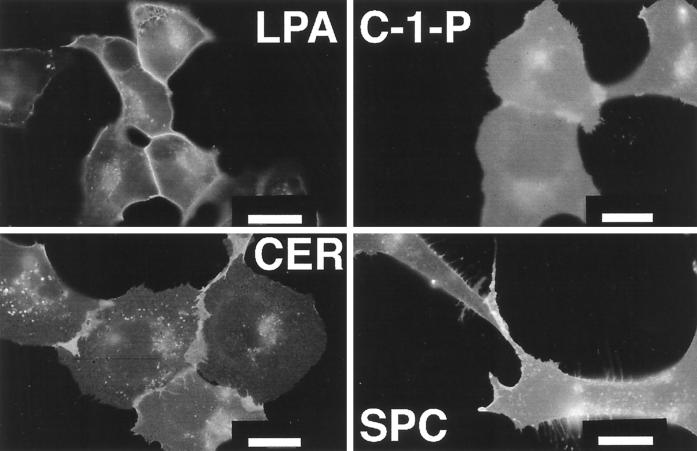
We recently showed that related sphingolipids, ceramide, ceramide-1-phosphate, and sphingosylphosphorylcholine, did not compete for high-affinity SPP binding (Lee et al., 1998b). Treatment of EDG-1–GFP–expressing cells with these lipids, even at micromolar concentrations, did not induce intracellular receptor trafficking (Figure 3). LPA, a low-affinity agonist for EDG-1 (Kd of ∼2.3 μM), did not induce EDG-1–GFP internalization even at high (50 μM) concentrations (Figure 3). LPA treatment, however, enhanced the intensity of EDG-1–GFP fluorescence on the cell surface, which may indicate receptor aggregation on the lateral plane of the plasma membrane after ligand binding (Lee et al., 1998a). These data suggest that the high-affinity ligand–receptor interaction is necessary to induce receptor internalization.
Figure 3.
Effect of bioactive lipids on EDG-1–GFP localization. HEK293–EDG-1–GFP cells were grown in CFBS for 2 d and treated for 60 min at 37°C with 50 μM LPA (LPA), 10 μM sphingosylphosphorylcholine (SPC), 2 μM ceramide (CER), and 20 μM ceramide-1-phosphate (C-1-P); and fluorescence micrographs were taken. Bars, 10 μm.
Recycling of EDG-1–GFP from Intracellular Vesicles to the Plasma Membrane
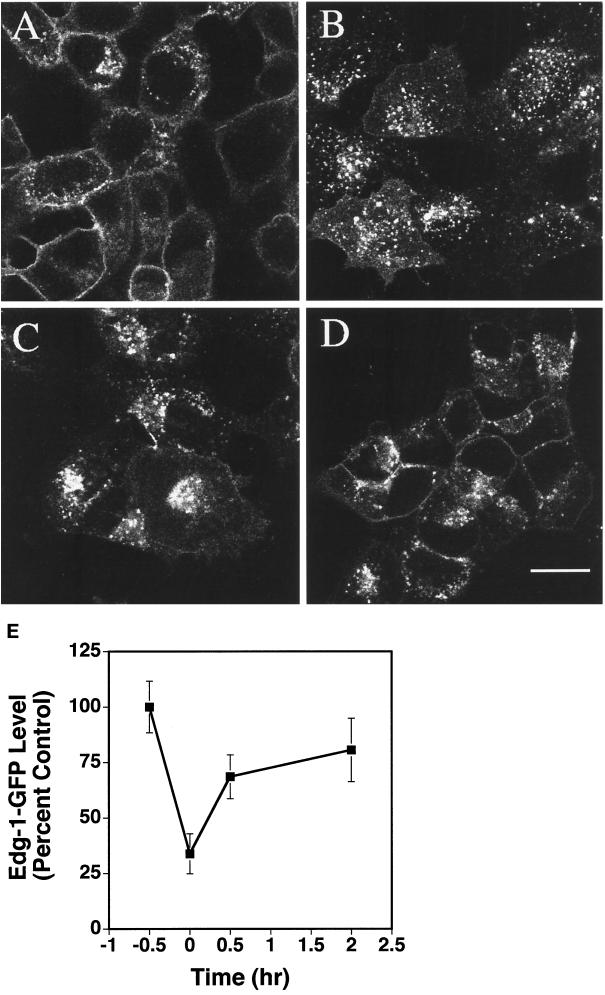
To determine whether SPP-induced EDG-1–GFP trafficking is reversible, HEK293–EDG-1–GFP cells were treated with SPP for 0.5 h, and the ligand was washed out. Cells were preincubated for 0.5 h and incubated with cycloheximide (15 μg/ml) to block the synthesis of new EDG-1–GFP receptors. Quantitation of plasma membrane-localized EDG-1–GFP signals indicated that ∼70% of EDG-1–GFP was internalized 30 min after SPP exposure. At 120 min after SPP washout, ∼80% of the pretreatment level of the EDG-1–GFP molecules returned to the cell surface (Figure 4). Because protein synthesis was inhibited, these data strongly suggest that EDG-1 recycles from the intracellular vesicles to the plasma membrane.
Figure 4.
Recycling of the EDG-1–GFP polypeptide. HEK293 cells expressing EDG-1–GFP polypeptide were subjected to various treatments and imaged by confocal microscopy. (A) Cells were treated with cycloheximide (CHX) for 30 min to block protein synthesis. (B) Cells were then stimulated with 100 nM SPP for 30 min in the presence of CHX. Note the accumulation of EDG-1–GFP in intracellular vesicles. (C and D) Exogenous SPP was washed out, and media containing CHX was replaced and incubated for 30 min (C) and 120 min (D) at 37°C. Note that the EDG-1–GFP polypeptide recycles back to the plasma membrane at 120 min. Bars, 10 μm. (E) Quantitative analysis of internalization and recycling of EDG-1–GFP is shown. Digitized fluorescence intensity from the plasma membrane was quantitated by the imaging software and normalized to the prestimulation level. Data represent mean ± SD of fluorescence intensities of six randomly selected fields.
Colocalization Studies Suggest That EDG-1–GFP Traffics via the Endosomal Pathway
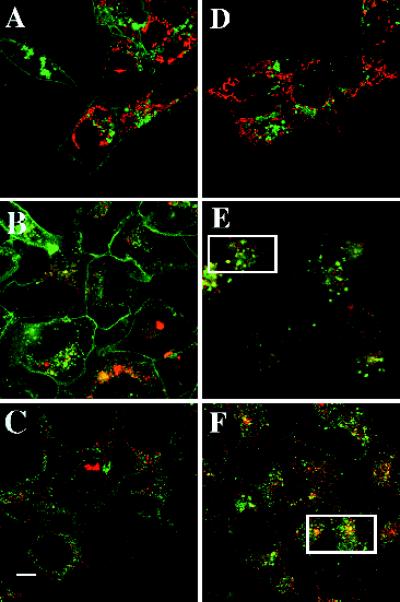
Because SPP induced trafficking of EDG-1–GFP into intracellular vesicles, the nature of these organelles was investigated next. To determine whether they are mitochondria, HEK293–EDG-1–GFP cells were labeled with the fluorescence dye TMRE, which accumulates in mitochondria and exhibits a red fluorescence (Farkas et al., 1989). As shown in Figure 5, A and D, EDG-1–GFP fluorescence did not colocalize with the TMRE signals either before or after SPP treatment, suggesting that SPP-induced organelles are not mitochondria. The shapes of the mitochondria and EDG-1–GFP–containing vesicles are also different; mitochondria are elongated in shape, whereas the EDG-1–GFP–containing vesicles are oval. Similarly, the dye Lysotracker was used to determine whether some of the EDG-1–GFP–containing structures are lysosomal in nature. Lysosomal structures partially colocalized with SPP-induced EDG-1–GFP vesicles (Figure 5, B and E). In addition, after SPP treatment, EDG-1–GFP–containing vesicles colocalized significantly with fluorescently labeled transferrin, which is a probe for receptor-mediated endocytosis via the clathrin-coated pathway (Figure 5, C and F) (Richardson and Ponka, 1997). These data suggest that SPP induces trafficking of EDG-1 into a receptor-mediated endosomal pathway and that a minor population of these vesicles are targeted to lysosomes. However, most of the receptor molecules traffic to the perinuclear locale and recycle back to the plasma membrane with a half-life of 0.5 h (Figure 4).
Figure 5.
Colocalization of intracellular organelles and EDG-1–GFP. HEK293 cells expressing EDG-1–GFP were grown in 10% FBS, incubated with various organelle-specific dyes in the presence or absence of SPP (100 nM) for 1 h, and imaged by confocal microscopy to localize the EDG-1–GFP polypeptide (488 nm) and the organelle-specific dyes (568 nm). (A and D) Untreated (A) and SPP-treated (D) cells were incubated with TMRE dye (50 nM) for 15 min at 37°C to label the mitochondria. (B and E) Untreated (B) and SPP-treated (E) cells were labeled with Lysotracker dye to label the lysosomal structures. (C and F) Untreated (C) and SPP-treated (F) cells were preincubated for 0.5 h with Texas red–labeled transferrin to visualize endosomal structures. Rectangles in E and F indicate cells in which significant colocalization is observed. Bar, 10 μm.
C-Terminal Domain of EDG-1 Is Critical for Ligand-induced Internalization
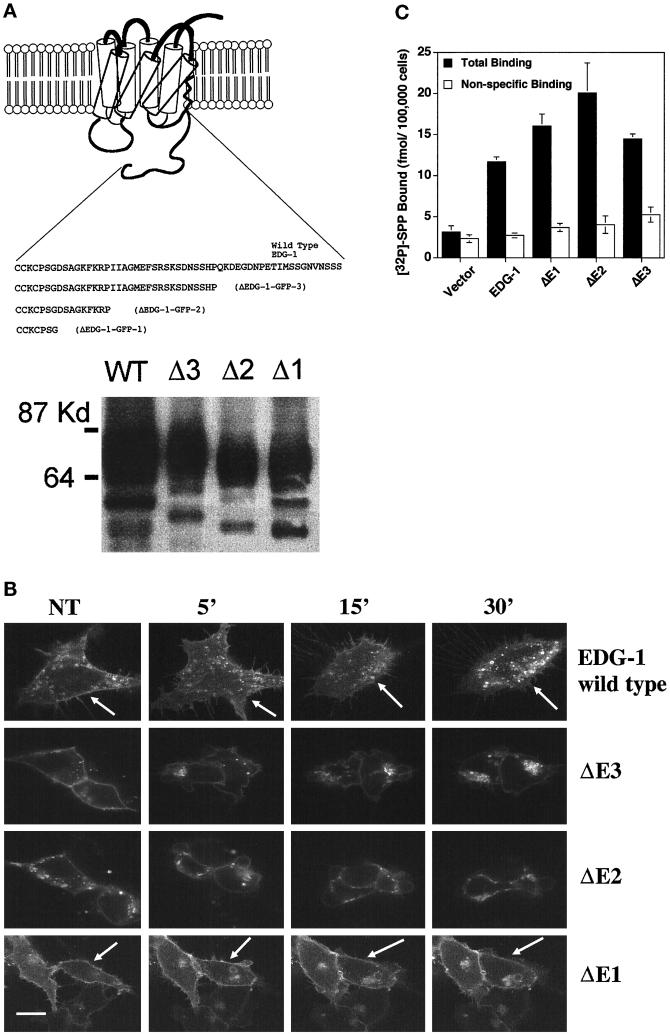
Studies on the β-adrenergic receptor have implicated the critical role of ligand-induced phosphorylation of the C-terminal domain in GPR internalization via the clathrin-coated endocytic pathway (Carman and Benovic, 1998). In this model, ligand activation of the GPR activates the phosphorylation of the C terminus by the G-protein–coupled receptor kinase proteins. Phosphorylated receptor interacts with arrestin proteins that allow the rapid internalization of the receptors via the clathrin-coated endocytic pathway. Indeed, the C terminus of EDG-1 is highly enriched in serine residues, and ligand activation rapidly induces the phosphorylation of EDG-1 (Lee et al., 1998a). Three deletion constructs of the EDG-1 were made as fusion proteins with the GFP as schematically depicted in Figure 6A. HEK293 cells were stably transfected with these constructs. As shown in Figure 6A, immunoblot analysis of the EDG-1–GFP–transfected cells as well as the ΔEDG-1–GFP cells indicates the production of appropriately sized fusion proteins. The ΔEDG-1–GFP proteins were expressed primarily on the plasma membrane, similar to the distribution of the wild-type receptor (Figure 6B). However, upon treatment with SPP, EDG-1–GFP and ΔEDG-1–GFP-3 were internalized, whereas the smaller constructs (ΔEDG-1–GFP-2 and ΔEDG-1–GFP-1) did not internalize after ligand stimulation. All of the receptor mutants exhibited high-affinity SPP binding (Figure 6C). Thus, the C terminus of the EDG-1 is required for SPP-induced internalization.
Figure 6.
C-terminal–deleted EDG-1–GFP chimeras. (A) Top, schematic representation of C-terminal–deleted EDG-1–GFP chimeras. Deleted ΔEDG-1 clones (1–3) were fused to the GFP polypeptide at the C terminus, and stable clones of HEK293 cells were derived as described. Bottom, the production of appropriately sized EDG-1–GFP and ΔEDG-1–GFP polypeptides determined by immunoblot analysis of cell extracts. Thirty micrograms of extract from the wild-type EDG-1–GFP (WT)-transfected cells and 10 μg from Δ3-, Δ2-, and Δ1-transfected cells were used. (B) SPP-induced internalization of ΔEDG-1–GFP chimeras. HEK293 cells stably transfected with EDG-1–GFP, ΔEDG-1–GFP-3 (ΔE3), ΔEDG-1–GFP-2 (ΔE2), and ΔEDG-1–GFP-1 (ΔE1) were treated with 100 nM SPP for the indicated times at 37°C and imaged by confocal microscopy. Note the reduction of plasma membrane fluorescence in the wild-type (indicated by arrows) and ΔE3-transfected cells but not in ΔE2 and ΔE1 cells (indicated by arrows). Bar, 10 μm. (C) Binding of [32P]SPP to HEK293 cells transiently transfected with EDG-1–GFP, ΔEDG-1–GFP-3 (ΔE3), ΔEDG-1–GFP-2 (ΔE2), and ΔEDG-1–GFP-1 (ΔE1).
DISCUSSION
Data in this study indicate that fusion of the GFP polypeptide to the extreme C terminus of the EDG-1 molecule did not interfere with ligand binding or signal transduction. The EDG-1–GFP polypeptide bound its ligand SPP with high affinity (apparent Kd of ∼7.4 nM). In addition, similar to its effects on the wild-type EDG-1, nanomolar concentrations of SPP activated EDG-1–GFP and regulated Gi-dependent ERK-2 activity. Furthermore, the majority of the receptor molecules are expressed on the cell surface. These data indicated that EDG-1–GFP functions in a manner similar to that of wild-type EDG-1. Because of the intrinsic fluorescence of the GFP molecule, this system provides a convenient means to visualize subcellular localization and trafficking of the receptor in live cells. This approach has been applied to other GPR molecules such as the cAMP receptor in the slime mold Dictyostelium (Xiao et al., 1997) as well as the β-adrenergic receptor (Barak et al., 1997) and the cholecystokinin receptor (Tarasova et al., 1997) in mammalian cells.
Although EDG-1–GFP is localized on the plasma membrane, it is internalized rapidly after the addition of exogenous SPP at ≥50 nM. The concentration of SPP required to induce EDG-1 internalization is significantly higher than the apparent Kd. This phenomenon, which has been observed in other systems (Mukherjee et al., 1997; Carman and Benovic, 1998), may be related to the fact that high receptor occupancy is required for efficient internalization, particularly in overexpressed systems. Receptor internalization was rapid, and quantitative analysis of confocal scanning fluorescence microscopy data indicated that τ1/2 is ∼15 min. Only the high-affinity ligand SPP was capable of receptor internalization; neither the low-affinity ligand LPA nor the related sphingolipids ceramide, ceramide-1-phosphate, or sphingosylphosphorylcholine induced receptor internalization, suggesting that high-affinity ligand–receptor interaction is necessary for receptor internalization. Immunoblot analysis of EDG-1–GFP before and after SPP treatment indicates that receptor redistribution, rather than degradation, is induced by the high-affinity ligand.
After SPP treatment, EDG-1–GFP is colocalized in part with internalized fluorescently labeled transferrin. It is well established that transferrin binds to its receptor and is internalized via clathrin-coated pits into the endosomal pathway, a phenomenon referred to as receptor-mediated endocytosis (Richardson and Ponka, 1997). Thus, EDG-1–GFP may also traffic via this pathway. Endosomal maturation occurs in intracellular vesicles; some vesicles return to the cell surface, and some fuse with lysosomes (Mukherjee et al., 1997). Some EDG-1–GFP–containing vesicles are colocalized with lysosomes, suggesting that a fraction of EDG-1–GFP was targeted to the lysosomes for degradation. It is noteworthy that lysosomes contain multiple sphingolipid-degrading enzymes (Furst and Sandhoff, 1992; Sandhoff and Klein, 1994). Indeed, lysosomal abnormalities are a prominent feature of inherited sphingolipid metabolic diseases, such as Nieman-Pick’s, Gaucher’s, and Fabry’s syndromes. The majority of EDG-1–GFP–containing vesicles, however, are at a distinct perinuclear location. This may be an endosomal compartment involved in the sorting of various vesicles. Alternatively, perinuclear localization of EDG-1 may be related to the potential intracellular role of SPP. Recently, the sphingosine kinase enzyme was cloned and was shown to be a cytosolic enzyme (Kohama et al., 1998). It will be of interest to investigate the role of intracellular SPP synthesis in EDG-1 localization and signaling. After internalization, most of the EDG-1–GFP recycle to the plasma membrane. This behavior is similar to that of the β-adrenergic receptor (Barak et al., 1997; Carman and Benovic, 1998) and is different from that of the thrombin receptor (Trejo et al., 1998).
Our data also demonstrate that the C-terminal domain of EDG-1 is required for SPP-induced EDG-1 internalization. Specifically, ΔEDG-1–GFP-2 and -1 did not internalize, whereas the ΔEDG-1–GFP-3 chimera does. Thus, deletion of the serine-rich domain (SRSKSDNSS) inhibits receptor internalization. Interestingly, this region is a potential phosphorylation site for casein kinase II and protein kinase C. Because EDG-1 is phosphorylated rapidly after SPP addition (Lee et al., 1998a), it is possible that phosphorylated C-terminal domain interacts with arrestin-like molecules to be internalized by the clathrin-coated pathway. In a related issue, two GPRs of the EDG-1 subfamily, EDG-3 and EDG-5/H218/AGR16, were shown to signal in response to SPP (An et al., 1997). It would be of interest to examine the subcellular localization of these molecules after ligand binding.
The role of receptor internalization in signal transduction is not completely resolved. It is generally assumed that receptor internalization is a mechanism of ligand-induced desensitization (Ferguson et al., 1996). However, recent data suggest that activation of specific signaling pathways requires receptor internalization (Daaka et al., 1998). In addition, it is also possible that internalization of EDG-1 may transport SPP to a specific subcellular locale, i.e., the lysosomal or the perinuclear endosomal compartment. EDG-1 is known to activate Gi- and Rho-dependent signaling pathways (Lee et al., 1996, 1998a,b). Further studies are necessary to define the role of EDG-1 internalization in receptor signaling.
In conclusion, the GFP reporter system defines the interaction of SPP with its receptor EDG-1 at the subcellular level. Our data indicate that exogenous SPP induces rapid, specific, and reversible trafficking of EDG-1 into a perinuclear endosomal compartment. Trafficking of EDG-1 by SPP may be important in the receptor-dependent intracellular-signaling functions of this potent lipid mediator.
ACKNOWLEDGMENTS
We thank Susan Krueger and Frank Morgan for expert help and advice on confocal microscopy and imaging. We also thank Nicolas Ancellin and Mark Terasaki for helpful comments. This work is supported by National Institutes of Health grants DK-45659 and HL-54710 to T.H. and GM-43880 to S.S. T.H. is an established investigator of the American Heart Association.
Abbreviations used:
- CFBS
charcoal-stripped FBS
- ERK
extracellular signal-regulated kinase
- FBS
fetal bovine serum
- GFP
green fluorescent protein
- GPR
G-protein–coupled receptor
- HEK293
human embryonic kidney 293
- LPA
lysophosphatidic acid
- MAP
mitogen-activated protein
- SPP
sphingosine-1-phosphate
- TMRE
tetramethylrhodamine ethyl ester
REFERENCES
- An S, Bleu T, Huang W, Hallmark OG, Coughlin SR, Goetzl EJ. Identification of cDNAs encoding two G protein-coupled receptors for lysosphingolipids. FEBS Lett. 1997;417:279–282. doi: 10.1016/s0014-5793(97)01301-x. [DOI] [PubMed] [Google Scholar]
- Barak LS, Ferguson SSG, Zhang J, Martenson C, Meyer T, Caron MG. Internal trafficking and surface mobility of a functionally intact β2-adrenergic receptor-green fluorescent protein conjugate. Mol Pharmacol. 1997;51:177–184. doi: 10.1124/mol.51.2.177. [DOI] [PubMed] [Google Scholar]
- Bornfeldt KE, et al. Sphingosine-1-phosphate inhibits PDGF-induced chemotaxis of human arterial smooth muscle cells: spatial and temporal modulation of PDGF chemotactic signal transduction. J Cell Biol. 1995;130:193–206. doi: 10.1083/jcb.130.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buehrer BM, Bell RM. Sphingosine kinase: properties and cellular functions. Adv Lipid Res. 1993;26:59–67. [PubMed] [Google Scholar]
- Carman CV, Benovic JL. G-protein-coupled receptors: turn-ons and turn-offs. Curr Opin Neurobiol. 1998;8:335–344. doi: 10.1016/s0959-4388(98)80058-5. [DOI] [PubMed] [Google Scholar]
- Choi OH, Kim JH, Kinet JP. Calcium mobilization via sphingosine kinase in signaling by the Fc ε RI antigen receptor. Nature. 1996;380:634–636. doi: 10.1038/380634a0. [DOI] [PubMed] [Google Scholar]
- Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind S, Spiegel S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381:800–803. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- Daaka Y, Luttrell LM, Ahn S, Della Rocca GJ, Ferguson SS, Caron MG, Lefkowitz RJ. Essential role for G protein-coupled receptor endocytosis in the activation of mitogen-activated protein kinase. J Biol Chem. 1998;273:685–688. doi: 10.1074/jbc.273.2.685. [DOI] [PubMed] [Google Scholar]
- Edsall LC, Pirianov GG, Spiegel S. Involvement of sphingosine 1-phosphate in nerve growth factor-mediated neuronal survival and differentiation. J Neurosci. 1997;17:6952–6960. doi: 10.1523/JNEUROSCI.17-18-06952.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas DL, Wei MD, Febbroriello P, Carson JH, Loew LM. Simultaneous imaging of cell and mitochondrial membrane potentials. Biophys J. 1989;56:1053–1069. doi: 10.1016/S0006-3495(89)82754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SS, Barak LS, Zhang J, Caron MG. G-protein-coupled receptor regulation: role of G-protein-coupled receptor kinases and arrestins. Can J Physiol Pharmacol. 1996;74:1095–1110. doi: 10.1139/cjpp-74-10-1095. [DOI] [PubMed] [Google Scholar]
- Furst W, Sandhoff K. Activator proteins and topology of lysosomal sphingolipid catabolism. Biochim Biophys Acta. 1992;1126:1–16. doi: 10.1016/0005-2760(92)90210-m. [DOI] [PubMed] [Google Scholar]
- Ghosh TK, Bian J, Gill DL. Intracellular calcium release mediated by sphingosine derivatives generated in cells. Science. 1990;248:1653–1656. doi: 10.1126/science.2163543. [DOI] [PubMed] [Google Scholar]
- Heringdorf DM, Lass H, Alemany R, Laser KT, Neumann E, Zhang C, Schmidt M, Rauen U, Jakobs KH, Van Koppen CJ. Sphingosine kinase-mediated Ca2+ signaling by G-protein-coupled receptors. EMBO J. 1998;17:2830–2837. doi: 10.1093/emboj/17.10.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hla, T., Lee, M., Ancellin, N., Liu, C.H., Thangada, S., Thompson, B.D., and Kluk, M. (1999). Sphingosine-1-phosphate: extracellular mediator or intracellular second messenger? Biochem. Pharmacol. (in press). [DOI] [PubMed]
- Hla T, Maciag T. An abundant transcript induced in differentiating human endothelial cells encodes a polypeptide with structural similarities to G-protein-coupled receptors. J Biol Chem. 1990;265:9308–9313. [PubMed] [Google Scholar]
- Hung WC, Chuang LY. Induction of apoptosis by sphingosine-1-phosphate in human hepatoma cells is associated with enhanced expression of bax gene product. Biochem Biophys Res Commun. 1996;229:11–15. doi: 10.1006/bbrc.1996.1750. [DOI] [PubMed] [Google Scholar]
- Kohama T, Olivera A, Edsall L, Nagiec MM, Dickson R, Spiegel S. Molecular cloning and functional characterization of murine sphingosine kinase. J Biol Chem. 1998;273:23722–23728. doi: 10.1074/jbc.273.37.23722. [DOI] [PubMed] [Google Scholar]
- Lee M-J, Evans M, Hla T. The inducible G protein-coupled receptor edg-1 signals via the G(i)/mitogen-activated protein kinase pathway. J Biol Chem. 1996;271:11272–11279. doi: 10.1074/jbc.271.19.11272. [DOI] [PubMed] [Google Scholar]
- Lee M-J, Thangada S, Liu CH, Thompson BD, Hla T. Lysophosphatidic acid stimulates the G-protein-coupled receptor EDG-1 as a low affinity agonist. J Biol Chem. 1998a;273:22105–22112. doi: 10.1074/jbc.273.34.22105. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Van Brocklyn JR, Thangada S, Liu CH, Hand AR, Menzeleev R, Spiegel S, Hla T. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science. 1998b;279:1552–1555. doi: 10.1126/science.279.5356.1552. [DOI] [PubMed] [Google Scholar]
- Liu CH, Hla T. The mouse gene for the inducible G-protein-coupled receptor EDG-1. Genomics. 1997;43:15–24. doi: 10.1006/geno.1997.4759. [DOI] [PubMed] [Google Scholar]
- Mattie M, Brooker G, Spiegel S. Sphingosine-1-phosphate, a putative second messenger, mobilizes calcium from internal stores via an inositol trisphosphate-independent pathway. J Biol Chem. 1994;269:3181–3188. [PubMed] [Google Scholar]
- Moolenaar WH, Kranenburg O, Postma FR, Zondag GC. Lysophosphatidic acid: G-protein signaling and cellular responses. Curr Opin Cell Biol. 1997;9:168–173. doi: 10.1016/s0955-0674(97)80059-2. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Ghosh RN, Maxfield FR. Endocytosis. Physiol Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- Okamoto H, Takuwa N, Gonda K, Okazaki H, Chang K, Yatomi Y, Shigematsu H, Takuwa Y. EDG1 is a functional sphingosine-1-phosphate receptor that is linked via a Gi/o to multiple signaling pathways, including phospholipase C activation, Ca2+ mobilization, Ras-mitogen-activated protein kinase activation, and adenylate cyclase inhibition. J Biol Chem. 1998;273:27104–27110. doi: 10.1074/jbc.273.42.27104. [DOI] [PubMed] [Google Scholar]
- Olivera A, Spiegel S. Sphingosine-1-phosphate as second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature. 1993;365:557–560. doi: 10.1038/365557a0. [DOI] [PubMed] [Google Scholar]
- Postma FR, Jalink K, Hengeveld T, Moolenaar WH. Sphingosine-1-phosphate rapidly induces Rho-dependent neurite retraction: action through a specific cell surface receptor. EMBO J. 1996;15:2388–2392. [PMC free article] [PubMed] [Google Scholar]
- Richardson DR, Ponka P. The molecular mechanisms of the metabolism and transport of iron in normal and neoplastic cells. Biochim Biophys Acta. 1997;1331:1–40. doi: 10.1016/s0304-4157(96)00014-7. [DOI] [PubMed] [Google Scholar]
- Sadahira Y, Ruan F, Hakomori S, Igarashi Y. Sphingosine 1-phosphate, a specific endogenous signaling molecule controlling cell motility and tumor cell invasiveness. Proc Natl Acad Sci USA. 1992;89:9686–9690. doi: 10.1073/pnas.89.20.9686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhoff K, Klein A. Intracellular trafficking of glycosphingolipids: role of sphingolipid activator proteins in the topology of endocytosis and lysosomal digestion. FEBS Lett. 1994;346:103–107. doi: 10.1016/0014-5793(94)00282-7. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Merrill AH., Jr Sphingolipid metabolism and cell growth regulation. FASEB J. 1996;10:1388–1397. doi: 10.1096/fasebj.10.12.8903509. [DOI] [PubMed] [Google Scholar]
- Su Y, Rosenthal D, Smulson M, Spiegel S. Sphingosine 1-phosphate, a novel signaling molecule, stimulates DNA binding activity of AP-1 in quiescent Swiss 3T3 fibroblasts. J Biol Chem. 1994;269:16512–16517. [PubMed] [Google Scholar]
- Takada Y, Kato C, Kondo S, Korenaga R, Ando J. Cloning of cDNAs encoding G protein-coupled receptor expressed in human endothelial cells exposed to fluid shear stress. Biochem Biophys Res Commun. 1997;240:737–741. doi: 10.1006/bbrc.1997.7734. [DOI] [PubMed] [Google Scholar]
- Tarasova NI, Stauber RH, Choi JK, Hudson EA, Czerwinski G, Miller JL, Pavlakis GN, Michedja CJ, Wank SA. Visualization of G protein-coupled receptor trafficking with the aid of the green fluorescent protein. Endocytosis and recycling of cholecystokinin receptor type A. J Biol Chem. 1997;272:14817–14824. doi: 10.1074/jbc.272.23.14817. [DOI] [PubMed] [Google Scholar]
- Trejo J, Hammes SR, Coughlin SR. Termination of signaling by protease-activated receptor-1 is linked to lysosomal sorting. Proc Natl Acad Sci USA. 1998;95:13698–13702. doi: 10.1073/pnas.95.23.13698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Brocklyn JR, et al. Dual actions of sphingosine-1-phosphate: extracellular through the Gi-coupled receptor edg-1 and intracellular to regulate proliferation and survival. J Cell Biol. 1998;142:229–240. doi: 10.1083/jcb.142.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Koppen C, Meyer ZU, Heringdorf M, Laser KT, Zhang C, Jakobs KH, Bunemann M, Pott L. Activation of a high affinity Gi protein-coupled plasma membrane receptor by sphingosine-1-phosphate. J Biol Chem. 1996;271:2082–2087. doi: 10.1074/jbc.271.4.2082. [DOI] [PubMed] [Google Scholar]
- Wu J, Spiegel S, Sturgill TW. Sphingosine 1-phosphate rapidly activates the mitogen-activated protein kinase pathway by a G protein-dependent mechanism. J Biol Chem. 1995;270:11484–11488. doi: 10.1074/jbc.270.19.11484. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Zhang N, Murphy DB, Devreotes PN. Dynamic distribution of chemoattractant receptors in living cells during chemotaxis and persistent stimulation. J Cell Biol. 1997;139:365–374. doi: 10.1083/jcb.139.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatomi Y, Igarashi Y, Yang L, Hisano N, Qi R, Asazuma N, Satoh K, Ozaki Y, Kume S. Sphingosine 1-phosphate, a bioactive sphingolipid abundantly stored in platelets, is a normal constituent of human plasma and serum. J Biochem. 1997;121:969–973. doi: 10.1093/oxfordjournals.jbchem.a021681. [DOI] [PubMed] [Google Scholar]
- Yatomi Y, Ruan F, Hakomori S, Igarashi Y. Sphingosine-1-phosphate: a platelet-activating sphingolipid released from agonist-stimulated human platelets. Blood. 1995;86:193–202. [PubMed] [Google Scholar]
- Zhang H, Desai NN, Olivera A, Seki T, Brooker G, Spiegel S. Sphingosine-1-phosphate, a novel lipid, involved in cellular proliferation. J Cell Biol. 1991;114:155–167. doi: 10.1083/jcb.114.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zondag GCM, Postma FR, Etten IV, Verlaan I, Moolenaar WH. Sphingosine 1-phosphate signaling through the G-protein-coupled receptor Edg-1. Biochem J. 1998;330:605–609. doi: 10.1042/bj3300605. [DOI] [PMC free article] [PubMed] [Google Scholar]