Abstract
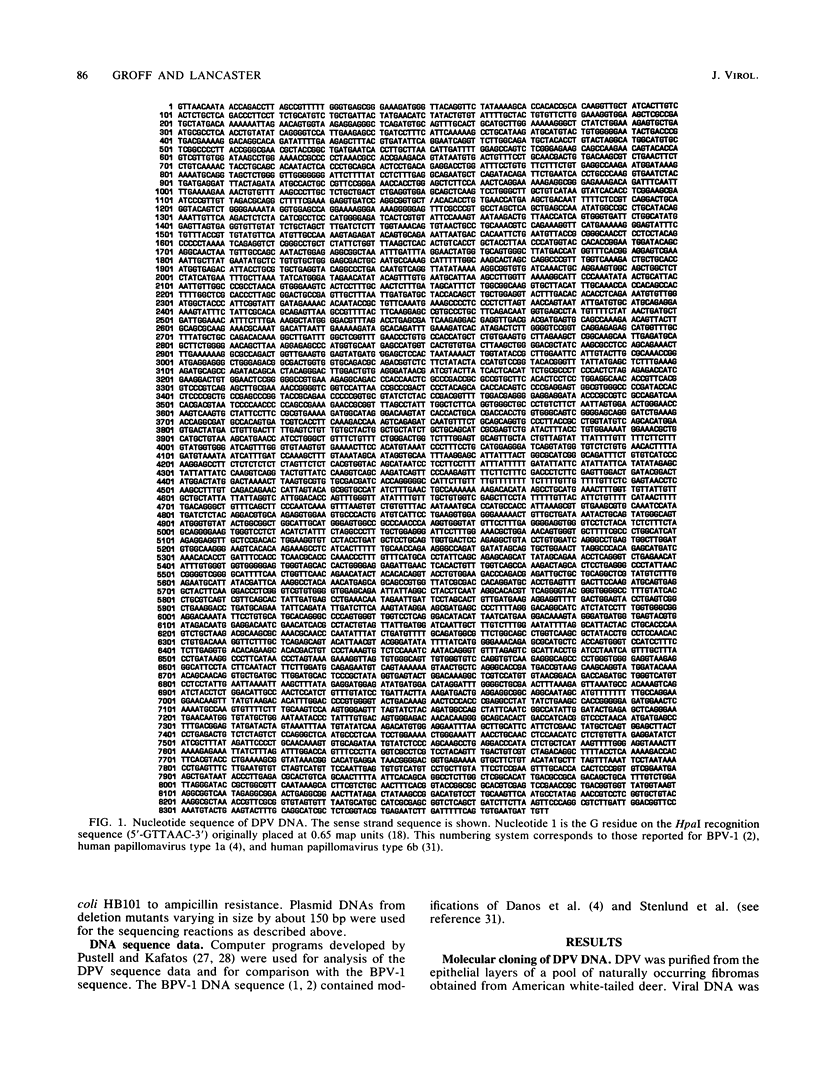
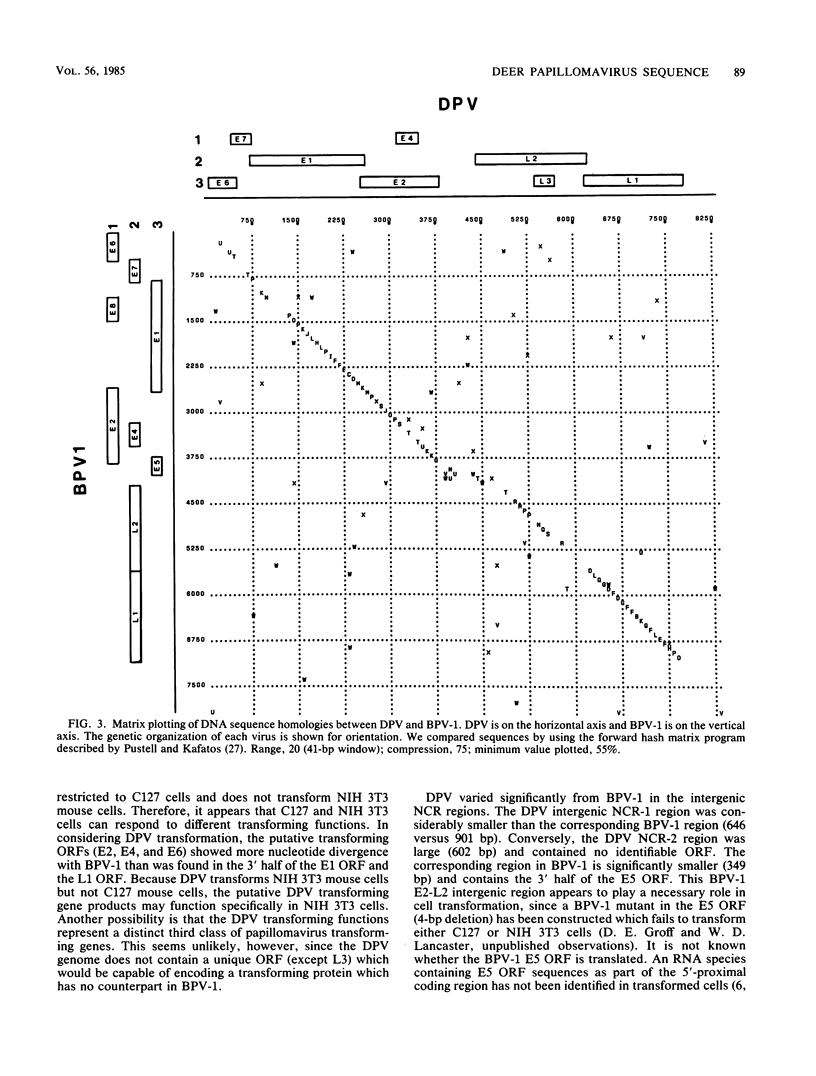
The genome of deer papillomavirus (DPV) isolated from American white-tailed deer was cloned into pBR322, and the entire nucleotide sequence of 8,374 base pairs was determined. The overall genetic organization of the DPV genome was similar to that of other papillomaviruses. All significant open reading frames were located on one strand, and the locations of putative promoters and polyadenylation signals were similar to those identified in the closely related bovine papillomavirus type 1 (BPV-1) genome. The DPV genome was approximately colinear with BPV-1 except for a noncoding region separating the early and late regions. The regions of highest nucleotide sequence homology between DPV and BPV-1 were found in the E1 open reading frame coding for BPV-1 DNA replication function and in the L1 open reading frame, which encodes the major capsid protein of BPV-1.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahola H., Stenlund A., Moreno-Lopez J., Pettersson U. Sequences of bovine papillomavirus type 1 DNA--functional and evolutionary implications. Nucleic Acids Res. 1983 May 11;11(9):2639–2650. doi: 10.1093/nar/11.9.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. Y., Howley P. M., Levinson A. D., Seeburg P. H. The primary structure and genetic organization of the bovine papillomavirus type 1 genome. Nature. 1982 Oct 7;299(5883):529–534. doi: 10.1038/299529a0. [DOI] [PubMed] [Google Scholar]
- Clertant P., Seif I. A common function for polyoma virus large-T and papillomavirus E1 proteins? Nature. 1984 Sep 20;311(5983):276–279. doi: 10.1038/311276a0. [DOI] [PubMed] [Google Scholar]
- Danos O., Engel L. W., Chen E. Y., Yaniv M., Howley P. M. Comparative analysis of the human type 1a and bovine type 1 papillomavirus genomes. J Virol. 1983 May;46(2):557–566. doi: 10.1128/jvi.46.2.557-566.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvoretzky I., Shober R., Chattopadhyay S. K., Lowy D. R. A quantitative in vitro focus assay for bovine papilloma virus. Virology. 1980 Jun;103(2):369–375. doi: 10.1016/0042-6822(80)90195-6. [DOI] [PubMed] [Google Scholar]
- Engel L. W., Heilman C. A., Howley P. M. Transcriptional organization of bovine papillomavirus type 1. J Virol. 1983 Sep;47(3):516–528. doi: 10.1128/jvi.47.3.516-528.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M., Shenk T. The sequence 5'-AAUAAA-3'forms parts of the recognition site for polyadenylation of late SV40 mRNAs. Cell. 1981 Apr;24(1):251–260. doi: 10.1016/0092-8674(81)90521-3. [DOI] [PubMed] [Google Scholar]
- Gingeras T. R., Sciaky D., Gelinas R. E., Bing-Dong J., Yen C. E., Kelly M. M., Bullock P. A., Parsons B. L., O'Neill K. E., Roberts R. J. Nucleotide sequences from the adenovirus-2 genome. J Biol Chem. 1982 Nov 25;257(22):13475–13491. [PubMed] [Google Scholar]
- Groff D. E., Lancaster W. D. Evidence that integration of virus DNA may not be necessary for maintenance of cell transformation. Prog Med Virol. 1984;29:218–230. [PubMed] [Google Scholar]
- Groff D. E., Sundberg J. P., Lancaster W. D. Extrachromosomal deer fibromavirus DNA in deer fibromas and virus-transformed mouse cells. Virology. 1983 Dec;131(2):546–550. doi: 10.1016/0042-6822(83)90519-6. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L. H., Yang R. C., Wu R. An improved strategy for rapid direct sequencing of both strands of long DNA molecules cloned in a plasmid. Nucleic Acids Res. 1983 Aug 25;11(16):5521–5540. doi: 10.1093/nar/11.16.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilman C. A., Engel L., Lowy D. R., Howley P. M. Virus-specific transcription in bovine papillomavirus-transformed mouse cells. Virology. 1982 May;119(1):22–34. doi: 10.1016/0042-6822(82)90061-7. [DOI] [PubMed] [Google Scholar]
- Hong G. F. A systemic DNA sequencing strategy. J Mol Biol. 1982 Jul 5;158(3):539–549. doi: 10.1016/0022-2836(82)90213-3. [DOI] [PubMed] [Google Scholar]
- Koller L. D., Olson C. Attempted transmission of warts from man, cattle, and horses and of deer fibroma, to selected hosts. J Invest Dermatol. 1972 Jun;58(6):366–368. doi: 10.1111/1523-1747.ep12540579. [DOI] [PubMed] [Google Scholar]
- Lancaster W. D., Olson C. Animal papillomaviruses. Microbiol Rev. 1982 Jun;46(2):191–207. doi: 10.1128/mr.46.2.191-207.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster W. D., Sundberg J. P. Characterization of papillomaviruses isolated from cutaneous fibromas of white-tailed deer and mule deer. Virology. 1982 Nov;123(1):212–216. doi: 10.1016/0042-6822(82)90307-5. [DOI] [PubMed] [Google Scholar]
- Law M. F., Lowy D. R., Dvoretzky I., Howley P. M. Mouse cells transformed by bovine papillomavirus contain only extrachromosomal viral DNA sequences. Proc Natl Acad Sci U S A. 1981 May;78(5):2727–2731. doi: 10.1073/pnas.78.5.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy D. R., Dvoretzky I., Shober R., Law M. F., Engel L., Howley P. M. In vitro tumorigenic transformation by a defined sub-genomic fragment of bovine papilloma virus DNA. Nature. 1980 Sep 4;287(5777):72–74. doi: 10.1038/287072a0. [DOI] [PubMed] [Google Scholar]
- Lusky M., Botchan M. R. Characterization of the bovine papilloma virus plasmid maintenance sequences. Cell. 1984 Feb;36(2):391–401. doi: 10.1016/0092-8674(84)90232-0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nakabayashi Y., Chattopadhyay S. K., Lowy D. R. The transforming function of bovine papillomavirus DNA. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5832–5836. doi: 10.1073/pnas.80.19.5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima Y., Suzuki Y. Cloning of the silk fibroin gene and its flanking sequences. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5363–5367. doi: 10.1073/pnas.74.12.5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister H. Biology and biochemistry of papillomaviruses. Rev Physiol Biochem Pharmacol. 1984;99:111–181. doi: 10.1007/BFb0027716. [DOI] [PubMed] [Google Scholar]
- Pustell J., Kafatos F. C. A convenient and adaptable package of computer programs for DNA and protein sequence management, analysis and homology determination. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):643–655. doi: 10.1093/nar/12.1part2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pustell J., Kafatos F. C. A high speed, high capacity homology matrix: zooming through SV40 and polyoma. Nucleic Acids Res. 1982 Aug 11;10(15):4765–4782. doi: 10.1093/nar/10.15.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHOPE R. E., MANGOLD R., MACNAMARA L. G., DUMBELL K. R. An infectious cutaneous fibroma of the Virginia whitetailed deer (Odocoileus virginianus). J Exp Med. 1958 Dec 1;108(6):797–802. doi: 10.1084/jem.108.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarver N., Rabson M. S., Yang Y. C., Byrne J. C., Howley P. M. Localization and analysis of bovine papillomavirus type 1 transforming functions. J Virol. 1984 Nov;52(2):377–388. doi: 10.1128/jvi.52.2.377-388.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller J. T., Vass W. C., Lowy D. R. Identification of a second transforming region in bovine papillomavirus DNA. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7880–7884. doi: 10.1073/pnas.81.24.7880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz E., Dürst M., Demankowski C., Lattermann O., Zech R., Wolfsperger E., Suhai S., zur Hausen H. DNA sequence and genome organization of genital human papillomavirus type 6b. EMBO J. 1983;2(12):2341–2348. doi: 10.1002/j.1460-2075.1983.tb01744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenlund A., Moreno-Lopez J., Ahola H., Pettersson U. European elk papillomavirus: characterization of the genome, induction of tumors in animals, and transformation in vitro. J Virol. 1983 Nov;48(2):370–376. doi: 10.1128/jvi.48.2.370-376.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts S. L., Phelps W. C., Ostrow R. S., Zachow K. R., Faras A. J. Cellular transformation by human papillomavirus DNA in vitro. Science. 1984 Aug 10;225(4662):634–636. doi: 10.1126/science.6330900. [DOI] [PubMed] [Google Scholar]
- Yang Y. C., Okayama H., Howley P. M. Bovine papillomavirus contains multiple transforming genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1030–1034. doi: 10.1073/pnas.82.4.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]


