Abstract
Proteins of the kinesin superfamily define a class of microtubule-dependent motors that play crucial roles in cell division and intracellular transport. To study the molecular mechanism of axonal transport, a cDNA encoding a new kinesin-like protein called KIF3C was cloned from a mouse brain cDNA library. Sequence and secondary structure analysis revealed that KIF3C is a member of the KIF3 family. In contrast to KIF3A and KIF3B, Northern and Western analysis indicated that KIF3C expression is highly enriched in neural tissues such as brain, spinal cord, and retina. When anti-KIF3C antibodies were used to stain the cerebellum, the strongest signal came from the cell bodies and dendrites of Purkinje cells. In retina, anti-KIF3C mainly stains the ganglion cells. Immunolocalization showed that the KIF3C motor in spinal cord and sciatic nerve is mainly localized in cytoplasm. In spinal cord, the KIF3C staining was punctate; double labeling with anti-giantin and anti-KIF3C showed a clear concentration of the motor protein in the Golgi complex. Staining of ligated sciatic nerves demonstrated that the KIF3C motor accumulated at the proximal side of the ligated nerve, which suggests that KIF3C is an anterograde motor. Immunoprecipitation experiments revealed that KIF3C and KIF3A, but not KIF3B, were coprecipitated. These data, combined with previous data from other labs, indicate that KIF3C and KIF3B are “variable” subunits that associate with a common KIF3A subunit, but not with each other. Together these results suggest that KIF3 family members combinatorially associate to power anterograde axonal transport.
INTRODUCTION
All cells require protein synthesis followed by transport and correct targeting of these proteins to their proper destinations (Vallee and Sheetz, 1996). This problem is particularly formidable in neural axons where various membranous components are transported bidirectionally along microtubules up to a meter or more long in large mammals (Brady and Sperry, 1995; Coy and Howard, 1994). Biochemical, genetic, and intracellular localization studies of kinesin and kinesin-like proteins have suggested that these motor proteins may power anterograde axonal transport (Goldstein, 1993; Bloom and Endow, 1995).
The founding member of the kinesin superfamily, kinesin, was first found in squid axoplasm where it is thought to play a role in axonal transport (Vale et al., 1985; Brady, 1985). Further work demonstrated that kinesin was but one member of a microtubule motor superfamily composed of evolutionarily conserved motor domains attached to cargo-binding tail domains (Yang et al., 1989), which have undergone structural and functional diversification during evolution. Thus, a series of genes encoding proteins related to the kinesin heavy chain (KHC)1 have been identified in several organisms including S. pombe, A. nidulans, S. cerevisiae, C. elegans, D. melanogaster, S. purpuratus, and M. musculus. Studies of the biochemistry, intracellular localization, and genetics have divided members of the kinesin superfamily into three broad groups, those apparently involved in vesicle transport, those thought to be involved in mitotic or meiotic spindle function, and those of unknown function (Goldstein, 1993; Bloom and Endow, 1995).
A prominent type of kinesin-like protein involved in vesicle transport is kinesin-II2 (Scholey, 1996), which was isolated first from S. purpuratus (Cole et al., 1993). Like kinesin, kinesin-II is composed of motor and nonmotor subunits. However, unlike kinesin, which is a heterotetramer of two heavy chain motor subunits and two light chain nonmotor subunits (Bloom et al., 1988; Kuznetsov et al., 1988), kinesin-II is generally a heterotrimeric protein containing two different motor subunits from the KIF32 family, complexed with a nonmotor subunit (KAP: kinesin accessory protein or kinesin associate protein) (Wedaman et al., 1996; Yamazaki et al., 1996). The KIF3 family currently includes KIF3A (Kondo et al., 1994) and KIF3B (Yamazaki et al., 1995) from M. musculus, KRP85 (Cole et al., 1993) and KRP95 (Rashid et al., 1995) from S. purpuratus, KLP64D (Stewart et al., 1991) and KLP68D (Pesavento et al., 1994) from D. melanogaster, FLA10 from C. reinharditi (Walther et al., 1994), and Osm3 from C. elegans (Shakir et al., 1993). Each member of this family shares greater than 60% amino acid sequence identity within their motor domains; in addition, each, except for Osm3, has significant similarity in the α-helical stalk regions. KIF3 family members have been suggested to play transport roles in axons, axonemes, and spindles (Scholey, 1996); however, the precise functions they carry out and the mechanisms by which they execute their functions remain unknown.
In this article we describe the properties of KIF3C, a new kinesin-like motor in M. musculus. Sequence and secondary structure analysis suggests that KIF3C is a new member of the KIF3 family. However, in contrast to KIF3A and KIF3B, KIF3C is mainly expressed in neural tissues. Staining of ligated sciatic nerves demonstrates that the KIF3C motor accumulates at the proximal side of the ligated nerve, which suggests that KIF3C is an anterograde motor. Intriguingly, immunoprecipitation experiments show that, like KIF3B, KIF3C also associates with KIF3A, but KIF3B and KIF3C do not associate with each other. Together, the data presented here suggest that kinesin-II subunits associate combinatorially to perform roles in anterograde axonal transport.
MATERIALS AND METHODS
Cloning and Sequence Analysis of KIF3C
Probes were used for isolating KIF3C cDNA clones from a BALB/c neonatal mouse brain cDNA library as described (Yang et al., 1997). A 4.0 kb, apparently full-length, KIF3C cDNA was completely sequenced on both strands. DNA sequence analysis was performed with the UWGCG Sequence Analysis Software Package (Devereux et al., 1984). α-helical coiled-coil probability was calculated by the conventional algorithm of Lupus (Lupas et al., 1991), by using a window of 28 amino acids.
Northern Blot Analysis
Total RNA was prepared from mouse tissues by guanidinium isothiocyanate extraction as described (Chomczynski and Sacchi, 1987) and analyzed in 1% formaldehyde agarose gels by standard methods (Sambrook et al., 1989). RNA was transferred to GeneScreen Plus membrane (NEN) in 10× SSC. Prehybridization and hybridization were performed in 6× SSC, 5× Denhardt’s solution, 1% SDS and 100 μg/ml single-stranded DNA in 50% formamide at 42°C. Final washes were carried out at 65°C in 0.2× SSC and 0.1% SDS.
Expression Constructs
DNA fragments encoding C-terminal 71 and 154 amino acid residues of the KIF3C motor were subcloned into pGEX-KG expression vectors (Guan and Dixon, 1991) to give pGEX-71C and pGEX-154C (see Figure 1A), respectively. pGEX-154C was made by cutting the KIF3C cDNA with Bsu36I, filling with Klenow, then cutting with XhoI. The 1.7 kb Bsu36I-XhoI fragment was ligated to the pGEX-KG vector, which had been cut with SmaI and XhoI. pGEX-71C was made by synthesizing a primer CCCCCCATGGAGTTTTCTCATGACCA, containing an NcoI site. This primer and a downstream primer (GCTCACCATCACACACAAGA) were used for PCR (94°C 30 sec, 55°C 1 min, 72°C 1 min for 30 cycles) to amplify a 600 bp DNA fragment from the KIF3C cDNA. The amplified DNA fragment was cut with NcoI and the 513 bp DNA fragment was isolated and ligated to NcoI cut pGEX-KG vector.
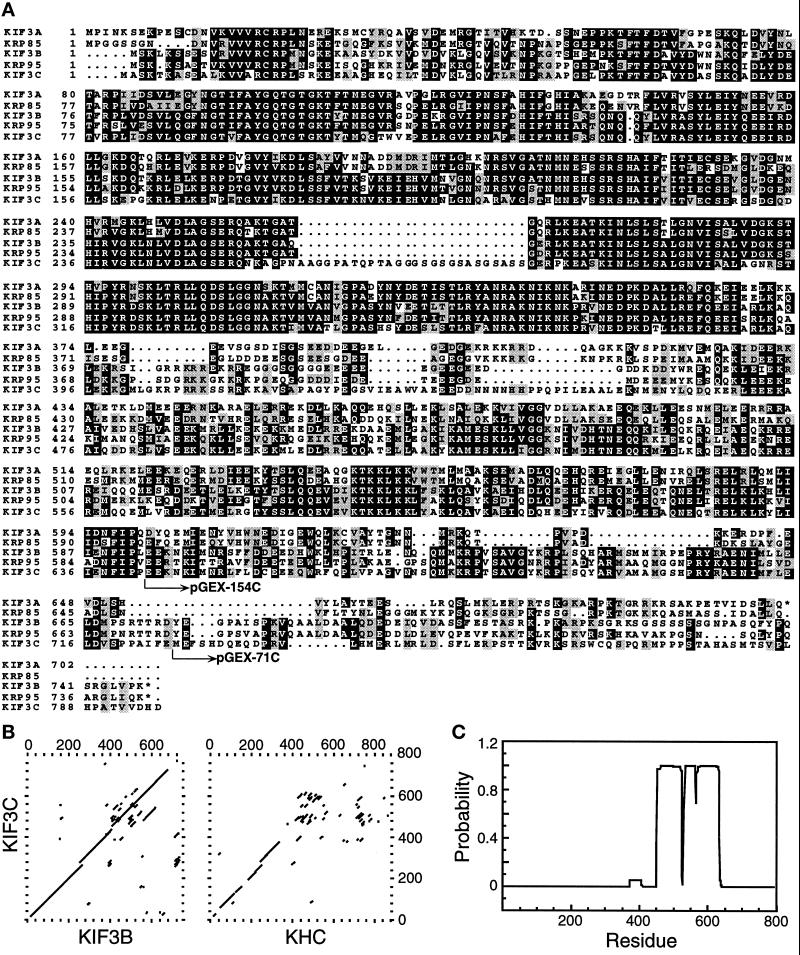
Figure 1.
Sequence analysis of KIF3C. (A) Predicted amino acid sequence alignment of KIF3C with KIF3A, KIF3B, KRP85, and KRP95. Identical amino acids, and conserved, but not identical amino acids, in the five polypeptides are shown in black and light boxes, respectively. pGEX-154C and pGEX-71C indicate the fusion points of GST protein and KIF3C protein regions, which were used for generating antigens and antibodies. The alignment was performed with the UWGCG software package (Devereux et al., 1984) and boxed by the program BOXSHADE 3.21 (http://ulrec3.unil.ch:80/software). These sequence data are available from EMBL/GenBank under accession number AF013116. (B) Dot matrix sequence comparisons of KIF3C with KIF3B and mouse KHC. Comparisons were done with the UWGCG program COMPARE (window = 30, stringency = 16), and were plotted with the program DOTPLOT. (C) The probability that each residue of KIF3C will participate in an α-helical coiled-coil structure is represented in a bar graph, as calculated by the algorithm of Lupas (Lupas et al., 1991).
Production and Affinity Purification of Antibodies
Glutathione-S-transferase (GST)1 fusion proteins were expressed by constructs pGEX-71C (GST-71C) and pGEX-154C (GST-154C) in E. coli. strain either BL21(DE3) or Xl-1 blue. Expression of both GST-154C and GST-71C proteins was induced by the addition of 0.4 mM IPTG and the expressed fusion proteins were detected by using antiserum recognizing GST protein. Cells were resuspended in lysis buffer (20 mM PO4, pH 7.4, 1 mM EDTA, and 0.1 mM PMSF) at 4°C; and cells were lysed in a French Press. Clarified lysates were obtained by centrifugation and incubated with glutathione-coupled beads (Sigma, St. Louis, MO) at 4°C for 30 min. After rinsing protein-coupled beads three times with the lysis buffer at 4°C, SDS-PAGE loading buffer (0.125 M Tris-Cl, pH 6.8, 2% SDS, 20% glycerol, 0.02% bromophenol blue, 0.71 M β-mercaptoethanol) was added to elute the protein. Expression and purification of GST protein from the vector pGEX-KG are essentially the same as those for GST-154C and GST-71C, except that the protein was eluted from the beads with 10 mM reduced glutathione in 50 mM Tris-Cl (pH 8.0). The eluted proteins were monitored by SDS-PAGE (Laemmli, 1970).
For antigen preparation, the eluted protein GST-154C was analyzed by SDS-PAGE. The gel was stained until the expected band was visible. The protein was electroeluted from the gel pieces in the SDS-PAGE running buffer in the presence 1 mM final concentration β-mercaptoethanol. The electroeluted protein was concentrated with a Centricon (Amicon, Beverly, MA) and then used for making antiserum.
For affinity-purification of antibodies, the purified proteins GST-154C and GST-71C were separated by SDS-PAGE and transferred to PVDF membrane (Bio-Rad, Hercules, CA). The membranes were quickly stained with Ponceau S (Sigma) and the expected bands were cut out. The purified GST proteins were coupled to affigel-10 (Bio-Rad) as suggested by the manufacturer. Antiserum was first passed through a GST column to remove anti-GST antibodies. Then the antiserum was sequentially incubated with strips of PVDF membranes with bound GST-71C or GST-154C, which had been blocked with 5% milk or BSA in TBST (50 mM Tris-Cl, pH 7.5, 150 mM NaCl, 0.05% Tween 20), for 1 h at room temperature. The membranes were washed with TBST there times. The antibodies were eluted from the membranes with 100 mM glycine (pH2.5) and neutralized with 1 M Tris-Cl (pH 8.0). The antibodies eluted from the GST-71C and GST-154C bound membranes were designated as anti-KIF3C and anti-KIF3BC, respectively.
On the basis of the published KIF3B sequence (Yamazaki et al., 1995), KIF3B cDNA was amplified from the above-mentioned mouse brain cDNA library by PCR. A 758-bp EcoO109I fragment, encoding C-terminal 71 amino acid residues of the KIF3B polypeptide, was filled in to give a flush end with Klenow and cloned into EcoRI site of pGEX-KG expression vector (Guan and Dixon, 1991). Expression and purification of the KIF3B fusion protein are essentially same as those for GST-154C and GST-71C. For affinity-purification of antibodies, the purified KIF3B fusion protein was separated by SDS-PAGE and transferred to PVDF membrane. The membranes were used for purifying anti-KIF3B from anti-KIF3B serum (BAbCO, Richmond, CA) as described earlier.
Western Blot Analysis
Mouse tissues were homogenized in RIPA (50 mM Tris-Cl, pH 8.0, 1% NP-40, 0.1% SDS, 0.5% sodium deoxycholate, 150 mM NaCl) buffer. The supernatant was obtained after centrifugation in a Ti1270 rotor (Sorvall, Wilmington, DE) at 35 krpm for 30 min at 4°C. The protein was quantitated by Bradford assay (Bio-Rad). Thirty micrograms of protein from each tissue type was loaded on a 10% SDS-PAGE. The proteins were transferred to PVDF membrane (Bio-Rad) in the SDS-PAGE running buffer in the presence of 10% methanol without SDS. The blots were blocked with 5% milk in TBST, then probed with primary and secondary antibodies in the milk TBST solution. The secondary antibodies were horseradish peroxidase-conjugated goat anti-rabbit IgG or anti-mouse IgG (Jackson ImmunoResearch, West Grove, PA). An ECL kit (Amersham, Arlington Heights, IL) was used for developing the blots as suggested by the manufacture.
Immunoprecipitation
One hundred microliters (about 500 μg total protein) mouse brain lysate prepared as described above was incubated with 5 μg affinity-purified antibodies at 4°C for one hour. Twenty microliters of Sepharose protein A beads (Sigma), which had been blocked with 5% BSA made in RIPA buffer for one hour, were added and incubated at 4°C for another hour. The protein A beads were washed with RIPA buffer three times and the proteins were eluted from the beads with SDS-PAGE loading buffer. The immunoprecipitated proteins were analyzed by SDS-PAGE and Western blots.
Taxol-assisted Assembly of Microtubules and Microtubule Sedimentation Assays
Taxol-assisted assembly of microtubules and microtubule sedimentation assays were performed as described (Barton et al., 1995, Hanlon et al., 1997). KIF3C, KHC, KIF3A, and KIF3B were detected by Western blot analysis.
Subcellular Fractionation of Brain Tissue
Brain cellular fraction was achieved via differential centrifugations to obtain fractions S1, P1, S2, P2, S3, P3, LS1, LP1, LS2, and LP2 as previously described (Hanlon et al., 1997; Huttner et al., 1983). (S1, supernatant of the homogenate at low-speed centrifugations and corresponding pellet P1; P2, crude synaptosomes pellet of S1 and corresponding supernatant S2 at medium-speed centrifugations; P3, pellet of S2 and corresponding supernatant S3 at high-speed centrifugations; LP1, pellet obtained after hypotonic lysis of P2 and corresponding supernatant LS1; and LP2, vesicle-enriched pellet of LS1 and corresponding supernatant LS2). Same amount of protein from each fractions was analyzed by SDS-PAGE and Western blots.
Ligation of Mouse Sciatic Nerves
The sciatic nerves of anthesthetized mice were tightly ligated as described (Smith, 1980; Hirokawa et al., 1990). After 3 h, the mouse was transcardially perfused with 4% paraformaldehyde. Small pieces of the nerve, which contained regions both proximal and distal to the ligated site, were frozen and sectioned on a cryostat (10 μm). Sections were used for immunofluorescent staining. The unligated nerves were treated in parallel as controls.
Immunofluorescence Microscopy
Adult mice were transcardially perfused with 4% paraformaldehyde. Brain, spinal cord, sciatic nerves, and eyes were dissected, postfixed with the same fixative, cryoprotected with 20% sucrose in PBS, frozen, and sectioned on a cryostat (10 μm). The sections were processed for immunofluorescence microscopy by using affinity-purified anti-KIF3C as primary and FITC-labeled goat anti-rabbit IgG (Jackson ImmunoResearch) as secondary antibodies. For double staining, Texas red-labeled goat anti-mouse IgG (Jackson ImmunoResearch) was used as secondary antibodies. Protein A purified preimmune rabbit IgG containing equivalent amounts of the anti-KIF3C was used as the primary antibody in control experiments. All images were collected with a Bio-Rad MRC-1000 confocal imaging system.
RESULTS
Cloning and Sequence Analysis of the KIF3C cDNA
As described (Yang et al., 1997), several cDNA clones encoding new kinesin-like motors were identified and cloned from a mouse brain cDNA library. One of those clones was designated as KIF3C because of its closer sequence relationship to KIF3A and KIF3B than to other members of the mouse kinesin superfamily (see below). This clone has a total length of 4017 bp and contains a single open reading frame that predicts a 796 amino acid polypeptide (90 kDa) with the conserved motor domain at its N-terminus (Figure 1A).
Comparison of the sequence of KIF3C to other members of the kinesin superfamily with the UWGCG program PILEUP (Devereux, et al., 1984) indicated that KIF3C is a member of the KIF3 family, which currently includes KIF3A (Kondo et al., 1994) and KIF3B (Yamazaki et al., 1995) from M. musculus, KRP85 (Cole et al., 1993) and KRP95 (Rashid et al., 1995) from S. purpuratus, KLP64D (Stewart et al., 1991) and KLP68D (Pesavento et al., 1994) from D. melanogaster, FLA10 (Walther et al., 1994) from C. reinharditi, and Osm3 from C. elegans (Shakir et al., 1993). The full sequence alignment of KIF3C with KIF3A, KIF3B, KRP85, and KRP95 (Figure 1A) suggests that KIF3C is more closely related to KIF3B and KRP95 than to other members of the KIF3 family. For example, KIF3C is 83%/66% (motor domain/whole protein, respectively) identical to KIF3B, 79%/59% to KRP95, 66%/46% to KIF3A, and 66%/44% to KRP85. As a comparison, KIF3C is 48% and 30% identical to mouse KHC (Gudkov et al., 1994) in the motor domain and the whole protein, respectively. Dot matrix comparisons of KIF3C with KIF3B and mouse KHC indicated that sequence identity was shared almost the entire length of KIF3C and KIF3B, but only in the N-terminal 380 amino acids of KIF3C and mouse KHC (Figure 1B).
Further analysis of the KIF3C sequence revealed that it is likely to be composed of three domains. First, homology to the kinesin motor region is included within the N-terminal 380 amino acids of the KIF3C motor (Figure 1B). In the motor domain, compared with other members of the KIF3 family, KIF3C has an extra 26 amino acids from residue 260 to 285 (Figure 1A); strikingly, the rat homologue of KIF3C also has these extra amino acids (Muresan et al., in press). Similar extra amino acid residues have not been found in other members of the kinesin superfamily. Several cDNA clones encoding KIF3C all contain the 26 amino acid sequence and RT-PCR products of KIF3C also contain the 26 amino acids. Recently, the three dimensional structure of the motor domain of human KHC was reported (Kull et al., 1996). Using the conserved amino acids between KIF3C and human KHC as markers for comparison, the 26 amino acid residues would be localized in loop 11, which has not been clearly defined in terms of structure and function. These residues may be located in a flexible region whose role has yet to be defined.
The central stalk domain of KIF3C was predicted to have a high α-helical content. A structure prediction program (Lupas et al., 1991) designed to estimate the probability that a given sequence will form an α-helical coiled-coil structure predicts that within this second domain, KIF3C has a high probability of forming an α coiled-coil structure from residues 455 to 633 (Figure 1C), suggesting that native KIF3C may be form a dimer via interactions within this region. Although many kinesin-like proteins contain a predicted α-helical coiled-coil, such regions vary greatly in both size and sequence. This region of KIF3C, however, shares very high sequence similarity with a comparable region of KIF3B (Figure 1B), and other members of the KIF3 family. Alternatively, when mouse KHC is compared with KIF3C (Figure 1B), no obvious similarity beyond the first 380 amino acids is seen. Instead, a scatter pattern typical of comparisons between unrelated α-helical coiled-coil regions is apparent.
The third structural domain of KIF3C consists of the C-terminal 150 amino acids (beyond the α-helical coiled-coil region) and is predicted to encode a globular tail domain. Although such globular tail regions have no demonstrated function at present, it has been suggested that these regions mediate interactions with other proteins or cargoes (Yang et al., 1989). Comparison of the KIF3C tail sequence with other members of the KIF3 family revealed that there is almost no similarity in the second half of the tail, although there is significant similarity in the first half between KIF3C and KIF3B or KRP95, but neither KIF3A nor KRP85 (Figure 1). This finding is consistent with the suggestion that KIF3C is most closely related to KIF3B and KRP95. Because KIF3A and KIF3B have been suggested to be homologues of KRP85 and KRP95 (Yamazaki et al., 1995), respectively, our sequence analysis may indicate that KIF3C could be another homologue of KRP95 in mouse.
KIF3C Expression Patterns
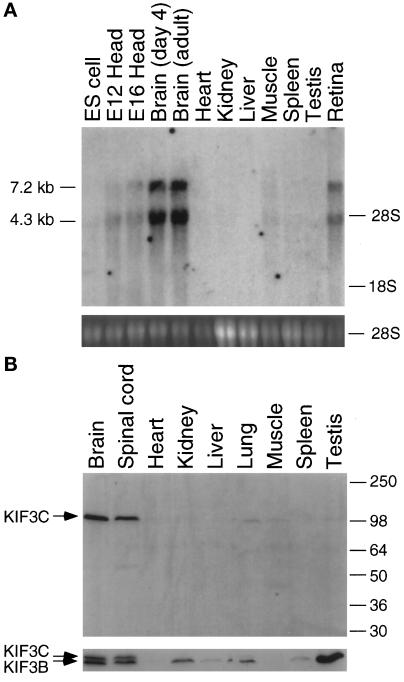
To probe the biological role of KIF3C, Northern analysis was used to examine its expression patterns in various mouse tissues and developmental stages (Figure 2A). KIF3C encodes two transcripts of 7.2 and 4.3 kb (Figure 2A, upper panel). When DNA sequences from the motor, tail, or 3′ untranslated region of the KIF3C cDNA were used as probes, the same two transcripts of 7.2 and 4.3 kb were observed, which renders unlikely the possibility that one of the two bands comes from cross-hybridization to a different gene’s product. KIF3C is mainly expressed in neural tissues such as brain and retina; little expression is apparent in other tissues. Although total RNA from kidney, liver, and spleen was overloaded (Figure 2A, lower panel), neither transcript was detected in these tissues. In comparison with mouse brain, embryonic stem cell (ES cells) and mouse embryo heads at day 12 (E12) and 16 (E16) have only a very low level of expression of KIF3C. However, there is no obvious difference between 4 d postnatal and adult mouse brain in the expression of KIF3C. This finding is in contrast to previous reports about KIF3A and KIF3B, which were expressed in kidney, testis, and other tissues besides brain (Yamazaki et al., 1995).
Figure 2.
Analysis of expression of KIF3C in mouse tissues and cell lines. (A) Analysis of KIF3C transcripts in mouse tissues or cell lines by Northern blots. Upper panel shows that two transcripts of 7.2 and 4.4 kb were detected in brain and retina. Lower panel is ethidium bromide staining of 28S ribosomal RNA as a control for integrity and loading of total RNA from all tissues and cell line. ES cell: embryonic stem cell. E12, E16: mouse embryo heads at day 12 and day 16, respectively. (B) Analysis of KIF3C expression in mouse tissues by Western blots. The same blots were first probed with anti-KIF3C (upper panel), then stripped and probed with anti-KIF3BC (lower panel).
Two antibodies, anti-KIF3C and anti-KIF3BC were generated to examine the localization of the KIF3C motor (see MATERIALS AND METHODS). When these antibodies were tested for specificity with Western blots of E. coli expressed proteins (the tail regions of KIF3A, KIF3B, and KIF3C), anti-KIF3C was found to be specific for the KIF3C protein, whereas anti-KIF3BC was reactive to both the KIF3C and KIF3B motors as expected. Neither anti-KIF3C nor anti-KIF3BC was reactive with the KIF3A protein. These findings are consistent with the knowledge that 48 out of the 74 amino acids from residues 643 to 717 of the KIF3C polypeptide are identical between KIF3B and KIF3C (Figure 1A). Thus, anti-KIF3BC, which was generated and purified with the protein GST-154C (from the construct pGEX-154C), is reactive with both the KIF3C and KIF3B polypeptides. In contrast, as the C-terminal 71 amino acid sequence of the KIF3C is virtually unique compared with that of KIF3B, anti-KIF3C, which was purified with the protein GST-71C, is specific for the KIF3C protein.
When anti-KIF3C was used for Western analysis of mouse tissues (Figure 2B, upper panel), a 100-kDa band was detected in brain and spinal cord, but not in other tissues (although a very low level was observed in lung). Because the size deduced from the KIF3C cDNA is 90 kDa, the 100-kDa band is reasonable for the KIF3C motor. When the same blot was stripped and probed with anti-KIF3BC, besides the KIF3C band (upper), one slightly smaller band (KIF3B, about 95 kDa) was detected in brain, spinal cord, kidney, testis, and other tissues (Figure 2B, lower panel). These results suggest that, in contrast to KIF3A (Kondo et al., 1994) and KIF3B (Yamazaki et al., 1995), KIF3C is mainly expressed in neural tissues, which is consistent with the results from Northern analysis (Figure 2A). Therefore, although the KIF3C amino acid sequence is very similar to KIF3B and KIF3A, its different expression pattern may suggest that KIF3C plays distinct roles compared with other members of the KIF3 family.
Cellular Localization of the KIF3C Motor
The cellular location of a protein often provides likely candidates for the site of in vivo function of the protein. Therefore, the affinity-purified anti-KIF3C antibodies, which are specific for KIF3C (Figure 2B), were used for immunofluorescence to localize the KIF3C motor in mouse tissues.
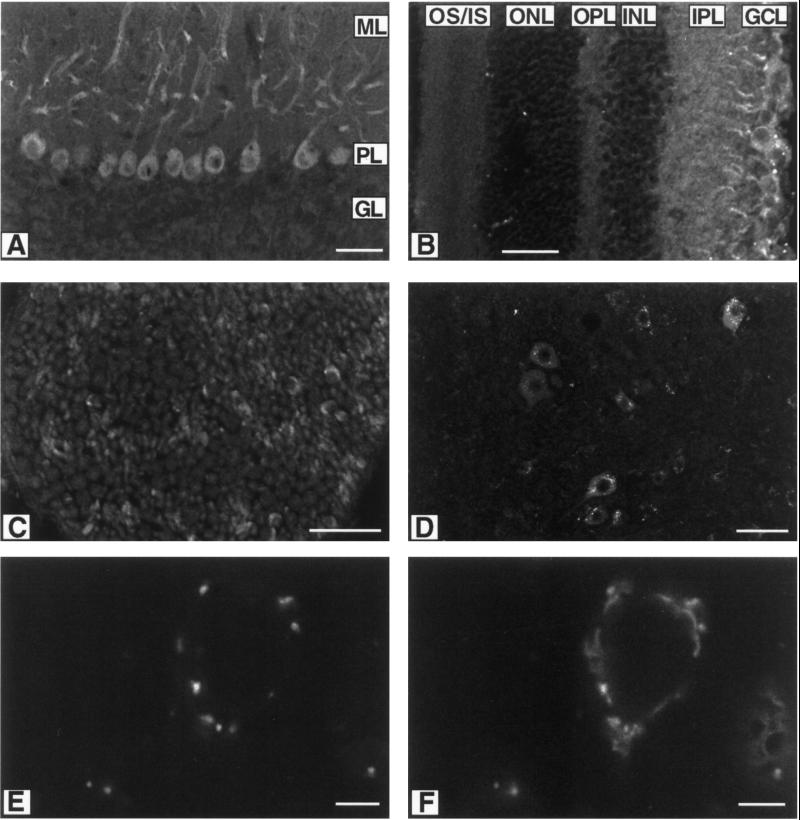
When anti-KIF3C was used to stain sections of cerebellum, the strongest signal came from Purkinje cells (Figure 3A). Under the conditions used, no signal was detected in the cells of the molecular and granular layers of cerebellum. In Purkinje cells, the antibodies produced very strong signals in cell bodies and dendrites, but not axons (Figure 3A). When anti-KIF3C was used for staining retina, the strongest signal came from the ganglion cells (GCL, Figure 3B). In sciatic nerve, anti-KIF3C gave weak signals in axons, but strong signals in the form of a half circle (Figure 3C). These half circles are probably Schwann cell cytoplasm because they overlap perfectly with antibody staining of Schwann cell marker protein S100. In cross sections of spinal cord (Figure 3D), anti-KIF3C apparently stains more intensely in cell bodies than in axons and dendrites. In control experiments, preimmune rabbit IgG gave no signal in cross sections of spinal cord. Thus, the KIF3C motor is apparently present in neural cell bodies, dendrites, and axons, with a stronger signal from cell bodies.
Figure 3.
Cellular localization of the KIF3C motor by immunofluorescence microscopy. Anti-KIF3C stain: (A) section of cerebellum, (B) section of retina, (C) cross section of sciatic nerve, and (D) cross sections of spinal cord. Double staining of cross section of spinal cord with anti-KIF3C (E) and anti-giantin (F). ML: molecular layer, PL: Purkinje cell layer, GL: granular layer; OS/IS; outer and inner segments, ONL: outer nuclear layer, OPL: outer plexiform layer, INL: inner nuclear layer, IPL: inner plexiform layer, GCL: ganglion cell layer. Scale bar: 50 μm in A–D; 5 μm in E and F.
Because the strongest signals in spinal cord came from a spot-like structure that looks like the Golgi apparatus, the possibility that KIF3C proteins are mainly localized in the Golgi apparatus was examined. When cross sections of spinal cord were doubly stained with anti-KIF3C and a monoclonal antibody against giantin, they partially overlapped (Figure 3E and F). Because giantin is a Golgi apparatus membrane protein (Linstedt and Hauri, 1993), these data suggest that KIF3C may be a component of the Golgi apparatus.
Direction of Transport of the KIF3C Motor
Most kinesin-like proteins with the motor domain at the N-terminus are plus-end–directed microtubule motors (Bloom and Endow, 1995). As KIF3C is an N-terminal motor closely related to other plus-end–directed motors, it is reasonable to suggest that KIF3C is a plus-end–directed microtubule motor. To test this view, mouse sciatic nerve was ligated and stained with ant-KIF3C. Previous studies demonstrated that after ligation of peripheral nerves, membranous organelles conveyed by anterograde or retrograde fast axonal transport accumulated at the proximal only, or proximal and distal regions of a restricted axon, respectively (Smith, 1980; Hirokawa et al., 1990, 1991). One of the two sciatic nerves was ligated in a mouse and the other was left as a control. When both ligated and control nerves were stained with anti-KIF3C, KIF3C obviously accumulated on the proximal but not on the distal side of the ligation (Figure 4A), which is consistent with the suggestion that KIF3C is an anterograde or plus-end–directed motor. In the unligated nerve, there is apparently no regional difference (Figure 4B).
Figure 4.
KIF3C accumulation at the proximal region of a ligated sciatic nerve. (A) Ligated and (B) unligated sciatic nerves were stained with anti-KIF3C. After ligation, KIF3C significantly accumulated at the proximal region (left side) of ligation, whereas almost no accumulation was observed at the distal region (right side). The ligated site is indicated by arrows. Ligated sciatic nerve double-stained with anti-KIF3C (C) and a monoclonal antibody against synaptobrevin (D). Only the proximal region with the ligated site on the right is shown in (C) and (D). Scale bar: 50 μm in (A) and (B); 20 μm in (C) and (D).
To examine whether KIF3C may be associated with organelles, double-staining experiments were performed with antibodies against KIF3C and proteins specific for various organelles transported within the axon. Ligated sciatic nerves were stained with antibodies to synaptic vesicle precursor proteins (synaptophysin, syntaxin 1A, synaptobrevin, and synaptotagmin). This type of experiment showed some, but not complete, overlap of the distribution of KIF3C with a subset of synaptic vesicle precursor proteins including synaptobrevin (Figure 4C and D).
Although KIF3C exhibited an apparently overlapping distribution with some synaptic precursor proteins in immunofluorescence double-labeling ligation experiments, owing to the resolution limits of light microscopy, there is a possibility that KIF3C is associated with other organelles, which accumulate at similar rates as these vesicles in the ligated axon. Thus, we investigated whether KIF3C biochemically fractionates with purified synaptic vesicles from the nerve terminals. Previous studies showed that the low-speed pellet (P1) contains the nuclear fraction and mitochondria, medium-speed pellet (P2) the synaptosomes and lysosomes, and high-speed pellet (P3) mainly microsomes, which usually contain Golgi complex, endoplasmic reticulum, and other organelle membranes (Huttner et al., 1983; Yamazaki et al., 1995). Figure 5 shows the distribution of KIF3C in fractions obtained by differential centrifugation during the purification of synaptic vesicles. KIF3C immunoreactivity was detected in all fractions, although much less in P1 and LP1 (Figure 5A). Like that of KIF3B (Figure 5B; Yamazaki et al., 1995), the level of KIF3C, as observed by Western blots, does not correspond to the levels of the synaptic precursor proteins synaptotagmin and synaptophysin (Figure 5C and D), which may suggest that most KIF3C is not associated with synaptic vesicles. Because KIF3C was detected at a higher level in fraction S2, P3, and S3, it may suggest that most of KIF3C are associated with microsomes, or some other kind of vesicles. These results are consistent with the prominent staining of KIF3C around the Golgi complex in spinal cord.
Figure 5.
Subcellular fractionation of KIF3C. Brain subcellular fractions were obtained by differential centrifugation. Same amount of protein from each fraction was analyzed by Western blots for the presence of KIF3C (A), KIF3B (B), synaptotagmin (C), and synaptophysin (D) with specific antibodies.
Microtubule Binding Characteristics of the KIF3C Motor
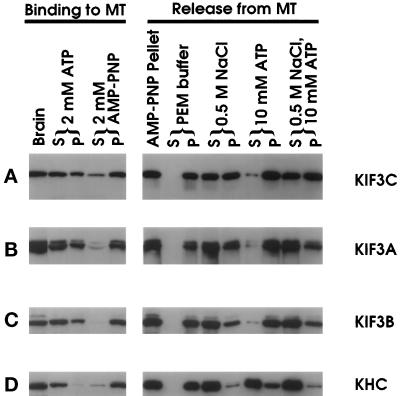
Anti-KIF3C was used to analyze the microtubule-binding characteristics of native KIF3C as shown in Figure 6. A binding assay was carried out with the taxol-based microtubule assembly and sedimentation protocols that have been used to purify conventional kinesin from a variety of sources. KHC, KIF3A, and KIF3B were followed as controls. In the presence of AMP-PNP, both KHC and KIF3C sediment with taxol-stabilized microtubules (Figure 6A and D). However, when the AMP-PNP pellet is incubated with ATP, most KHC, but little KIF3C, is released into the supernatant during subsequent centrifugation (Figure 6, right panels). Incubation of microtubule-bound KIF3C with PEM (80 mM PIPES, pH 6.9, 2 mM EGTA, 2 mM MgCl2) buffer plus 500 mM NaCl results in considerable release of KIF3C into the supernatant. This behavior of KIF3C is markedly different from the extraction properties of KHC (Figure 6A and D). KIF3A, KIF3B, and KIF3C apparently have a slight difference in binding to MT with AMP-PNP and also in the release from MT with salt and ATP (Figure 6A-C). However, compared with KHC, the microtubule-binding characteristics of KIF3A, KIF3B, and KIF3C are generally quite similar to each other. Interestingly, KRP95 and KRP85 from both S. purpuratus (Cole et al., 1992) and Xenopus (Rogers et al., 1997) were previously shown to be quantitatively released from microtubules by ATP. This property is similar to KHC, but different from KIF3A, KIF3B, and KIF3C. The significance of this difference among the members of the KIF3 family between different species is not clear.
Figure 6.
Microtubule sedimentation analysis of KIF3C. KIF3C binding to microtubules (left panels): soluble protein from total brain extract was incubated with GTP and Taxol to boost microtubule polymerization. After microtubule assembly, extracts were supplemented with ATP or AMP-PNP. KIF3C release from microtubules (right panels): the microtubule pellet (AMP-PNP pellet) was extracted with PEM buffer with or without 0.5 M NaCl, 10 mM ATP, or 0.5 M NaCl plus 10 mM ATP. Pellet (P) and supernatant (S) were separated by centrifugation. An equal percentage of each fraction was analyzed. KIF3C was detected with anti-KIF3C by Western blot analysis (A). The same blots were used for detecting KIF3A (B), KIF3B (C), KHC (D) with anti-KIF3A (Kondo et al., 1994), anti-KIF3B, and a monoclonal antibody Suk4 (Ingold et al., 1994), respectively.
KIF3C Associates with KIF3A, but not KIF3B
Kinesin-II has been reported to be a composed of two different motor subunits, KRP95 and KRP85, and one nonmotor subunit KAP115 (Cole et al., 1993); a similar structure exists with the mouse homologues KIF3A and KIF3B (Yamazaki et al., 1995). Because KIF3C is very similar in amino acid sequence to KRP95, KRP85, KIF3A, and KIF3B, we examined whether KIF3C participates in formation of heterotrimers with KIF3A and KIF3B.
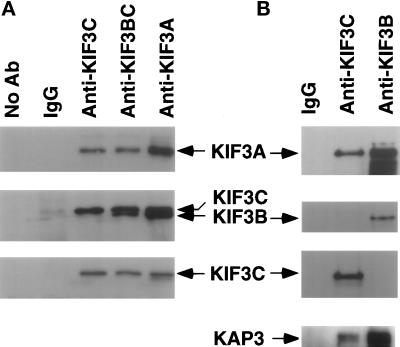
To test this hypothesis, we used anti-KIF3A, anti-KIF3BC, anti-KIF3C, and preimmune rabbit IgG for immunoprecipitation from mouse brain lysate (Figure 7A). After immunoprecipitation, Western blots were used to analyze the precipitated proteins with different antibodies. The blot was first probed with anti-KIF3BC (Figure 7A, middle panel), then stripped and probed with anti-KIF3C (Figure 7A, bottom panel), and finally stripped and probed with anti-KIF3A (Figure 7A, top panel). It is apparent that anti-KIF3A, anti-KIF3BC, and anti-KIF3C all can precipitate KIF3C and KIF3A, which suggests that KIF3C and KIF3A associate with each other. Intriguingly, KIF3B can be precipitated by anti-KIF3BC and anti-KIF3A, but not anti-KIF3C, which suggests that KIF3B and KIF3C do not associate with each other. In control experiments, neither KIF3A, KIF3B, nor KIF3 can be precipitated by either protein A beads alone or protein A beads with preimmune rabbit IgG (Figure 7A). When anti-KIF3B instead of anti-KIF3BC was used for a similar experiment, the same results were obtained (Figure 7B). Anti-KIF3C precipitated KIF3A and KIF3C, but not KIF3B. In contrast, anti-KIF3B brought down KIF3A and KIF3B, but not KIF3C.
Figure 7.
KIF3C associates with KIF3A, but not KIF3B. (A). Mouse brain lysate was used for immunoprecipitation with antibodies: IgG, anti-KIF3A (Kondo et al., 1994), anti-KIF3BC, and anti-KIF3C. The immunoprecipitated samples were analyzed by SDS-PAGE and Western blots. The same blot was probed with anti-KIF3C (bottom panel), anti-KIF3BC (middle panel), and anti-KIF3A (top panel). (B). The immunoprecipitation of the mouse brain lysate with IgG, anti-KIF3B, and anti-KIF3C. The same blot was probed with anti-KIF3C (second to bottom panel), anti-KIF3B (second to top panel), and anti-KIF3A (top panel). A similar blot was probed with anti-KAP3 (bottom panel).
Kinesin-II composed of KRP85 and KRP95 from S. purpuratus, or KIF3A/KIF3B from M. musculus. were each reported to contain a nonmotor kinesin-associated protein (KAP) subunit (Core et al., 1993; Yamazaki et al., 1995). To test whether Kinesin-II composed of KIF3A and KIF3C contains a similar KAP, Western blots of the immunoprecipitated samples with anti-KIF3C and anti-KIF3B were probed with anti-KAP3 (Yamazaki et al., 1996). Like anti-KIF3B, anti-KIF3C also precipitated apparently similar forms of KAP3 (Figure 7B, bottom panel). These results suggest that, although KIF3B and KIF3C selectively associate with KIF3A, kinesin-II composed of KIF3A and KIF3B or KIF3A and KIF3C have the same KAP.
DISCUSSION
A number of proteins similar to KHC have been identified from a variety of organisms (Goldstein, 1993; Bloom and Endow, 1995). Using a molecular genetic strategy we identified KIF3C, a novel kinesin-like motor from the mouse. Sequence analysis demonstrated that KIF3C is a new member of the KIF3 family and most closely related to KIF3B and KRP95. Northern and Western analysis showed that KIF3C is mainly expressed in neural tissues.
Combinatorial Diversity in the KIF3 Family
One distinguishable feature of kinesin-II is that it generally contains two different motor subunits from the KIF3 family (Cole et al., 1993; Yamazaki et al., 1995). To date, three members (KIF3A, KIF3B, and KIF3C) belonging to the KIF3 family have been identified in the mouse. Each of them has the potential to serve as a subunit of the kinesin-II complex. Previously, KIF3A and KIF3B were reported to form a heterodimer (Yamazaki et al., 1995). Our results show that, like KIF3B, KIF3C also associates with KIF3A. However, intriguingly, we found that KIF3C and KIF3B do not associate with each other. Thus, KIF3C and KIF3B may be “variable” subunits that associate with a common KIF3A subunit, but not with each other. These combinatorial associations of the KIF3 members might generate additional functional diversity of kinesin-like motors. Like the leucine-zipper transcription factors (Landshulz et al., 1988), selective association of the KIF3 members might provide a mechanism to associate with diverse targets selectively.
Many kinesin-like polypeptides have been suggested to form a homodimer through an α-helical coiled-coil interaction in the central stalk regions (reviewed by Goldstein, 1993). Besides the motor domains, members of the KIF3 family also share significant sequence similarity within the central stalk region, predicted to form an α-helical coiled-coil structure, which may explain why members of the KIF3 family form a heterodimeric structure. One interesting question is how KIF3C selectively associates with KIF3A, but not KIF3B. One suggestion is that opposing charge interactions within the stalk domains, in a region not predicted to form an α-helical coiled-coil are important for generating a KRP85/KRP95 heterodimer (Rashid et al., 1995). However, when a similar region of KIF3C was deleted, KIF3C still selectively associated with KIF3A (Yang and Goldstein, unpublished results). Thus, other interactions in addition to interactions within the stalk domains may play more important roles in selective associations among the subunits of the KIF3 family.
KIF3C may Play Roles in Axonal Transport
Members of the KIF3 family have been suggested to have transport functions in axons, axonemes, and spindles. KRP85/KRP95 staining in the mitotic apparatus associated with membranous vesicles has led to the suggestion that kinesin-II is a membrane-translocating motor protein (Henson et al., 1995). FLA-10 has been suggested to play a role in protein transport of membrane-bound or raft-associated cargoes, including axoneme-stabilizing factors, to their site of assembly at the axonemal tip (Kozminski et al., 1995; Walther et al., 1994). Becaise KLP68D is expressed primarily in the CNS and in a subset of the peripheral nervous system during embryogenesis, it has been suggested to play a role in anterograde axonal transport (Pesavento et al., 1994). The expression pattern of the Osm-3 gene and its mutant phenotype indicated that Osm-3 plays a role in chemosensation (Tabish et al., 1995; Shakir et al., 1993). Finally, antibodies to KIF3A and KIF3B bind specifically to 90–160 nm neuronal vesicles that differ from synaptic vesicle precursors, suggesting that KIF3A and KIF3B play roles in the anterograde fast axonal transport of a subset of membrane-bounded vesicles (Yamazaki et al., 1995).
The results presented here suggest that KIF3C may have a function in anterograde fast axonal transport of membrane-bounded vesicles. The expression pattern of KIF3C is highly enriched in neural tissues, suggesting that KIF3C is a neural motor. In retina, a part of the CNS, anti-KIF3C mainly stains the ganglion cells, which establish contact with the bipolar cells through their dendrites and send their axons to the brain. This immunostaining result may imply that KIF3C plays an axonal transport role in optic nerve. In peripheral nervous system, KIF3C was shown to accumulate at the proximal side of ligated sciatic nerve. This accumulation of KIF3C motor is consistent with KIF3C having a role in fast axonal transport where material has been shown to be transported within the axon at rates of several μm/sec (Smith, 1980). Although staining of ligated sciatic nerve showed some, but not complete, overlap of the distribution of KIF3C with a subset of synaptic vesicle precursor proteins, the cellular fractionation experiments suggest that the majority of KIF3C is not associated with synaptic vesicles, but with microsomes, or some other kind of membranous vesicles. Therefore, like KIF3A and KIF3B (Yamazaki et al., 1995), synaptic vesicles are probably not the main cargoes for the KIF3C motor. In contrast, KIF3C may move some distinct membrane-bounded vesicles.
Considering the difference between KIF3C and KIF3B expression patterns, slightly different microtubule-binding characteristics, and selective association with KIF3A, their functions could be carried out in distinct tissues and cell types, or selectively power different types of cargoes. However, despite these differences between KIF3C and KIF3B, we have not yet distinguished KIF3C from KIF3B in cellular localization and biochemical characterization (Yamazaki et al., 1995). Because KIF3C and KIF3B are similar in sequence and association with KIF3A, it is possible that the KIF3A/KIF3C and KIF3A/KIF3B heterodimers may play a similar role in anterograde fast transport. KIF3A/KIF3C may have transport activities only in neural tissues, whereas KIF3A/KIF3B may have a more general role in transport in neural and other tissues. Thus, it is possible that the two motors each participate in the movement of overlapping types of cargoes, in which case they might be redundant in neural tissues. Further genetic and biochemical analysis are needed to resolve these issues.
ACKNOWLEDGMENTS
We thank Dr. R. Jahn for anti-synaptotagmin, anti-synaptobrevin, anti-synaptophysin, and anti-syntaxin 1A; Dr. J. Yucel for anti-giantin, Dr. N. Hirokawa for anti-KIF3A and anti-KAP3, and Dr. B. Schnapp for sharing unpublished rat KIF3C amino acid sequences. We owe a debt to E. Roberts for invaluable assistance and Drs. J. Lee and H. Matthies for assistance with confocal microscopy. Z.Y. is a National Institute of Health postdoctoral fellow and L.S.B.G. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Abbreviations used: GST, glutathione-S-transferase; KHC, kinesin heavy chain; RIPA buffer, 50 mM Tris-Cl, pH 8.0, 1% NP-40, 0.1% SDS, 0.5% sodium deoxycholate, 150 mM NaCl; TBST, TBS containing 0.05% Tween 20.
The term KIF3 is used for describing the individual motor subunits, while the term kinesin-II refers to the holoenzyme.
REFERENCES
- Barton NR, Pereira AJ, Goldstein LSB. Motor activity and mitotic spindle localization of the Drosophila kinesin-like protein KLP61F. Mol Biol Cell. 1995;6:1563–1574. doi: 10.1091/mbc.6.11.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom GS, Endow SA. Motor proteins 1: kinesins. Protein Profile. 1995;2:1105–1171. [PubMed] [Google Scholar]
- Bloom GS, Gagner MC, Pfister KK, Brady ST. Native structure and physical properties of bovine brain kinesin and identification of the ATP-bindind subunit polypeptide. Biochemistry. 1988;27:3409–3416. doi: 10.1021/bi00409a043. [DOI] [PubMed] [Google Scholar]
- Brady ST, Sperry AO. Biochemical and functional diversity of microtubule motors in the nervous system. Curr Opin Neurobiol. 1995;5:551–558. doi: 10.1016/0959-4388(95)80058-1. [DOI] [PubMed] [Google Scholar]
- Brady ST. A novel brain ATPase with properties expected for the fast axonal transport motor. Nature. 1985;317:73–75. doi: 10.1038/317073a0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cole DG, Cande WZ, Baskin RD, Skoufias DA, Hogan CJ, Scholey JM. Isolation of a sea urchin egg kinesin-related protein using peptide antibodies. J Cell Sci, 1992;101:291–301. doi: 10.1242/jcs.101.2.291. [DOI] [PubMed] [Google Scholar]
- Cole DG, Chinn SW, Wedaman KP, Hall K, Vuong T, Scholey JM. Novel heterotrimeric kinesin-related protein purified from sea urchin eggs. Nature. 1993;366:268–270. doi: 10.1038/366268a0. [DOI] [PubMed] [Google Scholar]
- Coy DL, Howard J. Organelle transport and sorting in axons. Curr Opin Neurobiol. 1994;4:662–667. doi: 10.1016/0959-4388(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein LSB. With apologies to scheherazade: tails of 1001 kinesin motors. Annu Rev Genet. 1993;27:319–351. doi: 10.1146/annurev.ge.27.120193.001535. [DOI] [PubMed] [Google Scholar]
- Guan K, Dixon JE. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991;192:262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- Gudkov AV, Kazarov AR, Thimmapaya R, Axenovich S, Mazo IA, Roninson IB. Cloning mammalian genes by expression selection of genetic suppressor elements: association of kinesin with drug resistance and cell immortalization. Proc Natl Acad Sci USA. 1994;91:3744–3748. doi: 10.1073/pnas.91.9.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon DW, Yang Z, Goldstein LSB. Characterization of KIFC2, a neuronal kinesin superfamily member in mouse. Neuron. 1997;18:439–451. doi: 10.1016/s0896-6273(00)81244-1. [DOI] [PubMed] [Google Scholar]
- Henson JH, Cole DG, Terasaki M, Rashid D, Scholey JM. Immunolocalization of the heterotrimeric kinesin-related protein KRP(85/95) in the mitotic apparatus of sea urchin embryos. Dev Biol. 1995;171:182–194. doi: 10.1006/dbio.1995.1270. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Sato-Yoshitake R, Kobayashi N, Pfister KK, Bloom GH, Brady ST. Kinesin associated with anterogradely transported membranous organelles. J Cell Biol. 1991;114:295–302. doi: 10.1083/jcb.114.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N, Yoshida Y, Sato-Yoshitake R, Kawashima T. Brain dynein localizes on both anterogradely and retrogradely transported membranous organelles. J Cell Biol. 1990;111:1027–1037. doi: 10.1083/jcb.111.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner WB, Schiebler W, Greengard P, De Camilli P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J Cell Biol. 1983;96:1374–1388. doi: 10.1083/jcb.96.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingold AL, Cohn SA, Scholey JM. Inhibition of kinesin-driven microtubule motility by monoclonal antibodies to kinesin heavy chains. J Cell Biol. 1988;107:2657–2667. doi: 10.1083/jcb.107.6.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S, Sato-Yoshitake R, Noda Y, Aizawa H, Nakata T, Matsuura Y, Hirokawa N. KIF3A is a new microtubule-based anterograde motor in the nerve axon. J Cell Biol. 1994;125:1095–1107. doi: 10.1083/jcb.125.5.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozminski KG, Beech PL, Rosenbaum JL. The Chlamydomonas kinesin-like protein FLA10 is involved in motility associated with the flagellar membrane. J Cell Biol. 1995;131:1517–1527. doi: 10.1083/jcb.131.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kull FJ, Sablin EP, Lau R, Fletterick RJ, Vale RD. Crystal structure of the kinesin motor domain reveals a structural similarity to myosin. Nature. 1996;380:550–555. doi: 10.1038/380550a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsov SA, Vaisberg YA, Shanina NA, Magretova NN, Chernyak VY, Gelfand VI. The quarternary structure of bovine brain kinesin. EMBO J. 1988;7:353–356. doi: 10.1002/j.1460-2075.1988.tb02820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landschulz WH, Johnson PF, McKnight SL. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988;240:1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of the bacteriophage T-4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linstedt AD, Hauri H. Giantin, a novel conserved Golgi membrane protein containing a cytoplasmic domain of at least 350 kDa. Mol Biol Cell. 1993;4:679–693. doi: 10.1091/mbc.4.7.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupas A, VanDyke M, Stock J. Predicting coiled coils from protein sequences. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- Muresan, V., Abramson, T., Lyass, A., Winter, D., Porro, E., Hong, F., Chamberlin, N.L., and Schnapp, B.J. (1998). KIF3C and KIF3A form a novel neuronal heteromeric kinesin that associates with membrane vesicles. Mol. Biol. Cell (in press). [DOI] [PMC free article] [PubMed]
- Pesavento PA, Stewart RJ, Goldstein LSB. Characterization of the KLP68D kinesin-like protein in Drosophila: possible roles in axonal transport. J Cell Biol. 1994;127:1041–1048. doi: 10.1083/jcb.127.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid DJ, Wedaman KP, Scholey JM. Heterodimerization of the two motor subunits of the heterotrimeric kinesin, KRP85/95. J Mol Biol. 1995;252:157–162. doi: 10.1006/jmbi.1995.0484. [DOI] [PubMed] [Google Scholar]
- Rogers SL, Tint IS, Fanapour PC, Gelfand VI. Regulated bidirectional motility of melanophore pigment granules along microtubules in vitro. Proc Natl Acad Sci USA. 1997;94:3720–3725. doi: 10.1073/pnas.94.8.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Second Edition. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Scholey JM. Kinesin-II, a membrane traffic motor in axons, axonemes, and spindles. J Cell Biol. 1996;133:1–4. doi: 10.1083/jcb.133.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakir MA, Fukushige T, Yasuda H, Miwa J, Siddiqui SS. C. elegans osm-3 gene mediating osmotic avoidance behaviour encodes a kinesin-like protein. Neuroreport. 1993;4:891–894. doi: 10.1097/00001756-199307000-00013. [DOI] [PubMed] [Google Scholar]
- Smith RS. The short term accumulation of axonally transported organelles in the region of localized lesions of single myelinated axons. J Neurocytol. 1980;9:39–65. doi: 10.1007/BF01205226. [DOI] [PubMed] [Google Scholar]
- Stewart RJ, Pesavento PA, Woerpel DN, Goldstein LSB. Identification and partial characterization of six members of the kinesin superfamily in Drosophila. Proc Natl Acad Sci USA. 1991;88:8470–8474. doi: 10.1073/pnas.88.19.8470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabish M, Siddiqui ZK, Nishikawa K, Siddiqui SS. Exclusive expression of C. elegans osm-3 kinesin gene in chemosensory neurons open to the external environment. J Mol Biol. 1995;247:377–389. doi: 10.1006/jmbi.1994.0146. [DOI] [PubMed] [Google Scholar]
- Vale RD, Reese TS, Sheetz MP. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell. 1985;42:39–50. doi: 10.1016/s0092-8674(85)80099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee RB, Sheetz MP. Targeting of motor proteins. Science. 1996;271:1539–1544. doi: 10.1126/science.271.5255.1539. [DOI] [PubMed] [Google Scholar]
- Walther Z, Vashishtha M, Hall JL. The Chlamydomonas FLA10 gene encodes a novel kinesin-homologous protein. J Cell Biol. 1994;126:175–188. doi: 10.1083/jcb.126.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedaman KP, Meyer DW, Rashid DJ, Cole DG, Scholey JM. Sequence and submolecular localization of the 115-kD accessory subunit of the heterotrimeric kinesin-II (KRP85/KRP95) complex. J Cell Biol. 1996;132:371–380. doi: 10.1083/jcb.132.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki H, Nakata T, Okada Y, Hirokawa N. KIF3A/B: a heterodimeric kinesin superfamily protein that works as a microtubule plus end-directed motor for membrane organelle transport. J Cell Biol. 1995;130:1387–1399. doi: 10.1083/jcb.130.6.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki H, Nakata T, Okada Y, Hirokawa N. Cloning and characterization of KAP3: a novel kinesin superfamily-associated protein of KIF3A/3B. Proc Natl Acad Sci UAS. 1996;93:8443–8448. doi: 10.1073/pnas.93.16.8443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JT, Laymon RA, Goldstein LSB. A three domain structure of kinesin heavy chain revealed by DNA sequence and microtubule binding analyses. Cell. 1989;56:879–880. doi: 10.1016/0092-8674(89)90692-2. [DOI] [PubMed] [Google Scholar]
- Yang Z, Hanlon DW, Marszalek JR, Goldstein LSB. Identification, partial characterization, and genetic mapping of kinesin-like protein genes in mouse. Genomics. 1997;45:123–131. doi: 10.1006/geno.1997.4901. [DOI] [PubMed] [Google Scholar]