Abstract
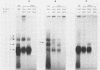
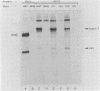
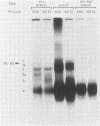
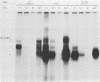
The SVT2 line of simian virus 40-transformed mouse cells expresses little or no wild-type-size A protein (T antigen). Instead, a variant form is produced in these cells that is larger than normal-size A protein. This variant form has an Mr of 100,000 (100K super-T antigen) and is found primarily in complexes with the host-cell-coded p53 protein. Binding of the 100K super-T antigen to simian virus 40 origin region DNA was assayed by immunoprecipitation of super-T antigen-DNA complexes and then digestion with DNase I. DNA sequences associated with super-T antigen were protected from digestion and retained in the immune complex, while unprotected sequences were digested and released. The 100K super-T antigen efficiently protects DNA sequences in the previously defined regions I and II (P. Tegtmeyer, B. A. Lewton, A. L. DeLucia, V. G. Wilson, and K. Ryder, J. Virol. 46:151-161, 1983). Within region II (the origin of replication), the pattern and size of protected fragments are identical for super-T antigen and purified wild-type A protein. Thus, even though super-T antigen is larger than wild-type A protein, both must bind with the same alignment on origin DNA. Furthermore, complexes between the host-cell-coded p53 protein and the 100K super-T antigen also retain the ability to bind in regions I and II.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chandler D. W., Gralla J. Specific binding and protection of form II SV40 deoxyribonucleic acid by ribonucleic acid polymerase II from wheat germ. Biochemistry. 1980 Apr 15;19(8):1604–1612. doi: 10.1021/bi00549a012. [DOI] [PubMed] [Google Scholar]
- Chaudry F., Belsham G. J., Smith A. E. Biochemical properties of the 145,000-dalton super-T antigen from simian virus 40-transformed BALB/c 3T3 clone 20 cells. J Virol. 1983 Mar;45(3):1098–1106. doi: 10.1128/jvi.45.3.1098-1106.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Blanck G., Pollack R. Reacquisition of a functional early region by a mouse transformant containing only defective simian virus 40 DNA. Mol Cell Biol. 1984 Apr;4(4):666–670. doi: 10.1128/mcb.4.4.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Grass D. S., Blanck G., Hoganson N., Manley J. L., Pollack R. E. A functional simian virus 40 origin of replication is required for the generation of a super T antigen with a molecular weight of 100,000 in transformed mouse cells. J Virol. 1983 Nov;48(2):492–502. doi: 10.1128/jvi.48.2.492-502.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton C. E., Lovett M., Rigby P. W. Functional analysis of a simian virus 40 super T-antigen. J Virol. 1982 Dec;44(3):974–982. doi: 10.1128/jvi.44.3.974-982.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. L., Wright P. J., DeLucia A. L., Lewton B. A., Anderson M. E., Tegtmeyer P. Critical spatial requirement within the origin of simian virus 40 DNA replication. J Virol. 1984 Jul;51(1):91–96. doi: 10.1128/jvi.51.1.91-96.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLucia A. L., Lewton B. A., Tjian R., Tegtmeyer P. Topography of simian virus 40 A protein-DNA complexes: arrangement of pentanucleotide interaction sites at the origin of replication. J Virol. 1983 Apr;46(1):143–150. doi: 10.1128/jvi.46.1.143-150.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMaio D., Nathans D. Cold-sensitive regulatory mutants of simian virus 40. J Mol Biol. 1980 Jun 15;140(1):129–142. doi: 10.1016/0022-2836(80)90359-9. [DOI] [PubMed] [Google Scholar]
- Gluzman Y., Sambrook J. F., Frisque R. J. Expression of early genes of origin-defective mutants of simian virus 40. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3898–3902. doi: 10.1073/pnas.77.7.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney E. G., Harrison R. O., Fenno J. Monoclonal antibodies against simian virus 40 T antigens: evidence for distinct sublcasses of large T antigen and for similarities among nonviral T antigens. J Virol. 1980 Jun;34(3):752–763. doi: 10.1128/jvi.34.3.752-763.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. A., Myers R. M., Tjian R. Mutational analysis of simian virus 40 large T antigen DNA binding sites. EMBO J. 1984 Dec 20;3(13):3247–3255. doi: 10.1002/j.1460-2075.1984.tb02286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Lovett M., Clayton C. E., Murphy D., Rigby P. W., Smith A. E., Chaudry F. Structure and synthesis of a simian virus 40 super T-antigen. J Virol. 1982 Dec;44(3):963–973. doi: 10.1128/jvi.44.3.963-973.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May E., Kress M., Daya-Grosjean L., Monier R., May P. Mapping of the viral mRNA encoding a super-T antigen of 115,000 daltons expressed in simian virus 40-transformed rat cell lines. J Virol. 1981 Jan;37(1):24–35. doi: 10.1128/jvi.37.1.24-35.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May E., Lasne C., Prives C., Borde J., May P. Study of the functional activities concomitantly retained by the 115,000 Mr super T antigen, an evolutionary variant of simian virus 40 large T antigen expressed in transformed rat cells. J Virol. 1983 Mar;45(3):901–913. doi: 10.1128/jvi.45.3.901-913.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay R. D. Binding of a simian virus 40 T antigen-related protein to DNA. J Mol Biol. 1981 Jan 25;145(3):471–488. doi: 10.1016/0022-2836(81)90540-4. [DOI] [PubMed] [Google Scholar]
- McKay R. Immunoassay for sequence-specific DNA-protein interaction. Methods Enzymol. 1983;92:138–146. doi: 10.1016/0076-6879(83)92013-x. [DOI] [PubMed] [Google Scholar]
- Myers R. M., Tjian R. Construction and analysis of simian virus 40 origins defective in tumor antigen binding and DNA replication. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6491–6495. doi: 10.1073/pnas.77.11.6491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich N. C., Levine A. J. Specific interaction of the SV40 T antigen-cellular p53 protein complex with SV40 DNA. Virology. 1982 Feb;117(1):286–290. doi: 10.1016/0042-6822(82)90531-1. [DOI] [PubMed] [Google Scholar]
- Shortle D. R., Margolskee R. F., Nathans D. Mutational analysis of the simian virus 40 replicon: pseudorevertants of mutants with a defective replication origin. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6128–6131. doi: 10.1073/pnas.76.12.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Andersen B. Partial purification of SV40 A protein and a related cellular protein from permissive cells. Virology. 1981 Nov;115(1):67–74. doi: 10.1016/0042-6822(81)90089-1. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P., Lewton B. A., DeLucia A. L., Wilson V. G., Ryder K. Topography of simian virus 40 A protein-DNA complexes: arrangement of protein bound to the origin of replication. J Virol. 1983 Apr;46(1):151–161. doi: 10.1128/jvi.46.1.151-161.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Schwartz M., Collins J. K., Rundell K. Regulation of tumor antigen synthesis by simain virus 40 gene A. J Virol. 1975 Jul;16(1):168–178. doi: 10.1128/jvi.16.1.168-178.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R. Protein-DNA interactions at the origin of simian virus 40 DNA replication. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):655–661. doi: 10.1101/sqb.1979.043.01.073. [DOI] [PubMed] [Google Scholar]
- Tjian R. The binding site on SV40 DNA for a T antigen-related protein. Cell. 1978 Jan;13(1):165–179. doi: 10.1016/0092-8674(78)90147-2. [DOI] [PubMed] [Google Scholar]
- Wilson V. G., Tevethia M. J., Lewton B. A., Tegtmeyer P. DNA binding properties of simian virus 40 temperature-sensitive A proteins. J Virol. 1982 Nov;44(2):458–466. doi: 10.1128/jvi.44.2.458-466.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright P. J., DeLucia A. L., Tegtmeyer P. Sequence-specific binding of simian virus 40 A protein to nonorigin and cellular DNA. Mol Cell Biol. 1984 Dec;4(12):2631–2638. doi: 10.1128/mcb.4.12.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]