Abstract
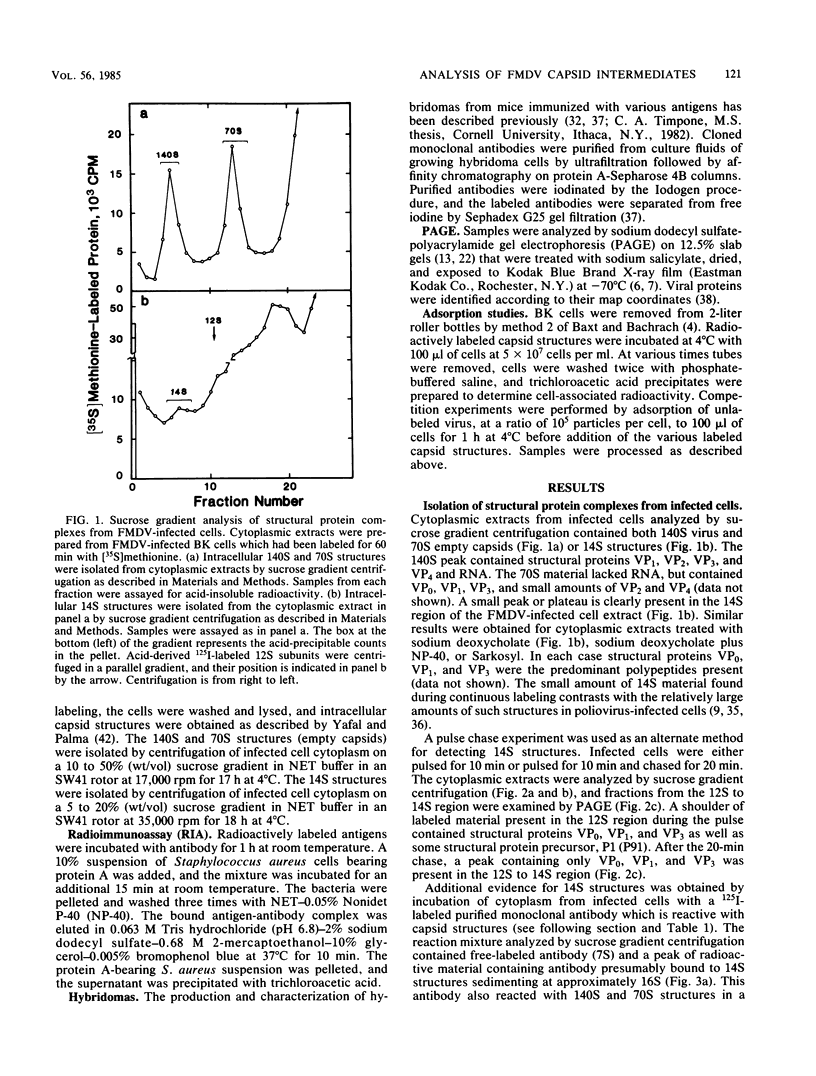
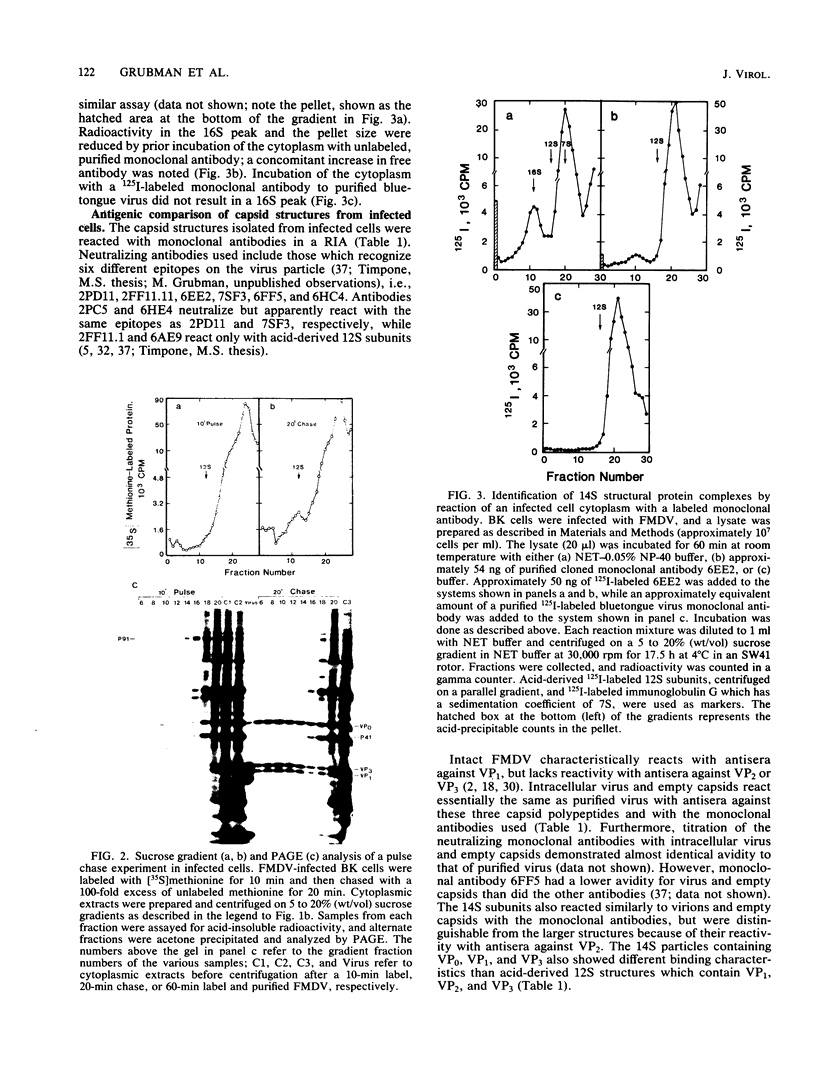
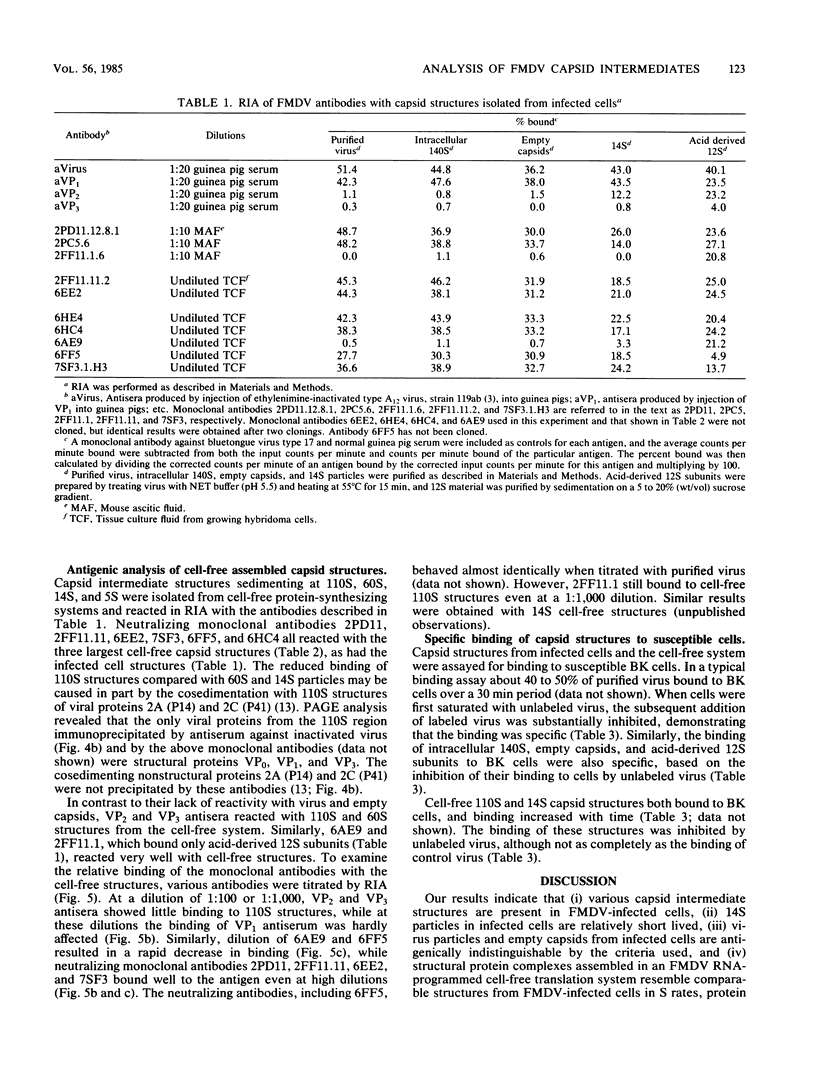
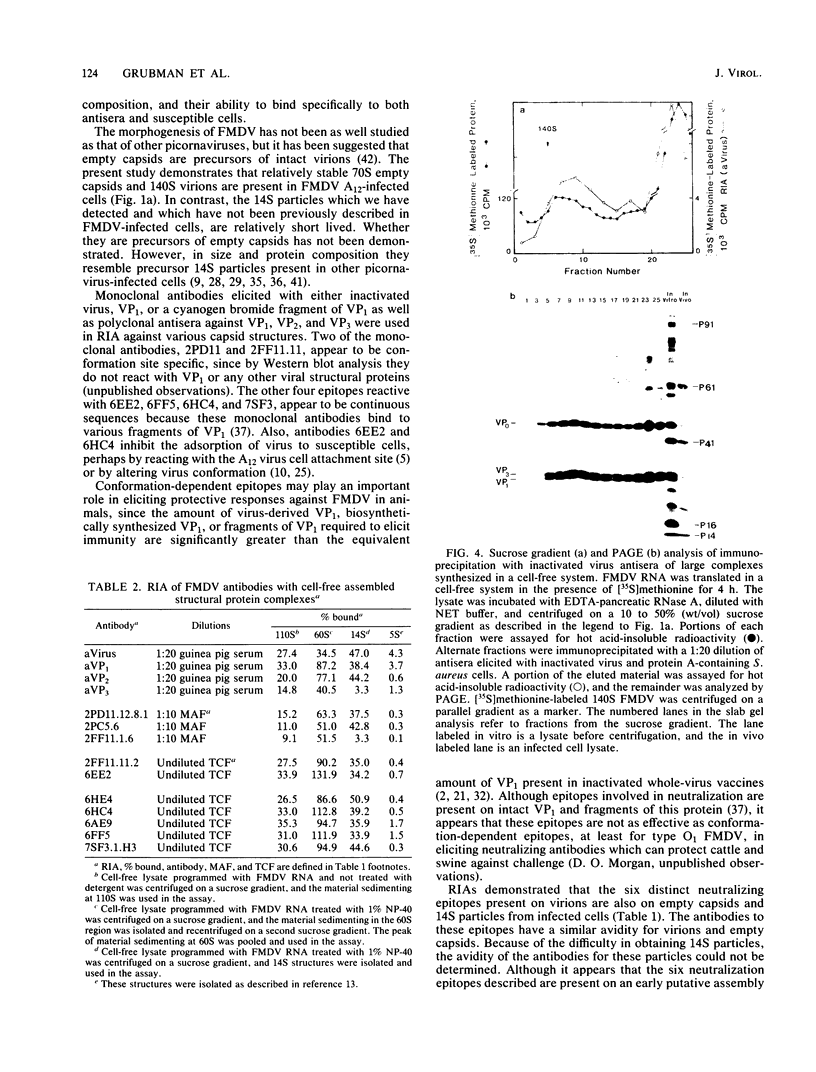
Structural protein complexes sedimenting at 140S, 70S (empty capsids), and 14S were isolated from foot-and-mouth disease virus-infected cells. The empty capsids were stable, while 14S complexes were relatively short-lived. Radioimmune binding assays involving the use of neutralizing monoclonal antibodies to six distinct epitopes on type A12 virus and polyclonal antisera to A12 structural proteins demonstrated that native empty capsids were indistinguishable from virus. Infected cell 14S particles possessed all the neutralizing epitopes and reacted with VP2 antiserum. Cell-free structural protein complexes sedimenting at 110S, 60S, and 14S containing capsid proteins VP0, VP3, and VP1 are assembled in a rabbit reticulocyte lysate programmed with foot-and-mouth viral RNA. These structures also contain the six epitopes, and cell-free 14S structures like their in vivo counterparts reacted with VP2 antiserum. Capsid structures from infected cells and the cell-free complexes adsorbed to susceptible cells, and this binding was inhibited, to various degrees, by saturating levels of unlabeled virus. These assays and other biochemical evidence indicate that capsid assembly in the cell-free system resembles viral morphogenesis in infected cells. In addition, epitopes on the virus surface possibly involved in interaction with cellular receptor sites are found early in virion morphogenesis.
Full text
PDF
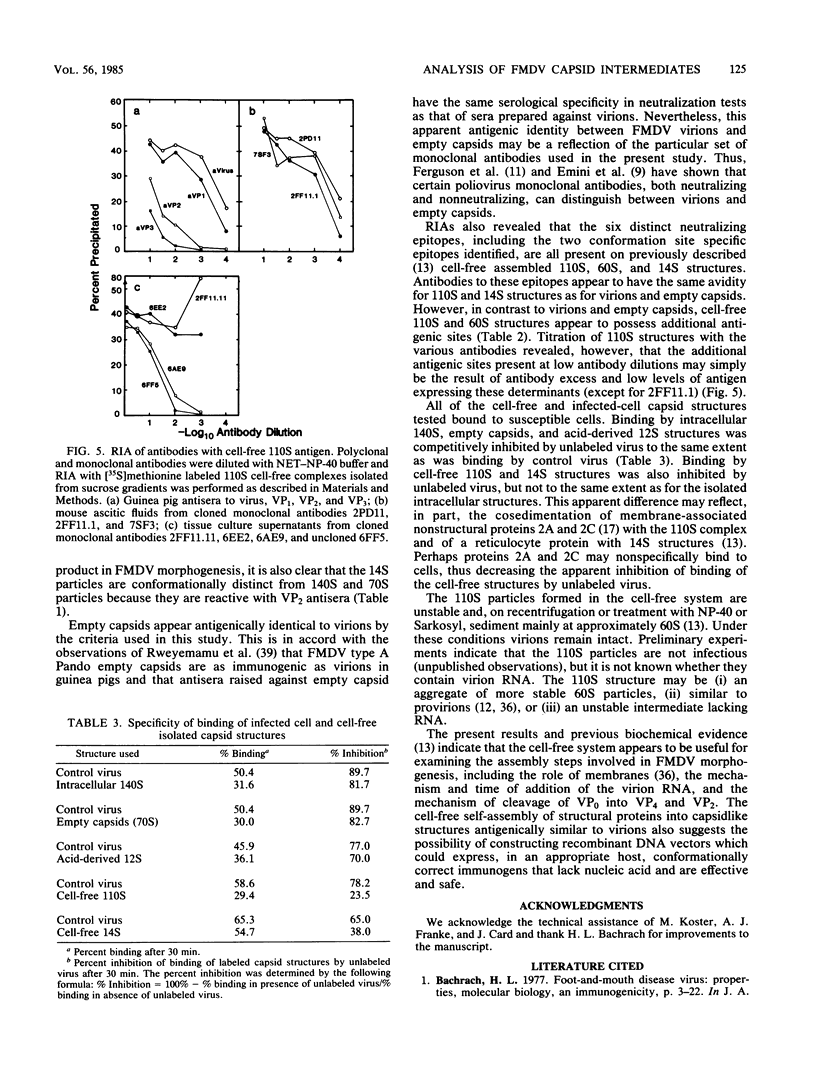

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachrach H. L., Moore D. M., McKercher P. D., Polatnick J. Immune and antibody responses to an isolated capsid protein of foot-and-mouth disease virus. J Immunol. 1975 Dec;115(6):1636–1641. [PubMed] [Google Scholar]
- Bahnemann H. G. Binary ethylenimine as an inactivant for foot-and-mouth disease virus and its application for vaccine production. Arch Virol. 1975;47(1):47–56. doi: 10.1007/BF01315592. [DOI] [PubMed] [Google Scholar]
- Baxt B., Bachrach H. L. Early interactions of foot-and-mouth disease virus with cultured cells. Virology. 1980 Jul 15;104(1):42–55. doi: 10.1016/0042-6822(80)90364-5. [DOI] [PubMed] [Google Scholar]
- Baxt B., Morgan D. O., Robertson B. H., Timpone C. A. Epitopes on foot-and-mouth disease virus outer capsid protein VP1 involved in neutralization and cell attachment. J Virol. 1984 Aug;51(2):298–305. doi: 10.1128/jvi.51.2.298-305.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Duchesne M., Cartwright T., Crespo A., Boucher F., Fallourd A. Localization of a neutralization epitope of foot-and-mouth disease virus using neutralizing monoclonal antibodies. J Gen Virol. 1984 Sep;65(Pt 9):1559–1566. doi: 10.1099/0022-1317-65-9-1559. [DOI] [PubMed] [Google Scholar]
- Emini E. A., Jameson B. A., Lewis A. J., Larsen G. R., Wimmer E. Poliovirus neutralization epitopes: analysis and localization with neutralizing monoclonal antibodies. J Virol. 1982 Sep;43(3):997–1005. doi: 10.1128/jvi.43.3.997-1005.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emini E. A., Kao S. Y., Lewis A. J., Crainic R., Wimmer E. Functional basis of poliovirus neutralization determined with monospecific neutralizing antibodies. J Virol. 1983 May;46(2):466–474. doi: 10.1128/jvi.46.2.466-474.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson M., Minor P. D., Magrath D. I., Qui Y. H., Spitz M., Schild G. C. Neutralization epitopes on poliovirus type 3 particles: an analysis using monoclonal antibodies. J Gen Virol. 1984 Jan;65(Pt 1):197–201. doi: 10.1099/0022-1317-65-1-197. [DOI] [PubMed] [Google Scholar]
- Fernandez-Tomas C. B., Baltimore D. Morphogenesis of poliovirus. II. Demonstration of a new intermediate, the proviron. J Virol. 1973 Nov;12(5):1122–1130. doi: 10.1128/jvi.12.5.1122-1130.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubman M. J., Bachrach H. L. Isolation of foot-and-mouth disease virus messenger RNA from membrane-bound polyribosomes and characterization of its 5' and 3' termini. Virology. 1979 Oct 30;98(2):466–470. doi: 10.1016/0042-6822(79)90570-1. [DOI] [PubMed] [Google Scholar]
- Grubman M. J., Baxt B., Bachrach H. L. Foot-and-mouth disease virion RNA: studies on the relation between the length of its 3'-poly(A) segment and infectivity. Virology. 1979 Aug;97(1):22–31. doi: 10.1016/0042-6822(79)90369-6. [DOI] [PubMed] [Google Scholar]
- Grubman M. J., Baxt B. Translation of foot-and-mouth disease virion RNA and processing of the primary cleavage products in a rabbit reticulocyte lysate. Virology. 1982 Jan 15;116(1):19–30. doi: 10.1016/0042-6822(82)90399-3. [DOI] [PubMed] [Google Scholar]
- Grubman M. J. In vitro morphogenesis of foot-and-mouth disease virus. J Virol. 1984 Mar;49(3):760–765. doi: 10.1128/jvi.49.3.760-765.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubman M. J., Robertson B. H., Morgan D. O., Moore D. M., Dowbenko D. Biochemical map of polypeptides specified by foot-and-mouth disease virus. J Virol. 1984 May;50(2):579–586. doi: 10.1128/jvi.50.2.579-586.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haresnape J. M., McCahon D. Four independent antigenic determinants on the capsid polypeptides of aphthovirus. J Gen Virol. 1983 Nov;64(Pt 11):2345–2355. doi: 10.1099/0022-1317-64-11-2345. [DOI] [PubMed] [Google Scholar]
- Icenogle J., Gilbert S. F., Grieves J., Anderegg J., Rueckert R. A neutralizing monoclonal antibody against poliovirus and its reaction with related antigens. Virology. 1981 Nov;115(1):211–215. doi: 10.1016/0042-6822(81)90103-3. [DOI] [PubMed] [Google Scholar]
- Icenogle J., Shiwen H., Duke G., Gilbert S., Rueckert R., Anderegg J. Neutralization of poliovirus by a monoclonal antibody: kinetics and stoichiometry. Virology. 1983 Jun;127(2):412–425. doi: 10.1016/0042-6822(83)90154-x. [DOI] [PubMed] [Google Scholar]
- Kleid D. G., Yansura D., Small B., Dowbenko D., Moore D. M., Grubman M. J., McKercher P. D., Morgan D. O., Robertson B. H., Bachrach H. L. Cloned viral protein vaccine for foot-and-mouth disease: responses in cattle and swine. Science. 1981 Dec 4;214(4525):1125–1129. doi: 10.1126/science.6272395. [DOI] [PubMed] [Google Scholar]
- La Torre J. L., Grubman M. J., Baxt B., Bachrach H. L. The structural polypeptides of aphthovirus are phosphoproteins. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7444–7447. doi: 10.1073/pnas.77.12.7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lund G. A., Ziola B. R., Salmi A., Scraba D. G. Structure of the Mengo virion. V. Distribution of the capsid polypeptides with respect to the surface of the virus particle. Virology. 1977 May 1;78(1):35–44. doi: 10.1016/0042-6822(77)90076-9. [DOI] [PubMed] [Google Scholar]
- MAYER M. M., RAPP H. J., ROIZMAN B., KLEIN S. W., COWAN K. M., LUKENS D. The purification of poliomyelitis virus as studied by complement fixation. J Immunol. 1957 Jun;78(6):435–455. [PubMed] [Google Scholar]
- Mandel B. Characterization of type 1 poliovirus by electrophoretic analysis. Virology. 1971 Jun;44(3):554–568. doi: 10.1016/0042-6822(71)90369-2. [DOI] [PubMed] [Google Scholar]
- McCullough K. C., Butcher R. Monoclonal antibodies against foot-and-mouth disease virus 146S and 12S particles. Arch Virol. 1982;74(1):1–9. doi: 10.1007/BF01320777. [DOI] [PubMed] [Google Scholar]
- McGregor S. Evidence for the existence of protomers in the assembly of encephalomyocarditis virus. J Virol. 1975 May;15(5):1107–1120. doi: 10.1128/jvi.15.5.1107-1120.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor S., Rueckert R. R. Picornaviral capsid assembly: similarity of rhinovirus and enterovirus precursor subunits. J Virol. 1977 Feb;21(2):548–553. doi: 10.1128/jvi.21.2.548-553.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloen R. H., Briaire J. A study of the cross-reacting antigens on the intact foot-and-mouth disease virus and its 12S Subunits with antisera against the structural proteins. J Gen Virol. 1980 Nov;51(Pt 1):107–116. doi: 10.1099/0022-1317-51-1-107. [DOI] [PubMed] [Google Scholar]
- Meloen R. H., Briaire J., Woortmeyer R. J., van Zaane D. The main antigenic determinant detected by neutralizing monoclonal antibodies on the intact foot-and-mouth disease virus particle is absent from isolated VPI. J Gen Virol. 1983 May;64(Pt 5):1193–1198. doi: 10.1099/0022-1317-64-5-1193. [DOI] [PubMed] [Google Scholar]
- Ouldridge E. J., Barnett P. V., Parry N. R., Syred A., Head M., Rweyemamu M. M. Demonstration of neutralizing and non-neutralizing epitopes on the trypsin-sensitive site of foot-and-mouth disease virus. J Gen Virol. 1984 Jan;65(Pt 1):203–207. doi: 10.1099/0022-1317-65-1-203. [DOI] [PubMed] [Google Scholar]
- Palmenberg A. C. In vitro synthesis and assembly of picornaviral capsid intermediate structures. J Virol. 1982 Dec;44(3):900–906. doi: 10.1128/jvi.44.3.900-906.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips B. A., Summers D. F., Maizel J. V., Jr In vitro assembly of poliovirus-related particles. Virology. 1968 Jun;35(2):216–226. doi: 10.1016/0042-6822(68)90262-6. [DOI] [PubMed] [Google Scholar]
- Putnak J. R., Phillips B. A. Picornaviral structure and assembly. Microbiol Rev. 1981 Jun;45(2):287–315. doi: 10.1128/mr.45.2.287-315.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson B. H., Morgan D. O., Moore D. M. Location of neutralizing epitopes defined by monoclonal antibodies generated against the outer capsid polypeptide, VP1, of foot-and-mouth disease virus A12. Virus Res. 1984 Sep;1(6):489–500. doi: 10.1016/0168-1702(84)90006-6. [DOI] [PubMed] [Google Scholar]
- Rueckert R. R., Wimmer E. Systematic nomenclature of picornavirus proteins. J Virol. 1984 Jun;50(3):957–959. doi: 10.1128/jvi.50.3.957-959.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rweyemamu M. M., Terry G., Pay T. W. Stability and immunogenicity of empty particles of foot-and-mouth disease virus. Arch Virol. 1979;59(1-2):69–79. doi: 10.1007/BF01317896. [DOI] [PubMed] [Google Scholar]
- Sherry B., Rueckert R. Evidence for at least two dominant neutralization antigens on human rhinovirus 14. J Virol. 1985 Jan;53(1):137–143. doi: 10.1128/jvi.53.1.137-143.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su R. T., Taylor M. W. Morphogenesis of picornaviruses: characterization and assembly of bovine enterovirus subviral particles. J Gen Virol. 1976 Mar;30(3):317–328. doi: 10.1099/0022-1317-30-3-317. [DOI] [PubMed] [Google Scholar]
- Yafal A. G., Palma E. L. Morphogenesis of foot-and-mouth disease virus. I. Role of procapsids as virion Precursors. J Virol. 1979 Jun;30(3):643–649. doi: 10.1128/jvi.30.3.643-649.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]