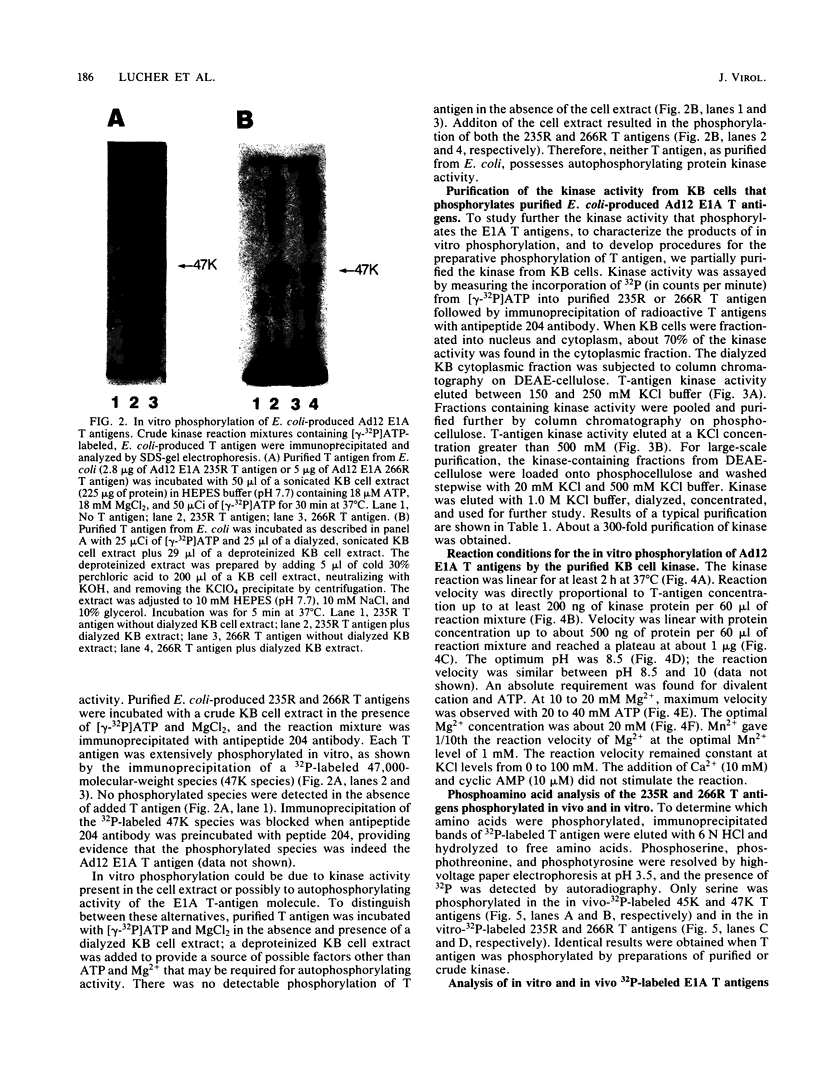
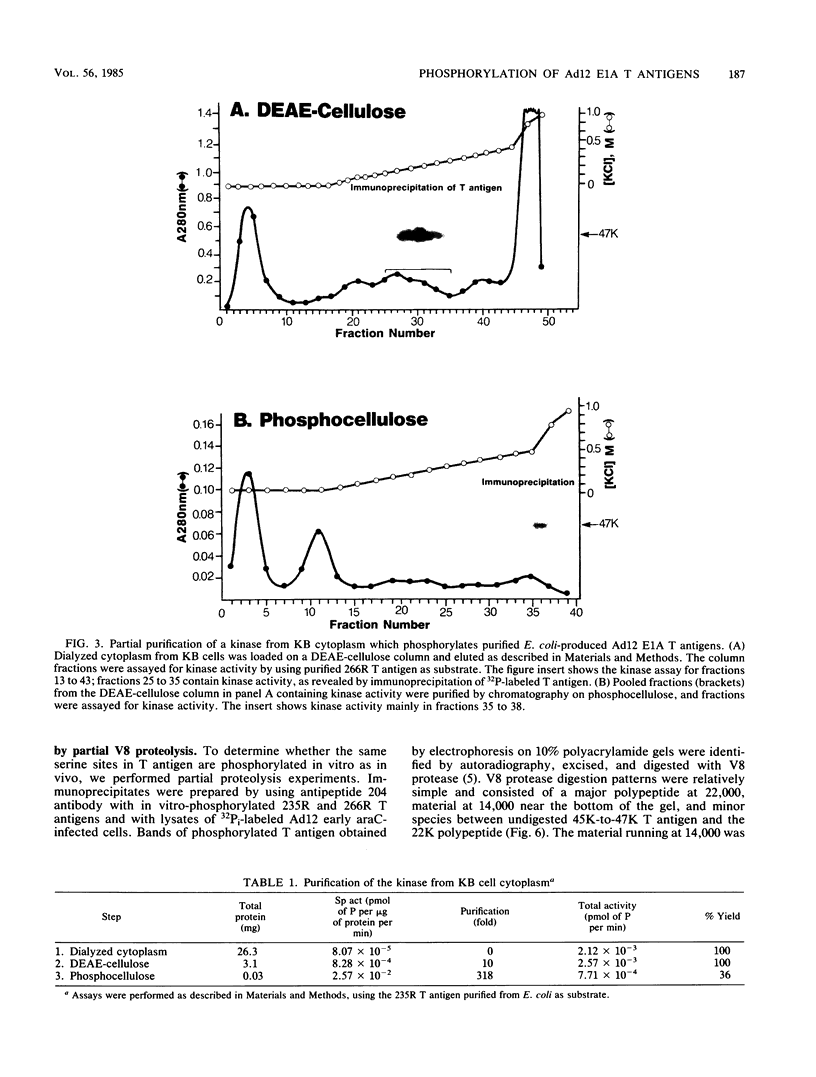
Abstract
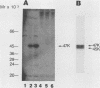
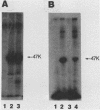
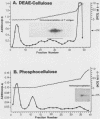
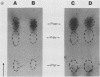
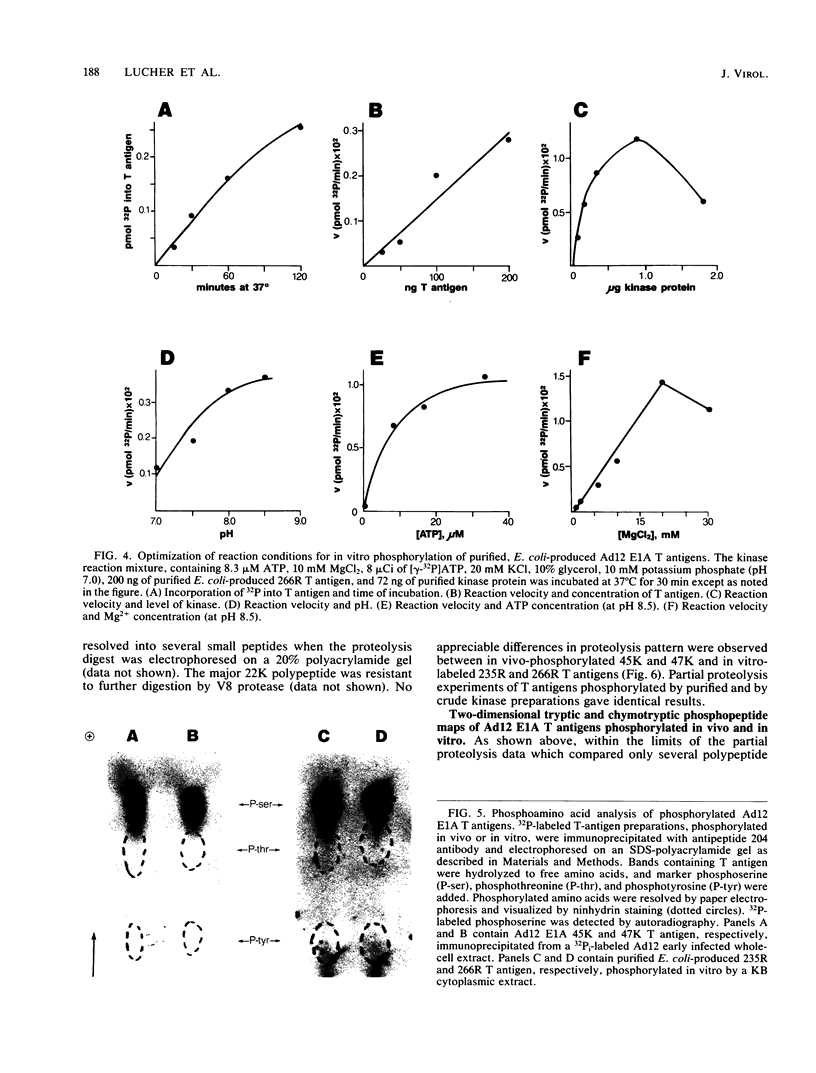
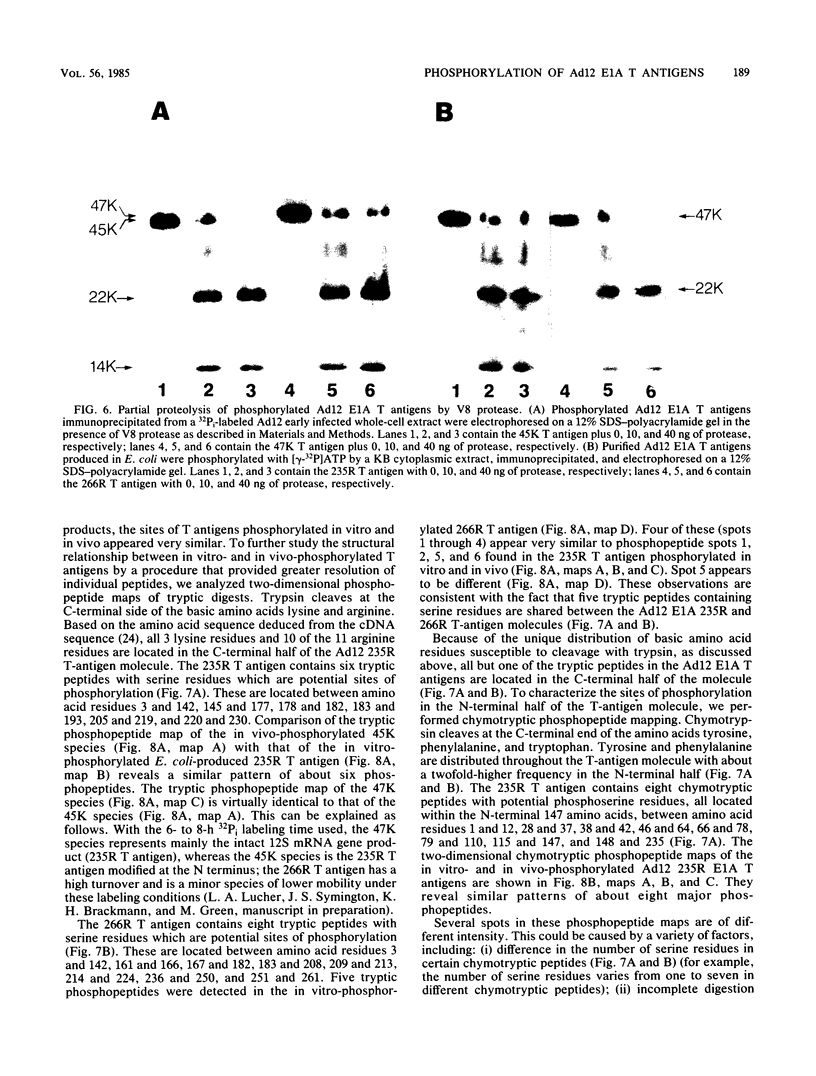
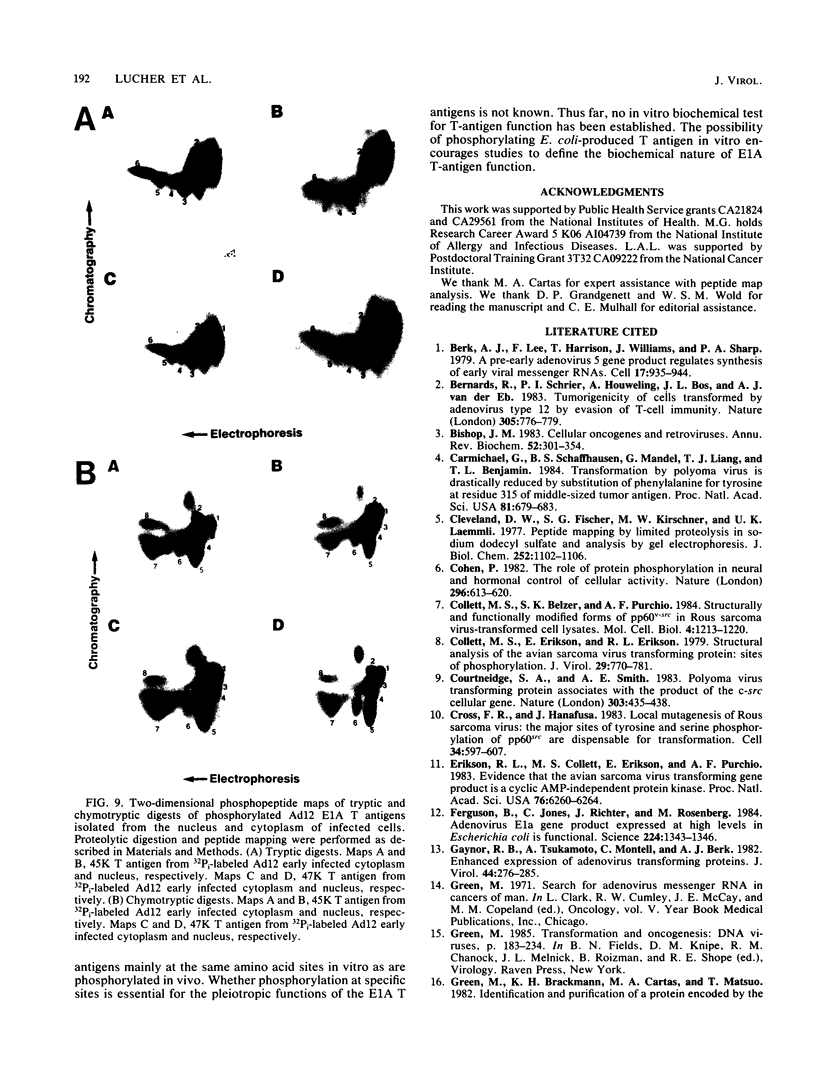
The tumor (T) antigens encoded by the human adenovirus early transforming region 1A (E1A) are gene regulatory proteins whose functions can immortalize cells. We have recently described the synthesis in Escherichia coli and the purification of the complete T antigens encoded by the adenovirus type 12 (Ad12) E1A 12S mRNA (235-residue [235R] T antigen) and 13S mRNA (266R T antigen). In this study, we show that the Ad12 E1A T antigens are extensively phosphorylated in Ad12-infected mammalian cells but are not phosphorylated in E. coli. Inasmuch as posttranslational phosphorylation at specific amino acid sites may be important for biological activity, we have studied the phosphorylation of the E. coli-produced T antigens in vitro by using a kinase activity isolated from cultured human KB cells. The kinase was purified about 300-fold and appears to be a cyclic AMP-independent, Ca2+-independent protein kinase requiring only ATP and Mg2+ for activity. To determine which amino acids are phosphorylated and whether phosphorylation in vitro occurs at the same amino acid sites that are phosphorylated in vivo, the Ad12 E1A T-antigen species synthesized by infected cells were metabolically labeled with 32Pi and compared with the E. coli-produced E1A T antigens labeled in vitro with [gamma-32P]ATP by using the partially purified kinase. Partial V8 proteolysis analysis gave similar patterns for in vivo- and in vitro-phosphorylated T antigen. Two-dimensional maps of tryptic phosphopeptides and of chymotryptic phosphopeptides suggested that mainly the same amino acid sites are phosphorylated in vitro and in vivo and that phosphorylation occurred at multiple sites distributed throughout the T-antigen molecule. Serine was the only amino acid that was phosphorylated both in vivo and in vitro, and, surprisingly, most serines appeared to be phosphorylated. The feasibility of faithfully phosphorylating T antigens in vitro suggests that the E. coli-produced Ad12 E1A 235R and 266R T antigens may prove useful for molecular studies on T-antigen function.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berk A. J., Lee F., Harrison T., Williams J., Sharp P. A. Pre-early adenovirus 5 gene product regulates synthesis of early viral messenger RNAs. Cell. 1979 Aug;17(4):935–944. doi: 10.1016/0092-8674(79)90333-7. [DOI] [PubMed] [Google Scholar]
- Bernards R., Schrier P. I., Houweling A., Bos J. L., van der Eb A. J., Zijlstra M., Melief C. J. Tumorigenicity of cells transformed by adenovirus type 12 by evasion of T-cell immunity. 1983 Oct 27-Nov 2Nature. 305(5937):776–779. doi: 10.1038/305776a0. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Cellular oncogenes and retroviruses. Annu Rev Biochem. 1983;52:301–354. doi: 10.1146/annurev.bi.52.070183.001505. [DOI] [PubMed] [Google Scholar]
- Carmichael G., Schaffhausen B. S., Mandel G., Liang T. J., Benjamin T. L. Transformation by polyoma virus is drastically reduced by substitution of phenylalanine for tyrosine at residue 315 of middle-sized tumor antigen. Proc Natl Acad Sci U S A. 1984 Feb;81(3):679–683. doi: 10.1073/pnas.81.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cohen P. The role of protein phosphorylation in neural and hormonal control of cellular activity. Nature. 1982 Apr 15;296(5858):613–620. doi: 10.1038/296613a0. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Belzer S. K., Purchio A. F. Structurally and functionally modified forms of pp60v-src in Rous sarcoma virus-transformed cell lysates. Mol Cell Biol. 1984 Jul;4(7):1213–1220. doi: 10.1128/mcb.4.7.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Erikson E., Erikson R. L. Structural analysis of the avian sarcoma virus transforming protein: sites of phosphorylation. J Virol. 1979 Feb;29(2):770–781. doi: 10.1128/jvi.29.2.770-781.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A., Smith A. E. Polyoma virus transforming protein associates with the product of the c-src cellular gene. Nature. 1983 Jun 2;303(5916):435–439. doi: 10.1038/303435a0. [DOI] [PubMed] [Google Scholar]
- Cross F. R., Hanafusa H. Local mutagenesis of Rous sarcoma virus: the major sites of tyrosine and serine phosphorylation of pp60src are dispensable for transformation. Cell. 1983 Sep;34(2):597–607. doi: 10.1016/0092-8674(83)90392-6. [DOI] [PubMed] [Google Scholar]
- Erikson R. L., Collett M. S., Erikson E., Purchio A. F. Evidence that the avian sarcoma virus transforming gene product is a cyclic AMP-independent protein kinase. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6260–6264. doi: 10.1073/pnas.76.12.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson B., Jones N., Richter J., Rosenberg M. Adenovirus E1a gene product expressed at high levels in Escherichia coli is functional. Science. 1984 Jun 22;224(4655):1343–1346. doi: 10.1126/science.6374895. [DOI] [PubMed] [Google Scholar]
- Gaynor R. B., Tsukamoto A., Montell C., Berk A. J. Enhanced expression of adenovirus transforming proteins. J Virol. 1982 Oct;44(1):276–285. doi: 10.1128/jvi.44.1.276-285.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. R., Treisman R., Maniatis T. Transcriptional activation of cloned human beta-globin genes by viral immediate-early gene products. Cell. 1983 Nov;35(1):137–148. doi: 10.1016/0092-8674(83)90216-7. [DOI] [PubMed] [Google Scholar]
- Green M., Brackmann K. H., Cartas M. A., Matsuo T. Identification and purification of a protein encoded by the human adenovirus type 2 transforming region. J Virol. 1982 Apr;42(1):30–41. doi: 10.1128/jvi.42.1.30-41.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M., Wold W. S., Brackmann K. H., Cartas M. A. Identification of families of overlapping polypeptides coded by early "transforming" gene region 1 of human adenovirus type 2. Virology. 1979 Sep;97(2):275–286. doi: 10.1016/0042-6822(79)90339-8. [DOI] [PubMed] [Google Scholar]
- Greengard P. Phosphorylated proteins as physiological effectors. Science. 1978 Jan 13;199(4325):146–152. doi: 10.1126/science.22932. [DOI] [PubMed] [Google Scholar]
- Jones N., Shenk T. An adenovirus type 5 early gene function regulates expression of other early viral genes. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3665–3669. doi: 10.1073/pnas.76.8.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelman D., Lucher L. A., Brackmann K. H., Symington J. S., Ptashne M., Green M. Synthesis in Escherichia coli of human adenovirus type 12 transforming proteins encoded by early region 1A 13S mRNA and 12S mRNA. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6300–6304. doi: 10.1073/pnas.81.20.6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucher L. A., Kimelman D., Symington J. S., Brackmann K. H., Cartas M. A., Thornton H., Green M. Identification of adenovirus 12-encoded E1A tumor antigens synthesized in infected and transformed mammalian cells and in Escherichia coli. J Virol. 1984 Oct;52(1):136–144. doi: 10.1128/jvi.52.1.136-144.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R. Induction of the synthesis of a 70,000 dalton mammalian heat shock protein by the adenovirus E1A gene product. Cell. 1982 Jul;29(3):913–919. doi: 10.1016/0092-8674(82)90453-6. [DOI] [PubMed] [Google Scholar]
- Perricaudet M., le Moullec J. M., Tiollais P., Pettersson U. Structure of two adenovirus type 12 transforming polypeptides and their evolutionary implications. Nature. 1980 Nov 13;288(5787):174–176. doi: 10.1038/288174a0. [DOI] [PubMed] [Google Scholar]
- Schaffhausen B., Benjamin T. L. Comparison of phosphorylation of two polyoma virus middle T antigens in vivo and in vitro. J Virol. 1981 Oct;40(1):184–196. doi: 10.1128/jvi.40.1.184-196.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidtmann K. H., Echle B., Walter G. Simian virus 40 large T antigen is phosphorylated at multiple sites clustered in two separate regions. J Virol. 1982 Oct;44(1):116–133. doi: 10.1128/jvi.44.1.116-133.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidtmann K. H., Hardung M., Echle B., Walter G. DNA-binding activity of simian virus 40 large T antigen correlates with a distinct phosphorylation state. J Virol. 1984 Apr;50(1):1–12. doi: 10.1128/jvi.50.1.1-12.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrier P. I., Bernards R., Vaessen R. T., Houweling A., van der Eb A. J. Expression of class I major histocompatibility antigens switched off by highly oncogenic adenovirus 12 in transformed rat cells. 1983 Oct 27-Nov 2Nature. 305(5937):771–775. doi: 10.1038/305771a0. [DOI] [PubMed] [Google Scholar]
- Segawa K., Ito Y. Differential subcellular localization of in vivo-phosphorylated and nonphosphorylated middle-sized tumor antigen of polyoma virus and its relationship to middle-sized tumor antigen phosphorylating activity in vitro. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6812–6816. doi: 10.1073/pnas.79.22.6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih T. Y., Papageorge A. G., Stokes P. E., Weeks M. O., Scolnick E. M. Guanine nucleotide-binding and autophosphorylating activities associated with the p21src protein of Harvey murine sarcoma virus. Nature. 1980 Oct 23;287(5784):686–691. doi: 10.1038/287686a0. [DOI] [PubMed] [Google Scholar]
- Snyder M. A., Bishop J. M., Colby W. W., Levinson A. D. Phosphorylation of tyrosine-416 is not required for the transforming properties and kinase activity of pp60v-src. Cell. 1983 Mar;32(3):891–901. doi: 10.1016/0092-8674(83)90074-0. [DOI] [PubMed] [Google Scholar]
- Weinmaster G., Hinze E., Pawson T. Mapping of multiple phosphorylation sites within the structural and catalytic domains of the Fujinami avian sarcoma virus transforming protein. J Virol. 1983 Apr;46(1):29–41. doi: 10.1128/jvi.46.1.29-41.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee S. P., Rowe D. T., Tremblay M. L., McDermott M., Branton P. E. Identification of human adenovirus early region 1 products by using antisera against synthetic peptides corresponding to the predicted carboxy termini. J Virol. 1983 Jun;46(3):1003–1013. doi: 10.1128/jvi.46.3.1003-1013.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Roy F., Fransen L., Fiers W. Improved localization of phosphorylation sites in simian virus 40 large T antigen. J Virol. 1983 Jan;45(1):315–331. doi: 10.1128/jvi.45.1.315-331.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]