Abstract
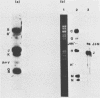
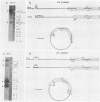
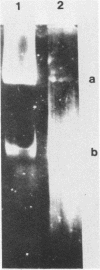
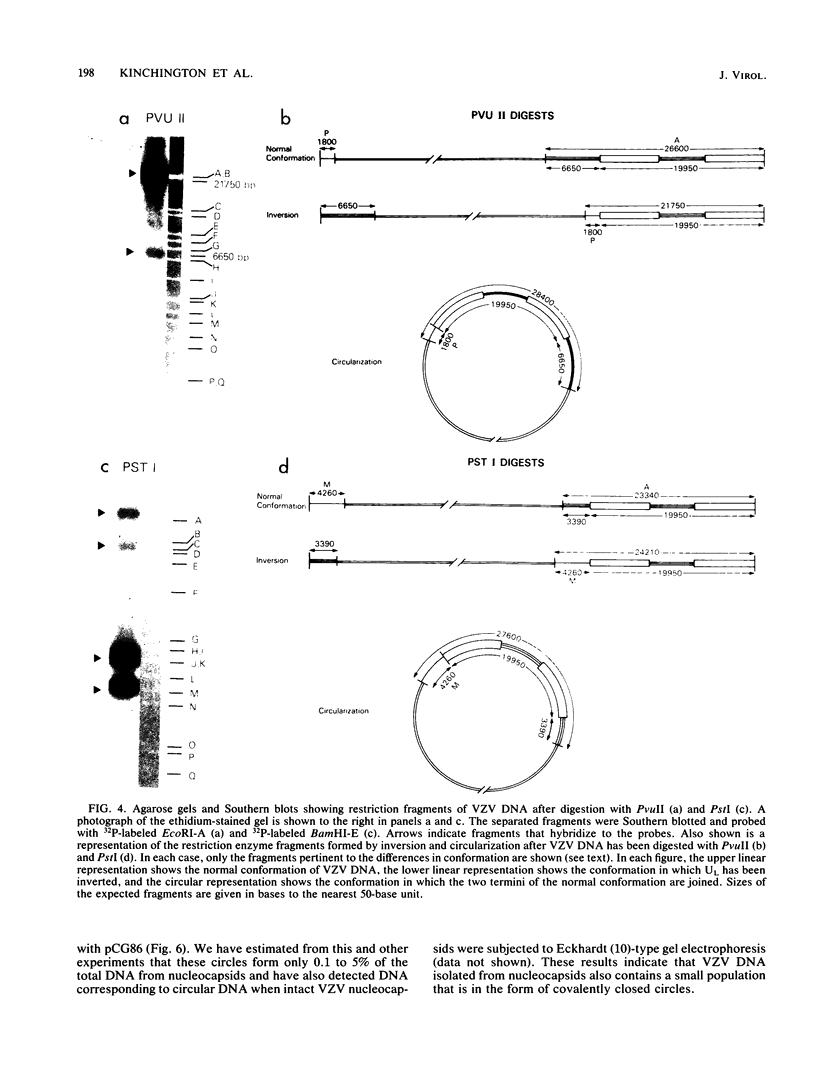
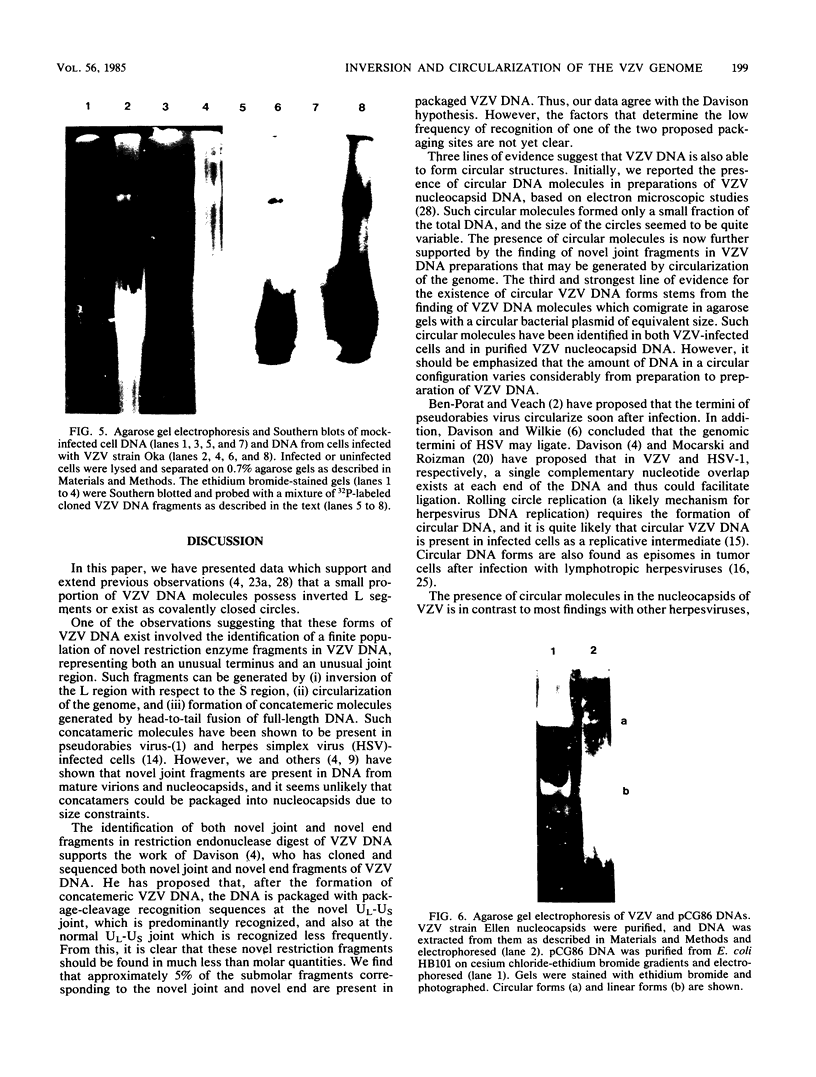
The genome of varicella-zoster virus (VZV) is a linear, double-stranded molecule of DNA composed of a long (L) region covalently linked to a short (S) region. The S region is capable of inverting relative to a fixed orientation of the L region, giving rise to two equimolar populations. We have investigated other forms of the VZV genome which are present in infected cells and packaged into nucleocapsids. That a small proportion of nucleocapsid DNA molecules also possess inverted L regions has been verified by the identification of submolar restriction fragments corresponding to novel joints and novel ends generated by such an inversion. The presence of circular molecules has been investigated by agarose gel electrophoresis. Bands corresponding to circular forms were present in small amounts in both VZV-infected cell DNA and nucleocapsid DNA. Southern blot analysis verified that these bands contained VZV sequences. We therefore conclude that the VZV genome may occasionally contain an inverted L region or exist in a circular configuration.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Porat T., Rixon F. J. Replication of herpesvirus DNA. IV: analysis of concatemers. Virology. 1979 Apr 15;94(1):61–70. doi: 10.1016/0042-6822(79)90438-0. [DOI] [PubMed] [Google Scholar]
- Ben-Porat T., Veach R. A. Origin of replication of the DNA of a herpesvirus (pseudorabies). Proc Natl Acad Sci U S A. 1980 Jan;77(1):172–175. doi: 10.1073/pnas.77.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison A. J. DNA sequence of the US component of the varicella-zoster virus genome. EMBO J. 1983;2(12):2203–2209. doi: 10.1002/j.1460-2075.1983.tb01724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison A. J., Scott J. E. Molecular cloning of the varicella-zoster virus genome and derivation of six restriction endonuclease maps. J Gen Virol. 1983 Aug;64(Pt 8):1811–1814. doi: 10.1099/0022-1317-64-8-1811. [DOI] [PubMed] [Google Scholar]
- Davison A. J. Structure of the genome termini of varicella-zoster virus. J Gen Virol. 1984 Nov;65(Pt 11):1969–1977. doi: 10.1099/0022-1317-65-11-1969. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Wilkie N. M. Inversion of the two segments of the herpes simplex virus genome in intertypic recombinants. J Gen Virol. 1983 Jan;64(Pt 1):1–18. doi: 10.1099/0022-1317-64-1-1. [DOI] [PubMed] [Google Scholar]
- Dumas A. M., Geelen J. L., Weststrate M. W., Wertheim P., van der Noordaa J. XbaI, PstI, and BglII restriction enzyme maps of the two orientations of the varicella-zoster virus genome. J Virol. 1981 Aug;39(2):390–400. doi: 10.1128/jvi.39.2.390-400.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker J. R., Hyman R. W. Varicella zoster virus DNA exists as two isomers. Proc Natl Acad Sci U S A. 1982 Jan;79(1):156–160. doi: 10.1073/pnas.79.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker J. R., Kudler L., Hyman R. W. Variation in the structure of varicella-zoster virus DNA. Intervirology. 1984;21(1):25–37. doi: 10.1159/000149500. [DOI] [PubMed] [Google Scholar]
- Eckhardt T. A rapid method for the identification of plasmid desoxyribonucleic acid in bacteria. Plasmid. 1978 Sep;1(4):584–588. doi: 10.1016/0147-619x(78)90016-1. [DOI] [PubMed] [Google Scholar]
- Gardella T., Medveczky P., Sairenji T., Mulder C. Detection of circular and linear herpesvirus DNA molecules in mammalian cells by gel electrophoresis. J Virol. 1984 Apr;50(1):248–254. doi: 10.1128/jvi.50.1.248-254.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilden D. H., Shtram Y., Friedmann A., Wellish M., Devlin M., Fraser N., Becker Y. The internal organization of the varicella-zoster virus genome. J Gen Virol. 1982 Jun;60(Pt 2):371–374. doi: 10.1099/0022-1317-60-2-371. [DOI] [PubMed] [Google Scholar]
- Hyman R. W. Identification of proteins tightly bound to herpes simplex virus DNA. Virology. 1980 Aug;105(1):254–255. doi: 10.1016/0042-6822(80)90174-9. [DOI] [PubMed] [Google Scholar]
- Jacob R. J., Morse L. S., Roizman B. Anatomy of herpes simplex virus DNA. XII. Accumulation of head-to-tail concatemers in nuclei of infected cells and their role in the generation of the four isomeric arrangements of viral DNA. J Virol. 1979 Feb;29(2):448–457. doi: 10.1128/jvi.29.2.448-457.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean J. H., Blankenship M. L., Ben-Porat T. Replication of herpesvirus DNA. I. Electron microscopic analysis of replicative structures. Virology. 1977 Jun 15;79(2):281–291. doi: 10.1016/0042-6822(77)90355-5. [DOI] [PubMed] [Google Scholar]
- Kaschka-Dierich C., Werner F. J., Bauer I., Fleckenstein B. Structure of nonintegrated, circular Herpesvirus saimiri and Herpesvirus ateles genomes in tumor cell lines and in vitro-transformed cells. J Virol. 1982 Oct;44(1):295–310. doi: 10.1128/jvi.44.1.295-310.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langridge J., Langridge P., Bergquist P. L. Extraction of nucleic acids from agarose gels. Anal Biochem. 1980 Apr;103(2):264–271. doi: 10.1016/0003-2697(80)90266-3. [DOI] [PubMed] [Google Scholar]
- Medveczky P., Kramp W. J., Sullivan J. L. Circular Herpesvirus sylvilagus DNA in spleen cells of experimentally infected cottontail rabbits. J Virol. 1984 Nov;52(2):711–714. doi: 10.1128/jvi.52.2.711-714.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra L., Dohner D. E., Wellinghoff W. J., Gelb L. D. Physical maps of varicella-zoster virus DNA derived with 11 restriction enzymes. J Virol. 1984 May;50(2):615–618. doi: 10.1128/jvi.50.2.615-618.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocarski E. S., Roizman B. Structure and role of the herpes simplex virus DNA termini in inversion, circularization and generation of virion DNA. Cell. 1982 Nov;31(1):89–97. doi: 10.1016/0092-8674(82)90408-1. [DOI] [PubMed] [Google Scholar]
- Poffenberger K. L., Roizman B. A noninverting genome of a viable herpes simplex virus 1: presence of head-to-tail linkages in packaged genomes and requirements for circularization after infection. J Virol. 1985 Feb;53(2):587–595. doi: 10.1128/jvi.53.2.587-595.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ruyechan W. T., Casey T. A., Reinhold W., Weir A. C., Wellman M., Straus S. E., Hay J. Distribution of G + C-rich regions in varicella-zoster virus DNA. J Gen Virol. 1985 Jan;66(Pt 1):43–54. doi: 10.1099/0022-1317-66-1-43. [DOI] [PubMed] [Google Scholar]
- Ruyechan W. T., Dauenhauer S. A., O'Callaghan D. J. Electron microscopic study of equine herpesvirus type 1 DNA. J Virol. 1982 Apr;42(1):297–300. doi: 10.1128/jvi.42.1.297-300.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rziha H. J., Bauer B. Circular forms of viral DNA in Marek's disease virus-transformed lymphoblastoid cells. Arch Virol. 1982;72(3):211–216. doi: 10.1007/BF01348966. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stevely W. S. Inverted repetition in the chromosome of pseudorabies virus. J Virol. 1977 Apr;22(1):232–234. doi: 10.1128/jvi.22.1.232-234.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus S. E., Aulakh H. S., Ruyechan W. T., Hay J., Casey T. A., Vande Woude G. F., Owens J., Smith H. A. Structure of varicella-zoster virus DNA. J Virol. 1981 Nov;40(2):516–525. doi: 10.1128/jvi.40.2.516-525.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus S. E., Owens J., Ruyechan W. T., Takiff H. E., Casey T. A., Vande Woude G. F., Hay J. Molecular cloning and physical mapping of varicella-zoster virus DNA. Proc Natl Acad Sci U S A. 1982 Feb;79(4):993–997. doi: 10.1073/pnas.79.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan D. C., Atherton S. S., Staczek J., O'Callaghan D. J. Structure of the genome of equine herpesvirus type 3. Virology. 1984 Jan 30;132(2):352–367. doi: 10.1016/0042-6822(84)90041-2. [DOI] [PubMed] [Google Scholar]
- Wu M., Hyman R. W., Davidson N. Electron microscopic mapping of proteins bound to herpes simplex virus DNA. Nucleic Acids Res. 1979 Aug 10;6(11):3427–3441. doi: 10.1093/nar/6.11.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]