Abstract
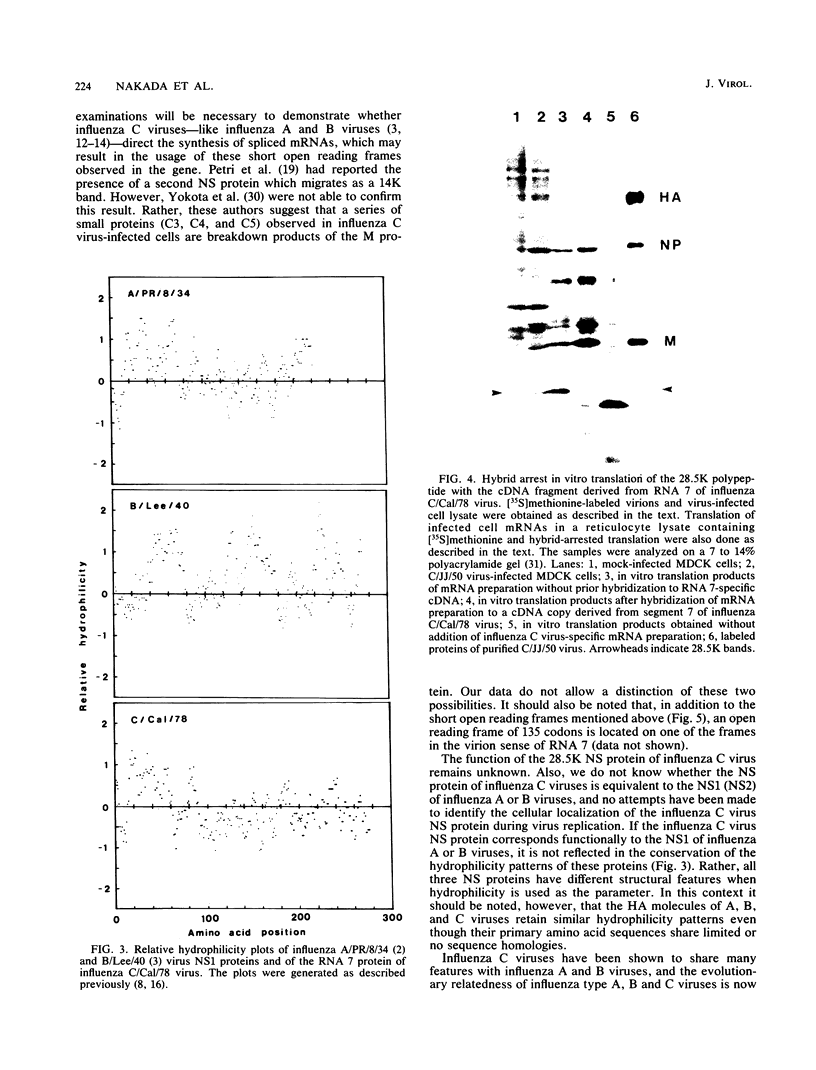
The complete nucleotide sequence of RNA segment 7 of influenza C/California/78 virus was determined by using cloned cDNA derived from viral RNA. The gene is 934 nucleotides long and possesses a long open reading frame which can code for a protein of 286 amino acids. Hybrid arrest translation experiments with the cloned cDNA fragment and poly(A)-containing RNA isolated from virus-infected cells showed that a 28,500-molecular-weight protein is coded for by RNA 7. Comparison of the proteins induced in the cell-free system and in virus-infected cells with those found in purified virus suggests that the 28,500-molecular-weight protein is a nonstructural protein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baez M., Taussig R., Zazra J. J., Young J. F., Palese P., Reisfeld A., Skalka A. M. Complete nucleotide sequence of the influenza A/PR/8/34 virus NS gene and comparison with the NS genes of the A/Udorn/72 and A/FPV/Rostock/34 strains. Nucleic Acids Res. 1980 Dec 11;8(23):5845–5858. doi: 10.1093/nar/8.23.5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briedis D. J., Lamb R. A. Influenza B virus genome: sequences and structural organization of RNA segment 8 and the mRNAs coding for the NS1 and NS2 proteins. J Virol. 1982 Apr;42(1):186–193. doi: 10.1128/jvi.42.1.186-193.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Compans R. W., Bishop D. H., Meier-Ewert H. Structural components of influenza C virions. J Virol. 1977 Feb;21(2):658–665. doi: 10.1128/jvi.21.2.658-665.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desselberger U., Racaniello V. R., Zazra J. J., Palese P. The 3' and 5'-terminal sequences of influenza A, B and C virus RNA segments are highly conserved and show partial inverted complementarity. Gene. 1980 Feb;8(3):315–328. doi: 10.1016/0378-1119(80)90007-4. [DOI] [PubMed] [Google Scholar]
- Etkind P. R., Krug R. M. Purification of influenza viral complementary RNA: its genetic content and activity in wheat germ cell-free extracts. J Virol. 1975 Dec;16(6):1464–1475. doi: 10.1128/jvi.16.6.1464-1475.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendal A. P. A comparison of "influenza C" with prototype myxoviruses: receptor-destroycing activity (neuraminidase) and structural polypeptides. Virology. 1975 May;65(1):87–99. doi: 10.1016/0042-6822(75)90009-4. [DOI] [PubMed] [Google Scholar]
- Krystal M., Elliott R. M., Benz E. W., Jr, Young J. F., Palese P. Evolution of influenza A and B viruses: conservation of structural features in the hemagglutinin genes. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4800–4804. doi: 10.1073/pnas.79.15.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal M., Young J. F., Palese P., Wilson I. A., Skehel J. J., Wiley D. C. Sequential mutations in hemagglutinins of influenza B virus isolates: definition of antigenic domains. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4527–4531. doi: 10.1073/pnas.80.14.4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. Segment 8 of the influenza virus genome is unique in coding for two polypeptides. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4908–4912. doi: 10.1073/pnas.76.10.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb R. A., Lai C. J. Sequence of interrupted and uninterrupted mRNAs and cloned DNA coding for the two overlapping nonstructural proteins of influenza virus. Cell. 1980 Sep;21(2):475–485. doi: 10.1016/0092-8674(80)90484-5. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nakada S., Creager R. S., Krystal M., Aaronson R. P., Palese P. Influenza C virus hemagglutinin: comparison with influenza A and B virus hemagglutinins. J Virol. 1984 Apr;50(1):118–124. doi: 10.1128/jvi.50.1.118-124.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada S., Creager R. S., Krystal M., Palese P. Complete nucleotide sequence of the influenza C/California/78 virus nucleoprotein gene. Virus Res. 1984 Sep;1(6):433–441. doi: 10.1016/0168-1702(84)90001-7. [DOI] [PubMed] [Google Scholar]
- Palese P., Racaniello V. R., Desselberger U., Young J., Baez M. Genetic structure and genetic variation of influenza viruses. Philos Trans R Soc Lond B Biol Sci. 1980 Feb 25;288(1029):299–305. doi: 10.1098/rstb.1980.0005. [DOI] [PubMed] [Google Scholar]
- Pfeifer J. B., Compans R. W. Structure of the influenza C glycoprotein gene as determined from cloned DNA. Virus Res. 1984;1(4):281–296. doi: 10.1016/0168-1702(84)90017-0. [DOI] [PubMed] [Google Scholar]
- Ritchey M. B., Palese P., Kilbourne E. D. RNAs of influenza A, B, and C viruses. J Virol. 1976 May;18(2):738–744. doi: 10.1128/jvi.18.2.738-744.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey M. B., Palese P., Schulman J. L. Mapping of the influenza virus genome. III. Identification of genes coding for nucleoprotein, membrane protein, and nonstructural protein. J Virol. 1976 Oct;20(1):307–313. doi: 10.1128/jvi.20.1.307-313.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. S., Schubert M., Lazzarini R. A. Polyadenylation sites for influenza virus mRNA. J Virol. 1981 Apr;38(1):157–163. doi: 10.1128/jvi.38.1.157-163.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. A strategy of DNA sequencing employing computer programs. Nucleic Acids Res. 1979 Jun 11;6(7):2601–2610. doi: 10.1093/nar/6.7.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. Further procedures for sequence analysis by computer. Nucleic Acids Res. 1978 Mar;5(3):1013–1016. doi: 10.1093/nar/5.3.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. Sequence data handling by computer. Nucleic Acids Res. 1977 Nov;4(11):4037–4051. doi: 10.1093/nar/4.11.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Feldman R., Shibuya M., Hanafusa H., Notter M. F., Balduzzi P. C. Genetic structure, transforming sequence, and gene product of avian sarcoma virus UR1. J Virol. 1981 Oct;40(1):258–267. doi: 10.1128/jvi.40.1.258-267.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota M., Nakamura K., Sugawara K., Homma M. The synthesis of polypeptides in influenza C virus-infected cells. Virology. 1983 Oct 15;130(1):105–117. doi: 10.1016/0042-6822(83)90121-6. [DOI] [PubMed] [Google Scholar]
- Young J. F., Palese P. Evolution of human influenza A viruses in nature: recombination contributes to genetic variation of H1N1 strains. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6547–6551. doi: 10.1073/pnas.76.12.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]