Abstract
The cell adhesion molecule L1 is a potent inducer of neurite outgrowth and it has been implicated in X-linked hydrocephalus and related neurological disorders. To investigate the mechanisms of neurite outgrowth stimulated by L1, attempts were made to identify the neuritogenic sites in L1. Fusion proteins containing different segments of the extracellular region of L1 were prepared and different neuronal cells were assayed on substrate-coated fusion proteins. Interestingly, both immunoglobulin (Ig)-like domains 2 and 6 (Ig2, Ig6) promoted neurite outgrowth from dorsal root ganglion cells, whereas neural retinal cells responded only to Ig2. L1 Ig2 contains a previously identified homophilic binding site, whereas L1 Ig6 contains an Arg-Gly-Asp (RGD) sequence. The neuritogenic activity of Ig6 was abrogated by mutations in the RGD site. The addition of RGD-containing peptides also inhibited the promotion of neurite outgrowth from dorsal root ganglion cells by glutathione S-transferase-Ig6, implicating the involvement of an integrin. The monoclonal antibody LM609 against αvβ3 integrin, but not an anti-β1 antibody, inhibited the neuritogenic effects of Ig6. These data thus provide the first evidence that the RGD motif in L1 Ig6 is capable of promoting neurite outgrowth via interaction with the αvβ3 integrin on neuronal cells.
INTRODUCTION
The development of the nervous system requires specific cell–cell and cell–substrate interactions that direct the extension of axons and dendrites to their precise synaptic targets. The basis of these processes involves receptors on the neuronal growth cone that can recognize specific environmental cues in the extracellular matrix or on the surface of other cells (Dodd and Jessell, 1988; Goodman and Shatz, 1993; Tessier-Lavigne and Goodman, 1996). The extension and orientation of growing neurites appear to result from the intracellular signaling cascades and cytoskeletal changes following receptor activation (Schuch et al., 1989; Ghosh and Greenberg, 1995). Several classes of cell adhesion molecules have been identified as receptors for the promotion of neurite outgrowth, including members of the immunoglobulin (Ig) superfamily (Edelman and Crossin, 1991), the integrins (Hynes, 1992), and cadherins (Takeichi, 1991).
The neural cell adhesion molecule L1 is predominantly expressed in the nervous system. The expression pattern, as well as the recent implication of L1 mutations in neurological diseases, point to a crucial role for L1 during neural development (Wong et al., 1995; Hortsch, 1996). In vertebrates, L1 is expressed on fasciculating axons, postmitotic neurons of the CNS, Schwann cells, and sensory neurons (Martini and Schachner, 1986; Persohn and Schachner, 1987; Giese et al., 1992). The differential expression of L1 at different stages of development suggests tightly regulated molecular interactions involving L1 (Daniloff et al., 1986; Moscoso and Sanes, 1995). Cellular processes that involve L1 include neurite outgrowth (Lagenaur and Lemmon, 1987), myelination (Martini and Schachner, 1986), growth cone morphology (Payne et al., 1992), cell migration (Lindner et al., 1983), and long-term potentiation in the hippocampus (Lüthi et al., 1994).
cDNAs of L1 and its homologues have been cloned from several vertebrate species. Their deduced amino acid sequences reveal that L1 is a large multidomain glycoprotein of ∼200 kDa (Grumet et al., 1984; Bock et al., 1985; Moos et al., 1988; Hlavin and Lemmon, 1991). It is a member of the Ig superfamily of recognition molecules, consisting of six Ig-like domains at the N-terminal region, followed by five fibronectin type III (FNIII) repeats, a transmembrane domain, and a cytoplasmic domain. L1 is known to mediate cell–cell adhesion by homophilic interactions (Miura et al., 1992). In addition to cell–cell adhesion, substrate-coated L1 has been found to be a potent inducer of neurite outgrowth from primary neurons (Hlavin and Lemmon, 1991).
Several pairs of the Ig-like domains of mouse L1 have been shown to possess cell adhesion and neuritogenic activities (Appel et al., 1993). However, in human L1, the homophilic binding site has been localized specifically to the second Ig-like domain (Ig2) (Zhao and Siu, 1995). Furthermore, a neuritogenic site has been colocalized with the homophilic binding site to Ig2 of human L1, suggesting that L1–L1 interactions may trigger a signaling cascade leading to neurite outgrowth (Zhao and Siu, 1995). L1 also interacts heterophilically with several extracellular matrix components and membrane proteins such as laminin (Grumet et al., 1993; Hall et al., 1997), the proteoglycans neurocan and phosphacan (Friedlander et al., 1994; Milev et al., 1995; Grumet et al., 1996), NCAM (Kadmon et al., 1990; Horstkorte et al., 1993), TAG-1/axonin-1 (Kuhn et al., 1991; Felsenfeld et al., 1994), F3/F11 (Brümmendorf et al., 1993), and integrins α5β1 and αvβ3 (Ruppert et al., 1995; Montgomery et al., 1996). Some of these interactions are also known to have an influence on neurite extension.
Recently, several reports have linked a group of heterogeneous mutations in L1 to several neurological disorders, such as X-linked hydrocephalus; mental retardation, aphasia, shuffling gait, and adducted thumbs (MASA) syndrome; and spastic paraplegia type 1 (Vits et al., 1994; Jouet et al. 1994, 1995; Kenwrick et al., 1996; Takechi et al., 1996), which are now collectively known as the CRASH syndrome (for corpus callosum hypoplasia, retardation, adducted thumbs, spastic paraplegia, and hydrocephalus) (Fransen et al., 1995). Most of them are missense mutations resulting in amino acid substitutions in either the extracellular domain or the cytoplasmic domain. The effects of these mutations on the interactions of L1 with L1 or with its heterophilic receptors may trigger a cascade of events leading to pathological development. Indeed, the deleterious effects of mutations in Ig2 on L1 homophilic binding and neuritogenic activities correlate very well with the severity of the neuropathological phenotypes of patients carrying these mutations (Zhao and Siu, 1996).
Evidently, L1 plays an important role during brain development. A better understanding of its mechanisms of action will depend on our knowledge of its structure/function relationships. In this article, we investigate the ability of fusion proteins containing one or more extracellular domains of human L1 to promote neurite outgrowth from different neuronal cell types. We discover that, in addition to Ig2, a second neuritogenic site of human L1 exists in Ig6. This latter site is centered around the Arg-Gly-Asp (RGD) motif in Ig6. Interestingly, although Ig6 promotes neurite outgrowth from dorsal root ganglion (DRG) cells, it fails to elicit any response from neural retinal cells. Because L1 is known to interact with integrins (Ruppert et al., 1995; Montgomery et al., 1996), antibody perturbation studies were carried out. The results suggest that the αvβ3 integrin is responsible for binding the RGD sequence in Ig6 and for the promotion of neurite outgrowth. These results indicate that L1 uses at least two distinct mechanisms to promote neurite outgrowth: one is mediated via L1–L1 homophilic interaction and the other via L1–integrin interaction.
MATERIALS AND METHODS
Materials
pGEX-3X plasmid and glutathione-Sepharose 4B were purchased from Pharmacia Biotech (Toronto, Ontario, Canada). Trypsin-EDTA and N2 supplement were purchased from Life Technologies (Toronto, Ontario, Canada). Poly-l-lysine, p-phenylenediamine, diazobicyclo(2, 2, 2)octane, and the monoclonal antibody (mAb) W1B10 against chick β1 integrin subunit were purchased from Sigma (St. Louis, MO). The mAb LM609 against αvβ3 was kindly provided by Dr. David Cheresh (The Scripps Research Institute, La Jolla, CA). DiI was purchased from Molecular Probes (Eugene, OR). The bicinchoninic acid protein assay kit was obtained from Pierce Chemical (Rockford, IL). Domain-specific antibodies against L1 were raised in our laboratory as described previously (Zhao and Siu, 1995).
Cell Lines and Culture Conditions
The Chinese hamster ovary cell line LR73 (Zhou et al., 1993) was provided by Dr. Clifford Stanners (McGill University, Montreal, Quebec, Canada). LR73 cells were cultured in α-minimum essential medium containing 10% fetal calf serum. The human L1 cDNA was obtained from Dr. Vance Lemmon (Case Western Reserve University, Cleveland, OH). The full-length L1 cDNA and L1 cDNA with the Ig2 coding region deleted (L1Δ2) were subcloned into the expression vector pRc/CMV (Invitrogen, San Diego, CA). An antisense-L1 construct was also made by inserting the L1 cDNA in the reverse orientation into the same vector. Standard recombinant DNA methods (Sambrook et al., 1989) were used in the construction of these expression vectors. The DNA constructs were transfected into LR73 cells as previously described (Zhao and Siu, 1996). Transfected clones were selected using 400 μg/ml of G418, followed by limiting dilution and clonal analysis for L1 expression. Stably transfected clones expressing comparable amounts of wild-type or mutant L1 were selected for further studies.
Construction and Expression of L1 Fusion Proteins
Construction of the expression vectors for the glutathione S-transferase (GST)-L1 fusion proteins containing the first three Ig-like domains (GST-Ig1-2-3), the fourth to sixth Ig-like domains (GST-Ig4-5-6), the five fibronectin type III repeats (GST-FNIII), and the second Ig-like domain (GST-Ig2) has been described elsewhere (Zhao and Siu, 1995). The GST-Ig1-2-3 construct contains the cDNA fragment encoding the amino acid sequence of L1 between Arg-24 and Gly-351, the GST-Ig4-5-6 construct encodes the segment between Ile-352 and Pro-595, the GST-FNIII construct encodes the segment between Val-596 and Pro-1094, and the GST-Ig2 construct contains the segment between Glu-114 and Arg-209 (amino acid numbering according to Hlavin and Lemmon, 1991). In all cases, the GST moiety was fused to the amino terminus of the fusion protein. The fragment coding for the fourth and fifth Ig-like domains (Ig4-5) between Ile-352 and Thr-499 was generated using the primers 5′-GAGAATTCACCGTACTGGCTGCACAAGC-3′ and 5′-CCAATTTCTACGTTGAGTCTTAAGAG-3′. The polymerase chain reaction (PCR) product was digested with BamHI and EcoRI and then subcloned into these two sites of pGEX-3X. The fragment coding for the sixth Ig-like domain (Ig6) between Gln-500 and Pro-595 was generated with the forward primer 5′-TGGGATCCAGATCACTCAGGGGC-3′ and the reverse primer 5′-GCGAATTCTGGGATCCCGGCCCAGGGCTCCCCAC-3′. The PCR product was digested with BamHI and subcloned into the BamHI site of pGEX-3X. To generate the mutated sixth Ig-like domain (Ig6(KGE)) containing the amino acid substitutions of Arg-554 with Lys and Asp-556 with Glu, the four-primer method (Higuchi, 1990) was employed, using the primers for Ig6 in conjunction with the mutant primers 5′-CATCACCTGGAAGGGGGAGGGTCGAGACC-3′ and 5′-GTAGTGGACCTTCCCCCTCCCAGCTCTGG-3′. The final amplified product was digested with BamHI and subcloned into the BamHI site of pGEX-3X. The nucleotide sequences of these inserts were confirmed by double-stranded DNA sequencing using the T7 Sequencing kit (Pharmacia Biotech). The Escherichia coli strain JM101 was used for transformation, and transformed cells were selected at 37°C in LB medium with 100 μg/ml ampicillin. Synthesis of fusion protein was induced by adding 0.1 mM isopropyl β-d-thiogalactopyranoside after A600 had reached 0.6–0.8, and cultures were shaken overnight at room temperature. After sonication of the cells, the GST-L1 fusion protein was purified from the soluble fraction of the cell lysate using a glutathione-Sepharose 4B column according to the manufacturer’s protocol. Eluted proteins were dialyzed against phosphate-buffered saline (PBS) at 4°C and stored at −20°C.
Isolation and Culture Conditions of Neuronal Cells
Neural retinal cells and DRG cells were isolated from the neural retinal and intact lumbar ganglia, respectively, from E10 chick embryos. Dissection was performed in α-minimum essential medium and the tissue was incubated in Ca2+/Mg2+-free Hanks’ balanced salt solution (HBSS) containing 0.25% trypsin and 1 mM EDTA. Cells were dissociated by gentle trituration. The cell suspension was centrifuged through a step gradient of 0.5 ml of 35% (wt/vol) bovine serum albumin in PBS and 1 ml of 3.5% (wt/vol) in PBS for 4 min at 400 × g. Cells were collected at the interface of the step gradient, washed once in HBSS, resuspended in 1 ml of HBSS containing 25 μM DiI, and incubated for 5 min at 37°C. Cells were washed twice in HBSS and resuspended in α-minimum essential medium containing N2 supplement. Neuronal cells were then seeded on protein-coated coverslips or monolayers of L1-transfected LR73 cells for the neurite outgrowth assay.
Neurite Outgrowth Assay
Round glass coverslips of 12-mm diameter were acid washed and autoclaved before being coated with 0.02% poly-l-lysine for 3 h at room temperature. After the coverslips were washed three times with water, they were coated with 80 μl of fusion protein at 1 μM concentration. The efficiency of protein adsorption to the substratum was estimated using the bicinchoninic acid protein assay after the coverslips were stripped with 50 μl of 0.2% SDS. Routinely, 10–20% of the input protein was found adsorbed to coverslips.
The neurite outgrowth assay was essentially as described by Sandig et al. (1994). The substrate-coated coverslips were blocked with 1% bovine serum albumin and washed before the neuronal cells were seeded. In inhibition studies, domain-specific IgG, anti-integrin IgG, or recombinant proteins were added to the culture at a final concentration of 40 μg/ml. Neurite extension was allowed to proceed for 20 h. Cells were then fixed for 20 min in 3.7% formaldehyde in PBS by gradually replacing the culture medium with the fixative. The coverslips were washed three times with PBS and mounted in Vinol, containing 1,4-diazobicyclo(2,2,2)octane and p-phenylenediamine, to retard photobleaching. Samples were examined by epifluorescence microscopy and DRG cells bearing neurites were recorded onto a video cassette. Approximately 100 neurites were measured in each experiment. Only neurites with a length greater than one cell body width were measured. Most DRG cells cultured on fusion protein-coated coverslips bore a single axon-like neurite, and approximately the same percentage of cells extended neurites for each substrate. However, DRG cells cultured on LR73 transfectants usually had several neurites. In both cases, only the longest predominant neurite was measured for each neuronal cell.
RESULTS
Differential Neuritogenic Effects of L1 Fusion Proteins on Neuronal Cells
The ability of various L1 fragments to promote neurite outgrowth was assessed with the use of different embryonic neuronal cell types. In addition to neural retinal cells, initial studies showed that the fusion protein GST-Ig1-2-3 stimulated neurite outgrowth from chick embryonic hippocampal cells, DRG cells, and cerebellar cells (our unpublished data). Surprisingly, GST-Ig4-5-6, which previously was found not to affect retinal cells (Zhao and Siu, 1995), stimulated the outgrowth of neurites from hippocampal cells and DRG cells. Because the neuritogenic activity of GST-Ig4-5-6 was most prominent with DRG cells, they were chosen for more detailed analyses.
DRG cells and neural retinal cells were isolated from E10 chick embryos and were cultured on substrate-coated fusion proteins containing different extracellular fragments of L1. After 20 h of culture, cells were fixed and the lengths of the longest neurite extended from neuronal cells were recorded. GST-Ig1-2-3 promoted neurite outgrowth from neural retinal cells whereas GST-Ig4-5-6 did not (Figure 1D). However, both GST-Ig1-2-3 and GST-Ig4-5-6 promoted neurite outgrowth from DRG cells (Figure 1, A and B). Similar to neural retinal cells, the majority of DRG cells extended a single, long, and slender neurite. Occasionally, one or two additional neurites were present, but usually one neurite was predominantly longer than the other. The neuritogenic effects of GST-Ig-4-5-6 were abrogated when the substrate was precoated with anti-Ig4-5-6 Fab before the seeding of DRG cells (Figure 1C), indicating that the neuritogenic activity was associated with the Ig4-Ig6 domains of L1.
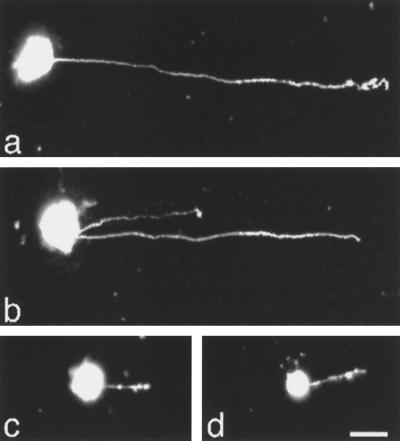
Figure 1.
Epifluorescence micrographs of neurite outgrowth from DRG cells cultured on different L1 fusion protein substrates. DRG cells and neural retinal cells were isolated from E10 chick embryos and labeled with DiI. Cells were cultured for 20 h on coverslips coated with different fusion proteins. DRG cells extended long neurites in response to substrate-coated GST-Ig1-2-3 (a) and GST-Ig4-5-6 (b). When the substrate was precoated with anti-Ig4-5-6 Fab, DRG cells failed to send out long neurites (c). Neural retinal cells cultured on GST-Ig4-5-6 did not extend long neurites (d). Bar, 10 μm.
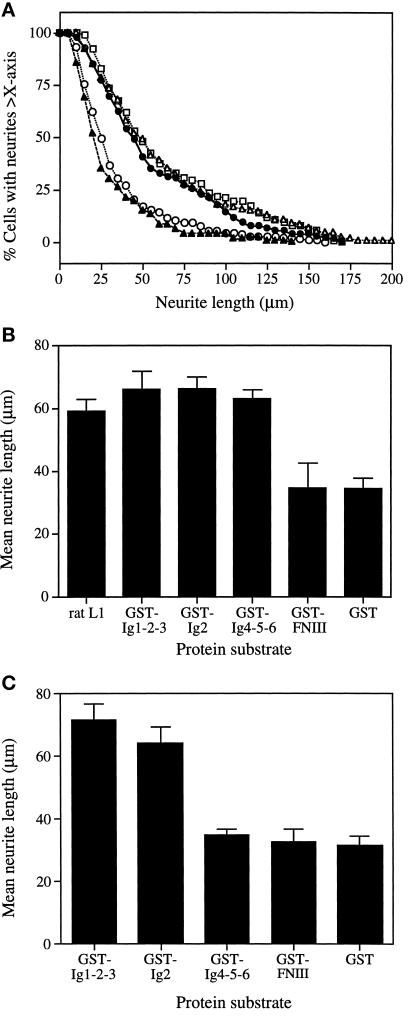
Quantitative data were obtained by measuring neurite lengths of neuronal cells cultured on different protein substrates. The cumulative plots for DRG cells are shown in Figure 2A. DRG cells cultured on GST-FNIII had only background levels of neurite outgrowth, similar to those cultured on GST. On both substrates, >50% of neurites were <25 μm in length, with the mean neurite lengths ranging between 30 and 35 μm (Figure 2B). On the stimulatory substrates, GST-Ig1-2-3 and GST-Ig4-5-6, neurites were longer and had a wider range of size distribution, whereas ∼80% of the cells had neurites >25 μm (Figure 2A). Similar results were obtained using DRG cells isolated from embryos between E5 and E12 (our unpublished data), suggesting that there was no stage-dependent difference in their response to these L1 fusion proteins.
Figure 2.
Promotion of neurite outgrowth from DRG and neural retinal cells by L1 fusion proteins. Glass coverslips were coated with 0.02% poly-l-lysine followed by one of the GST-L1 fusion proteins at 1 μM concentration. Purified rat L1 (1 μM) was used as the positive control. Cells were seeded on coverslips and cultured in N2 medium. Cultures were incubated at 37°C for 20 h and then fixed for fluorescence microscopy. Images were recorded for neurite length measurement. (A) Size distribution patterns of neurites extending from DRG cells cultured on GST-Ig1-2-3 (•), GST-Ig2 (□), GST-Ig4-5-6 (▵), GST-Fn (○), and GST (▴). (B) Mean neurite lengths of DRG cells cultured on different substrate-coated proteins. (C) Mean neurite lengths of neural retinal cells cultured on different fusion proteins. Data represent the mean ± SD of three experiments.
We previously found that L1 Ig2 contained both homophilic binding and neuritogenic activities (Zhao and Siu, 1995). When the GST-Ig2 fusion protein was assayed using DRG cells, it was as effective as GST-Ig1-2-3 and GST-Ig4-5-6, yielding similar distribution profiles of neurite lengths (Figure 2A) and mean neurite lengths of 66.2 μm, 65.6 μm, and 62.7 μm, respectively (Figure 2B). Similar results were obtained with intact rat L1, which was included as the positive control.
Neural retinal cells isolated from E10 embryos yielded mean neurite lengths of 71 μm and 64 μm when cultured on GST-Ig1-2-3 and GST-Ig2 substrates, respectively (Figure 2C). No significant neurite outgrowth above the GST control was observed for cells cultured on GST-Ig4-5-6 and GST-FNIII substrates. These results are essentially identical to those obtained with E5/E6 retinal cells (Zhao and Siu, 1995), suggesting that there is no change in the responsiveness of retinal cells to L1 fragments between these two embyonic stages.
Promotion of DRG Neurite Outgrowth by L1 Expressed on LR73 Cells
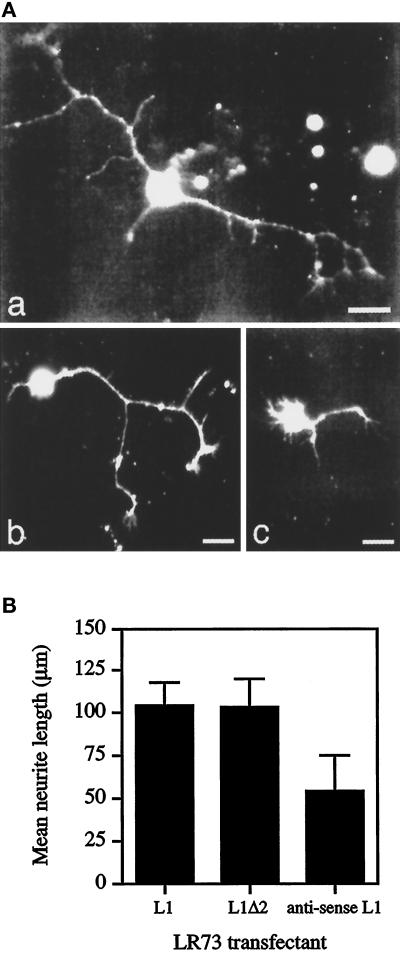
The ability of L1 to promote neurite outgrowth was also examined by the use of LR73 transfectants expressing either wild-type L1 or mutant L1 with Ig2 deleted (L1Δ2). LR73 cells do not express endogenous L1, but they provide a more physiological environment for L1 presentation. If a second neuritogenic site exists outside of Ig2, both L1 and L1Δ2 should be able to promote neurite outgrowth from DRG cells. Indeed, deletion of Ig2 did not abrogate the neuritogenic activity of the mutant L1. Both wild-type L1 and the mutant form L1Δ2 on the surface of LR73 cells promoted neurite outgrowth from DRG cells. Moreover, DRG cells cultured on monolayers of LR73 transfectants developed a more complex morphology than those cultured on substrate-coated L1 fusion proteins. They showed more spreading, and a much greater proportion of cells possessed multiple neurites (Figure 3A). Many neurites contained multiple branches and prominent growth cones at their tips.
Figure 3.
Neurite outgrowth from DRG cells cultured on LR73 transfectants. (A) Epifluorescence micrographs of neurites extending from DRG cells cultured on a monolayer of L1-expressing LR73 cells (a), L1Δ2-expressing LR73 cells (b), or LR73 cells transfected with an antisense L1 cDNA construct (c). Bar, 10 μm. (B) Mean neurite lengths of DRG cells cultured on monolayers of different LR73 transfectants. Data represent the mean ± SD of three experiments.
DRG cells cultured on a monolayer of L1-LR73 transfectants had a mean neurite length of 105 μm. Consistent with the above results, deletion of Ig2 did not abrogate the neuritogenic activity of L1, and cells cultured on L1Δ2-LR73 cells had a mean neurite length of 104 μm (Figure 3B). In contrast, L1Δ2-LR73 cells did not support neurite outgrowth from neural retinal cells (our unpublished data). These results confirm the presence of a second neuritogenic site outside Ig2, which can elicit a cell-type–specific response.
Localization of Neuritogenic Activity to Ig-like Domain 6
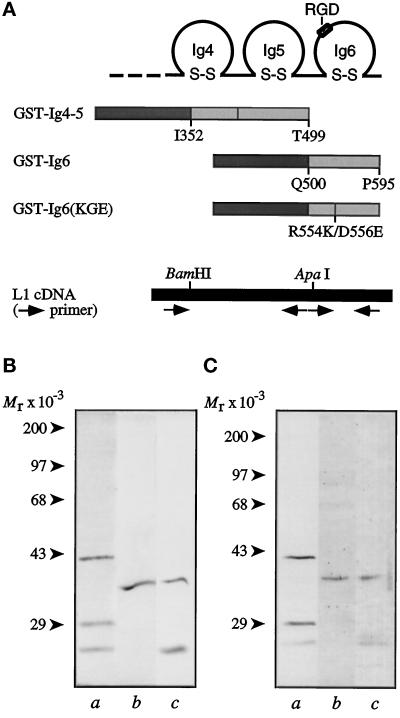
The results shown in Figures 1 and 2 indicate that the second neuritogenic site is present within Ig-like domains 4–6. A potential location of this site is Ig6 because it contains an RGD sequence, which is a known integrin-binding motif. To test this hypothesis, GST-L1 fusion proteins containing Ig4 and 5 (GST-Ig4-5) and Ig6 (GST-Ig6) were constructed and expressed in bacteria (Figure 4A). Soluble fusion proteins were purified and analyzed by SDS-PAGE. Under reducing conditions, the fusion proteins migrated close to their expected molecular sizes (Figure 4B). The lower molecular weight bands that copurified with the fusion protein showed immunoreactivity with anti-Ig4-5-6 antibodies (Figure 4C), suggesting that they were degradative products of the recombinant proteins.
Figure 4.
Construction and expression of GST-L1 fusion proteins. (A) Schematic drawings of GST fusion proteins: GST-Ig4-5, GST-Ig6, and GST-Ig6(KGE). The PCR fragment from nucleotide position 990 to the end of Ig-like domain 5 at nucleotide position 1564 was used to construct GST-Ig4-5. The PCR fragment from nucleotide position 1548 to 1848 was used to construct GST-Ig6. To construct GST-Ig6(KGE) with the RGD site mutated to KGE, the GST-Ig6 primers were used along with a pair of complementary mutagenic primers. All fragments were subcloned into the pGEX-3X vector. (B) Gel profiles of GST-L1 fusion proteins. Protein samples were separated on 10% polyacrylamide gels and stained with Coomassie brilliant Blue. (C) Immunoblots of GST-L1 fusion proteins, stained with rabbit antibodies raised against L1 Ig4-6 (Zhao and Siu, 1995). In B and C: lane a, GST-Ig4-5; lane b, GST-Ig6; lane c, GST-Ig6(KGE). Molecular weight markers are indicated on the left of each panel.
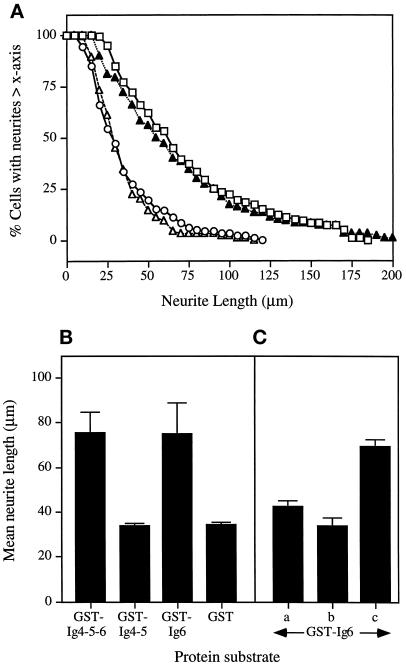
The fusion proteins were assayed for their ability to promote neurite outgrowth from DRG cells. When cultured on the GST-Ig6 substrate, both the pattern of neurite length distribution and the mean neurite length (75 μm) were almost identical to those obtained with the GST-Ig4-5-6 substrate (Figure 5, A and B). In contrast, GST-Ig4-5 did not support neurite outgrowth and the mean neurite length of cells cultured on the GST-Ig4-5 substrate was 34 μm, similar to that obtained with the GST-negative control (Figure 5B). Therefore, Ig6 alone is sufficient to promote neurite outgrowth from DRG cells.
Figure 5.
Localization of neuritogenic activity to the Ig-like domain 6 of L1. (A) Size distribution patterns of neurites extending from DRG cells cultured on GST-Ig4-5-6 (□), GST-Ig4-5 (▵), GST-Ig6 (▴), and GST (○). (B) Mean neurite length of DRG cells cultured on different protein substrates. (C) DRG cells cultured on substrate-coated GST-Ig6 in the presence of different competitors. In A, the GST-Ig6-coated coverslips were preincubated with anti-Ig4-5-6 Fab (250 μg/ml) for 15 min at room temperature, washed to remove unbound Fab, and seeded with DRG cells. In B, the assay was carried out in the presence of soluble GST-Ig6 (40 μg/ml). In C, GST (40 μg/ml) was added to the assay. Data represent the mean ± SD of three experiments.
Competition experiments were carried out to demonstrate the specific requirement for Ig6. When DRG cells were cultured in the presence of soluble GST-Ig6, neurite outgrowth was reduced to background level, whereas the addition of soluble GST did not result in any significant inhibitory effect (Figure 5C). Similarly, precoating the substratum with anti-Ig4-5-6 Fab before the seeding of cells reduced the neuritogenic activity of the GST-Ig6 substratum to background level (Figure 5C).
Inhibition of the Neuritogenic Activity of L1 Ig6 by RGD Peptides
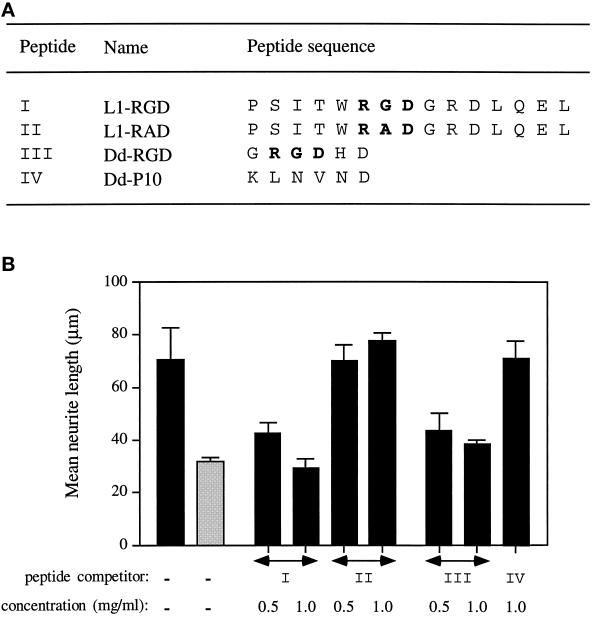
To determine whether the RGD sequence between amino acid positions 554 and 556 in L1 Ig6 is responsible for the neuritogenic activity, synthetic peptides containing an RGD sequence were tested for their ability to inhibit Ig6-dependent neurite outgrowth. The peptide L1-RGD contained the L1 sequence between amino acids Pro-549 and Leu-563 (Figure 6A). In the absence of peptide competitor, DRG cells extended neurites with a mean length of 70 μm on GST-Ig4-5-6 and 30 μm on GST (Figure 6B). The addition of peptide L1-RGD in the assay inhibited neurite outgrowth in a dose-dependent manner. The mean neurite length of DRG cells was reduced to the background level at 1 mg/ml peptide L1-RGD (Figure 6B). Substitution of Gly-555 with Ala in the peptide L1-RAD resulted in the loss of inhibition (Figure 6B), highlighting the importance of the RGD sequence. Similar inhibitory effects were observed when peptide L1-RGD was added to DRG cells cultured on LR73 transfectants expressing L1Δ2 (our unpublished data).
Figure 6.
Inhibition of GST-Ig4-5-6-stimulated neurite outgrowth by RGD-containing peptides. (A) Synthetic peptides used in competition studies. (B) Mean neurite lengths of DRG cells cultured on substrate-coated GST-Ig4-5-6 (black bars) or GST (gray bar). DRG cells were also assayed in the presence of different L1 peptide competitors. Data represent the mean ± SD of three experiments.
To further demonstrate the sole requirement for the RGD sequence, a short RGD-containing peptide, Dd-RGD (derived from the RGD site of the discoidin-I molecule of Dictyostelium discoideum; Poole et al., 1981), was tested in this assay. Peptide Dd-RGD inhibited neurite outgrowth on the GST-Ig4-5-6 substrate just as effectively as the peptide L1-RGD (Figure 6B). However, the control peptide Dd-P10 had no significant inhibitory effect.
Effects of Substitutions in the RGD Sequence in Ig6
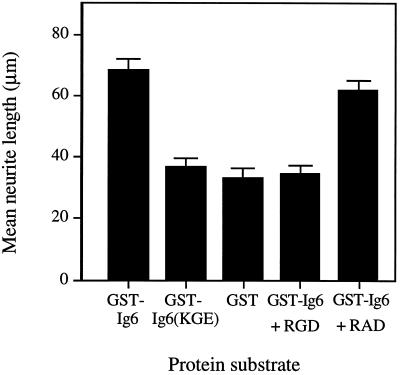
To confirm the pivotal role played by the RGD sequence in promoting neurite outgrowth from neuronal cells, the RGD sequence in L1 Ig6 was changed to KGE and then expressed as a GST fusion protein (Figure 4). The ability of the fusion protein GST-Ig6(KGE) to promote neurite outgrowth from DRG cells was examined. Cells cultured on the GST-Ig6 substrate produced neurites with a mean length of 68 μm, whereas the GST-Ig6(KGE) substrate yielded neurites with a mean length of 37 μm (Figure 7). The mean neurite length in the latter case was similar to that of cells plated on GST. Consistent with our observations for GST-Ig4-5-6, neurite outgrowth of DRG cells on substrate-coated GST-Ig6 was inhibited by the peptide L1-RGD but not by L1-RAD (Figure 7). Therefore, the ability of L1 Ig6 to stimulate neurite outgrowth is dependent on the RGD sequence.
Figure 7.
Effects of peptide competitors and mutation on the neuritogenic activity of L1 Ig6. DRG cells were cultured on substrate-coated GST-Ig6 or GST-Ig6(KGE) for 20 h and fixed. Approximately 100 neurites were measured and the mean neurite length was calculated. As a control, DRG cells were cultured on GST-Ig6 in the presence of L1 peptide competitors: 1.0 mg/ml of peptide L1-RGD or peptide L1-RAD. Data represent the mean ± SD of three experiments.
The RGD Sequence Interacts with αvβ3 Integrin to Promote Neurite Outgrowth
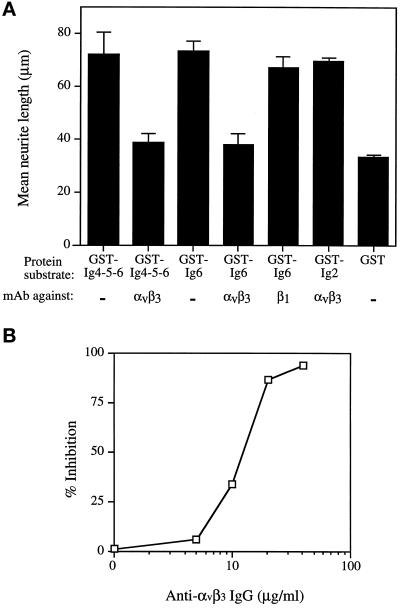
The RGD sequence in Ig6 of L1 has been shown to bind αvβ3 integrin (Ebeling et al., 1996; Montgomery et al., 1996). To determine whether the neuritogenic activity of the RGD sequence in L1 Ig6 was mediated by interaction with αvβ3 integrin, the mAb LM609, which specifically recognizes the αvβ3 integrin (Cheresh and Spiro, 1987), was added to DRG cells cultured on substrate-coated fusion proteins. The inclusion of mAb LM609 in the assay resulted in reduction of neurite outgrowth to the background level for both GST-Ig4-5-6 and GST-Ig6 substrates, such that their mean neurite lengths were reduced to 38.5 μm and 37.8 μm, respectively (Figure 8A). The inhibitory effect of mAb LM609 was dose dependent. Fifty percent inhibition was achieved at ∼15 μg/ml and maximum inhibition was achieved at 40 μg/ml IgG (Figure 8B). However, LM609 was unable to inhibit L1 Ig2-dependent neurite outgrowth from DRG cells (Figure 8A). These results suggest that L1 uses at least two distinct pathways to stimulate neurite outgrowth and that one of the pathways is triggered by its interaction with the αvβ3 integrin.
Figure 8.
Inhibition of neurite outgrowth by mAb LM609 directed against αvβ3 integrin. (A) Mean neurite lengths of DRG cells cultured on stimulatory substrates GST-Ig4-5-6, GST-Ig6, or GST-Ig2 in the presence of either mAb LM609 against αvβ3 integrin or mAb W1B10 against the chick β1 integrin subunit. Both antibodies were added at a final concentration of 40 μg/ml. Data represent the mean ± SD of three experiments. (B) Dose effects of mAb LM609 on neurite outgrowth of DRG cells cultured on substrate-coated GST-Ig6.
β1-dependent neurite outgrowth has been reported for chick DRG cells (Venstrom and Reichardt, 1995). Therefore, the effects of mAb W1B10, which recognizes the chick β1 integrin subunit, were also examined in the neurite outgrowth assay. In contrast to results obtained with LM609, mAb W1B10 did not significantly inhibit neurite outgrowth for DRG cells on the GST-Ig6 substrate (Figure 8A), indicating that the RGD sequence in L1 did not interact with β1 integrins to promote neurite outgrowth.
DISCUSSION
The data presented in this study indicate that the RGD sequence in the Ig-like domain 6 of human L1 promotes neurite outgrowth via interaction with αvβ3 integrin. L1 is expressed on the cell surface as an integral membrane protein, while L1 fragments of different sizes are shed by cells and become associated with the extracellular matrix (Faissner et al., 1985; Martini and Schachner, 1986; Sadoul et al., 1988; Poltorak et al., 1990, 1993; Montgomery et al., 1996). We have shown previously that Ig2 is capable of mediating L1–L1 homophilic binding and promoting neurite outgrowth from neural retinal cells (Zhao and Siu, 1995). Conceivably, L1 fragments associated with the extracellular matrix may elicit different responses from different neuronal cells and, therefore, serve as specific guidance cues for axonal migration. Indeed, the effects of L1 Ig6 on neuronal cells are cell-type specific. Whereas Ig2 promotes neurite outgrowth from all neuronal cells tested so far, Ig6 stimulates neurite outgrowth from DRG cells but not from retinal cells. Because these two neuritogenic sites are located on two distantly separated Ig-like domains, each site may function independently.
Previously, Appel et al. (1993) have shown that recombinant proteins consisting of mouse L1 Ig1-2, Ig3-4, or Ig5-6 exhibit varied degrees of neuritogenic activity when tested on small cerebellar neurons. Neither the neuritogenic sequences in these Ig pairs nor the mechanisms involved have been characterized. However, it is of interest to note that the human RGD motif in Ig6 is conserved in both mouse and rat L1 (Moos et al., 1988; Hlavin and Lemmon, 1991; Kobayashi et al., 1991; Prince et al., 1991). Further experiments will be required to determine whether mouse L1 uses the same RGD motif to elicit neurite outgrowth. The RGD motif in Ig6, however, is absent in the chicken homologue NgCAM (Burgoon et al., 1991), zebrafish L1 (Tongiorgi et al., 1995), and Drosophila melanogaster neuroglian (Bieber et al., 1989). Instead, chicken NgCAM has an RGD motif in its third FNIII domain. Because this RGD motif is not required for the neuritogenic activity of NgCAM (Burgoon et al., 1995), it remains to be determined whether the RGD motif in NgCAM can interact with RGD-specific integrins.
An outline structure of the Ig domains in human L1 has been proposed (Bateman et al., 1996) based on sequence alignment with telokin, a member of the I set of Ig molecules with a known structure (Holden et al., 1992; Harpaz and Chothia, 1994). This model suggests that the RGD motif would not be an active epitope because it is predicted to reside on the C β-strand and participate in intramolecular hydrogen bonding. Therefore, it is incompatible with our observation that the RGD motif can stimulate neurite outgrowth. Recently, a different model has been proposed for murine L1 based on electron microscopic analysis and computer-assisted modeling (Drescher et al., 1996). Although Ig-like domains 1–5 in L1 have greatest sequence homology with telokin, Ig6 shows closest homology with the Fv fragment of IgG Iia (Hohne et al., 1993). In addition to the conserved RGD motif found in human L1, murine L1 contains another RGD sequence in Ig6. The model predicts that both RGD sequences are located at the molecular surface within the turn structure between the C′ and E β-strands and are available for binding with receptors (Drescher et al., 1996). Furthermore, L1 Ig6 possesses greater surface hydrophobicity than the other Ig-like domains in their analysis, suggesting that it may participate more readily in intermolecular interactions.
The RGD motif was first discovered in fibronectin as a cell attachment site (Pierschbacher and Ruoslahti, 1984) and was subsequently found to be the recognition sequence for a number of integrin receptors (Ruoslahti, 1996). X-ray crystallographic analysis of fibronectin and other RGD-containing proteins active in cell adhesion reveals that the RGD sequences are found in loop regions and typically form a type II β-hairpin turn (Leahy et al., 1996). A glycine residue in the second position is generally required due to the conformational constraints of the turn (Richardson, 1981). Our peptide inhibition studies also point to the importance of the glycine residue and are consistent with the notion that the RGD sequence in human L1 Ig6 is localized to a loop region.
The RGD sequences in both human and rodent L1 have been reported to interact with integrins in lymphocytes and tumor cells (Ruppert et al., 1995; Ebeling et al., 1996; Montgomery et al., 1996). However, it is not known whether L1 binds RGD-specific integrins in the brain. Our study provides the first evidence that L1 interacts with integrin on neuronal cells and that this interaction can stimulate neurite outgrowth. Using specific blocking monoclonal antibodies, we identified the αvβ3 integrin as the neuritogenic receptor for the RGD sequence in L1. Although neurite outgrowth from chick DRG cells on fibronectin was shown to depend on the α8β1 integrin (Müller et al., 1995), we found that anti-β1 antibodies failed to inhibit the neuritogenic activity of L1 Ig6, thus confirming the specificity of the L1–αvβ3 interaction. Interestingly, immunostaining experiments show that the αvβ3 integrin is expressed in both retinal cells and DRG cells (Yip and Siu, unpublished data). The reason that retinal cells fail to respond to L1 Ig6 is not known. Possibly, one or more essential elements in the αvβ3-dependent signaling pathway may be absent in retinal cells.
The αv integrin subunit is predominantly located in nervous tissues with strong expression in the neural tube during mouse development, and it becomes down-regulated in the adult brain (Hirsch et al., 1994). The expression pattern suggests that αv integrins may play an important role during development of the nervous system. For example, the adhesion of avian neural crest cells to vitronectin is primarily via αvβ1, whereas migration involves αvβ3 and αvβ5 (Delannet et al., 1994). Furthermore, the migration of oligodendrocyte precursors depends on αvβ1 (Milner et al., 1996). We anticipate that αv-dependent functions will become increasingly important to neural development.
In addition to L1, several cell adhesion molecules of the Ig superfamily have been found to contain the RGD motif. Among these are TAG-1/axonin-1 (Furley et al., 1990; Hasler et al., 1993) and the neurofascins (Volkmer et al., 1992). Although all vertebrate neurofascins contain an RGD sequence in their third FNIII domain, the function of their RGD motif is not known. Similar to L1, the RGD sequence of TAG-1 is found in human and rat, but not in the chicken homologue axonin-1 (Zuellig et al., 1992). Also, TAG-1 has been reported to interact with β1 integrins to promote neurite outgrowth (Felsenfled et al., 1994). This interaction requires the participation of L1 and it is therefore not clear whether the TAG-1 RGD motif or the L1 RGD motif is involved in integrin binding.
The identification of the αvβ3 integrin as a neuritogenic receptor for L1 suggests a signaling pathway that may differ from the one triggered by L1–L1 homophilic binding. The signaling events of αvβ3 integrin probably involve the integrin-associated protein (IAP) CD47 (Lindberg et al., 1993; Mawby et al., 1994), which is known to complex with the αvβ3 integrin to form a signal transducing unit (Zhou and Brown, 1993; Reinhold et al., 1997). For example, IAP is required for integrin-dependent calcium entry in fibroblasts and endothelial cells (Schwartz et al., 1993; Tsao and Mousa, 1995). Since Ca2+ influx is a key step in integrin-dependent neurite outgrowth (Williams et al., 1992, 1994a), the initial steps of the αvβ3 signaling cascade likely involve the intimate association of IAP. Also, an alternatively spliced neural form of IAP is highly expressed in both central and peripheral nervous systems (Reinhold et al., 1995). Further studies will be required to determine whether this form of IAP is involved in integrin-related events during neuronal cell differentiation.
The use of specific pharmacological reagents in inhibitory studies has helped to distinguish between integrin-dependent and NCAM/L1-dependent signaling pathways involved in the promotion of neurite outgrowth (Williams et al., 1992, 1994a). One model proposes that clustering of cell adhesion molecules, such as L1, NCAM, or N-cadherin, induces the activation of the fibroblast growth factor receptor, which in turn activates a signaling cascade leading to neurite outgrowth (Williams et al., 1994b; Saffell et al., 1997). In other work, protein phosphorylation involving the nonreceptor tyrosine kinase pp60c-src has been shown to be an essential component in the early steps of the signaling pathway (Ignelzi et al., 1994). Recently, the activation of c-src in M21 melanoma cells by osteopontin was shown to be dependent on the αvβ3 integrin (Chellaiah et al., 1996). It will be of interest to determine whether the binding of L1 Ig6 with αvβ3 integrin will also lead to the activation of c-src in DRG cells. Since both NCAM/L1-dependent and integrin-dependent pathways lead to an influx of Ca2+, this may represent the point at which these two pathways converge.
L1 is a multidomain and multifunctional protein. In addition to the nervous system, L1 is expressed in epithelial cells of the intestine and the urogenital tract (Thor et al., 1987; Probstmeier et al., 1990; Kujat et al., 1995). L1 expression has also been reported in leukocytes (Kowitz et al., 1992; Ebeling et al., 1996) and in a variety of malignant cells (Mujoo et al., 1986; Linnemann and Bock, 1989; Reid and Hemperly, 1992). L1 shed into the matrix promotes the migration of lymphocytes and tumor cells (Montgomery et al., 1996; Duczmal et al., 1997). These observations indicate that the significance of the homophilic and heterophilic interactions of L1 may extend beyond neural development and that L1 may participate in the normal morphogenesis of tissues as well as in the metastatic events of tumor cells.
ACKNOWLEDGMENTS
We thank Drs. David Isenman and Derek van der Kooy for advice and discussion. This work was supported by Operating grant MT-11443 from the Medical Research Council of Canada. P.Y. is supported by an Ontario Graduate Scholarship and X.Z. is supported by a Studentship from the Medical Research Council of Canada.
Footnotes
Abbreviations used: DRG, dorsal root ganglion; FNIII, fibronectin type III repeat; GST, glutathione S-transferase; IAP, integrin-associated protein; mAb, monoclonal antibody.
REFERENCES
- Appel F, Holm J, Conscience J-F, Schachner M. Several extracellular domains of the neural cell adhesion molecule L1 are involved in neurite outgrowth and cell body adhesion. J Neurosci. 1993;13:4764–4775. doi: 10.1523/JNEUROSCI.13-11-04764.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman A, Jouet M, MacFarlane J, Du J-S, Kenwrick S, Chothia C. Outline structure of the human L1 cell adhesion molecule and the sites where mutations cause neurological disorders. EMBO J. 1996;15:6050–6059. [PMC free article] [PubMed] [Google Scholar]
- Bieber AJ, Snow PM, Hortsch M, Patel NH, Jacobs JR, Traquina ZR, Schilling J, Goodman CS. Drosophila neuroglian: a member of the immunoglobulin superfamily with extensive homology to the vertebrate neural adhesion molecule L1. Cell. 1989;59:447–460. doi: 10.1016/0092-8674(89)90029-9. [DOI] [PubMed] [Google Scholar]
- Bock E, Richter-Landsberg C, Faissner A, Schachner M. Demonstration of immunochemical identity between the nerve growth factor-inducible large external (NILE) glycoprotein and the cell adhesion molecule L1. EMBO J. 1985;4:2765–2768. doi: 10.1002/j.1460-2075.1985.tb04001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brümmendorf T, Hubert M, Treubert U, Leuschner R, Tárnok A, Rathjen FG. The axonal recognition molecule F11 is a multifunctional protein: specific domains mediate interactions with Ng-CAM and restrictin. Neuron. 1993;10:711–727. doi: 10.1016/0896-6273(93)90172-n. [DOI] [PubMed] [Google Scholar]
- Burgoon MP, Grumet M, Mauro V, Edelman GM, Cunningham BA. Structure of the chicken neuron-glia cell adhesion molecule, Ng-CAM: origin of the polypeptides and relation to the Ig superfamily. J Cell Biol. 1991;112:1017–1029. doi: 10.1083/jcb.112.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoon MP, Hazan RB, Phillips GR, Crossin KL, Edelman GM, Cunningham BA. Functional analysis of posttranslational cleavage products of the neuron-glia cell adhesion molecule, Ng-CAM. J Cell Biol. 1995;130:733–744. doi: 10.1083/jcb.130.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellaiah M, Fitzgerald C, Filardo EJ, Cheresh DA, Hruska KA. Osteopontin activation of c-src in human melanoma cells requires the cytoplasmic domain of the integrin αv-subunit. Endocrinology. 1996;137:2432–2440. doi: 10.1210/endo.137.6.8641196. [DOI] [PubMed] [Google Scholar]
- Cheresh DA, Spiro RC. Biosynthetic and functional properties of an Arg-Gly-Asp–directed receptor involved in human melanoma cell attachment to vitronectin, fibrinogen, and von Willebrand factor. J Biol Chem. 1987;262:17703–17711. [PubMed] [Google Scholar]
- Daniloff JK, Chuong C-M, Levi G, Edelman GM. Differential distribution of cell adhesion molecules during histogenesis of the chick nervous system. J Neurosci. 1986;6:739–758. doi: 10.1523/JNEUROSCI.06-03-00739.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delannet M, Martin F, Bossy B, Cheresh DA, Reichardt LF, Duband J-L. Specific roles of the αvβ1, αvβ3, and αvβ5 integrins in avian neural crest cell adhesion and migration on vitronectin. Development. 1994;120:2687–2702. doi: 10.1242/dev.120.9.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd J, Jessell TM. Axon guidance and the patterning of neuronal projections in vertebrates. Science. 1988;242:692–699. doi: 10.1126/science.3055291. [DOI] [PubMed] [Google Scholar]
- Drescher B, Spiess E, Schachner M, Probstmeier R. Structural analysis of the murine cell adhesion molecule L1 by electron microscopy and computer-assisted modelling. Eur J Neurosci. 1996;8:2467–2478. doi: 10.1111/j.1460-9568.1996.tb01541.x. [DOI] [PubMed] [Google Scholar]
- Duczmal A, Schollhammer S, Katich S, Ebeling O, Schwartz-Albiez R, Altevogt P. The L1 adhesion molecule supports αvβ3-mediated migration of human tumor cells and activated T lymphocytes. Biochem Biophys Res Commun. 1997;232:236–239. doi: 10.1006/bbrc.1997.6265. [DOI] [PubMed] [Google Scholar]
- Ebeling O, Duczmal A, Aigner S, Geiger C, Schöllhammer S, Kemshead JT, Möller P, Schwartz-Albiez R, Altevogt P. L1 adhesion molecule on human lymphocytes and monocytes: expression and involvement in binding to αvβ3 integrin. Eur J Immunol. 1996;26:2508–2516. doi: 10.1002/eji.1830261035. [DOI] [PubMed] [Google Scholar]
- Edelman GM, Crossin KL. Cell adhesion molecules: implications for a molecular histology. Annu Rev Biochem, 1991;60:155–190. doi: 10.1146/annurev.bi.60.070191.001103. [DOI] [PubMed] [Google Scholar]
- Faissner A, Teplow DB, Kübler D, Keilhauer G, Kinzel V, Schachner M. Biosynthesis and membrane topology of the neural cell adhesion molecule L1. EMBO J. 1985;4:3105–3113. doi: 10.1002/j.1460-2075.1985.tb04052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenfeld DP, Hynes MA, Skoler KM, Furley AJ, Jessell TM. TAG-1 can mediate homophilic binding, but neurite outgrowth on TAG-1 requires an L1-like molecule and β1 integrins. Neuron. 1994;12:675–690. doi: 10.1016/0896-6273(94)90222-4. [DOI] [PubMed] [Google Scholar]
- Fransen E, Lemmon V, Van Camp G, Vits L, Coucke P, Willems PJ. CRASH syndrome: clinical spectrum of corpus callosum hypoplasia, retardation, adducted thumbs, spastic paraparesis and hydrocephalus due to mutations in one single gene, L1. Eur J Hum Genet. 1995;3:273–284. doi: 10.1159/000472311. [DOI] [PubMed] [Google Scholar]
- Friedlander DR, Milev P, Karthikeyan L, Margolis RK, Margolis RU, Grumet M. The neuronal chondroitin sulfate proteoglycan neurocan binds to the neural cell adhesion molecules Ng-CAM/L1/NILE and N-CAM, and inhibits neuronal adhesion and neurite outgrowth. J Cell Biol. 1994;125:669–680. doi: 10.1083/jcb.125.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furley AJ, Morton SB, Manalo D, Karagogeos D, Dodd J, Jessell TM. The axonal glycoprotein TAG-1 is an immunoglobulin superfamily member with neurite outgrowth-promoting activity. Cell. 1990;61:157–170. doi: 10.1016/0092-8674(90)90223-2. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Greenberg ME. Calcium signaling in neurons: molecular mechanisms and cellular consequences. Science. 1995;268:239–247. doi: 10.1126/science.7716515. [DOI] [PubMed] [Google Scholar]
- Giese KP, Martini R, Lemke G, Soriano P, Schachner M. Mouse Po gene disruption leads to hypomyelination, abnormal expression of recognition molecules, and degeneration of myelin and axons. Cell. 1992;71:565–576. doi: 10.1016/0092-8674(92)90591-y. [DOI] [PubMed] [Google Scholar]
- Goodman CS, Shatz CJ. Developmental mechanisms that generate precise patterns of neuronal connectivity. Cell. 1993;72:77–98. doi: 10.1016/s0092-8674(05)80030-3. [DOI] [PubMed] [Google Scholar]
- Grumet M, Friedlander DR, Edelman GM. Evidence for the binding of Ng-CAM to laminin. Cell Adhes Commun. 1993;1:177–190. doi: 10.3109/15419069309095693. [DOI] [PubMed] [Google Scholar]
- Grumet M, Friedlander DR, Sakurai T. Functions of brain chondroitin sulfate proteoglycans during development: interactions with adhesion molecules. Perspect Dev Neurobiol. 1996;3:319–330. [PubMed] [Google Scholar]
- Grumet M, Hoffman S, Edelman GM. Two antigenetically related neuronal cell adhesion molecules of different specificities mediate neuron–neuron and neuron–glia adhesion. Proc Natl Acad Sci USA. 1984;81:267–271. doi: 10.1073/pnas.81.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall H, Carbonetto S, Schachner M. L1/HNK-1 carbohydrate- and β1 integrin-dependent neural cell adhesion to laminin-1. J Neurochem. 1997;68:544–553. doi: 10.1046/j.1471-4159.1997.68020544.x. [DOI] [PubMed] [Google Scholar]
- Harpaz Y, Chothia C. Many of the immunoglobulin superfamily domains in cell adhesion molecules and surface receptors belong to a new structural set which is close to that containing variable domains. J Mol Biol. 1994;238:528–539. doi: 10.1006/jmbi.1994.1312. [DOI] [PubMed] [Google Scholar]
- Hasler TH, Rader C, Stoeckli ET, Zuellig RA, Sonderegger P. cDNA cloning, structural features, and eucaryotic expression of human TAG-1/axonin-1. Eur J Biochem. 1993;211:329–339. doi: 10.1111/j.1432-1033.1993.tb19902.x. [DOI] [PubMed] [Google Scholar]
- Higuchi R. Recombinant PCR. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR Protocols: A Guide to Methods and Applications. San Diego: Academic Press; 1990. pp. 177–183. [Google Scholar]
- Hirsch E, Gullberg D, Balzac F, Altruda F, Silengo L, Tarone G. αv integrin subunit is predominantly located in nervous tissue and skeletal muscle during mouse development. Dev Dyn. 1994;201:108–120. doi: 10.1002/aja.1002010203. [DOI] [PubMed] [Google Scholar]
- Hlavin ML, Lemmon V. Molecular structure and functional testing of human L1CAM: an interspecies comparison. Genomics. 1991;11:416–423. doi: 10.1016/0888-7543(91)90150-d. [DOI] [PubMed] [Google Scholar]
- Hohne WE, et al. Structural base of the interaction of a monoclonal antibody against p24 of HIV-1 with its peptide epitope. Mol Immunol. 1993;30:1213–1221. doi: 10.1016/0161-5890(93)90140-7. [DOI] [PubMed] [Google Scholar]
- Holden HM, Ito M, Hartshorne DJ, Rayment I. X-ray structure determination of telokin, the C-terminal domain of myosin light chain kinase, at 2.8 Å resolution. J Mol Biol. 1992;227:840–851. doi: 10.1016/0022-2836(92)90226-a. [DOI] [PubMed] [Google Scholar]
- Horstkorte R, Schachner M, Magyar JP, Vorherr T, Schmitz B. The fourth immunoglobulin-like domain of NCAM contains a carbohydrate recognition domain for oligomannosidic glycans implicated in association with L1 and neurite outgrowth. J Cell Biol. 1993;121:1409–1421. doi: 10.1083/jcb.121.6.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortsch M. The L1 family of neural cell adhesion molecules: old proteins performing new tricks. Neuron. 1996;17:587–593. doi: 10.1016/s0896-6273(00)80192-0. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Ignelzi MA, Miller DR, Soriano P, Maness PF. Impaired neurite outgrowth of src-minus cerebellar neurons on the cell adhesion molecule L1. Neuron. 1994;12:873–884. doi: 10.1016/0896-6273(94)90339-5. [DOI] [PubMed] [Google Scholar]
- Jouet M, Moncla A, Paterson J, McKeown C, Fryer A, Carpenter N, Holmberg E, Wadelius C, Kenwrick S. New domains of neural cell-adhesion molecule L1 implicated in X-linked hydrocephalus and MASA syndrome. Am J Hum Genet. 1995;56:1304–1314. [PMC free article] [PubMed] [Google Scholar]
- Jouet M, Rosenthal A, Armstrong G, MacFarlane J, Stevenson R, Paterson J, Metzenberg A, Ionasescu V, Temple K, Kenwrick S. X-linked spastic paraplegia (SPG1), MASA syndrome and X-linked hydrocephalus result from mutations in the L1 gene. Nat Genet. 1994;7:402–407. doi: 10.1038/ng0794-402. [DOI] [PubMed] [Google Scholar]
- Kadmon G, Kowitz A, Altevogt P, Schachner M. The neural cell adhesion molecule N-CAM enhances L1-dependent cell–cell interactions. J Cell Biol. 1990;110:193–208. doi: 10.1083/jcb.110.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenwrick S, Jouet M, Donnai D. X linked hydrocephalus and MASA syndrome. J Med Genet. 1996;33:59–65. doi: 10.1136/jmg.33.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Miura M, Asou H, Uyemura K. Molecular cloning of cell adhesion molecule L1 from human nervous tissue: a comparison of the primary sequences of L1 molecules of different origin. Biochim Biophys Acta. 1991;1090:238–240. doi: 10.1016/0167-4781(91)90108-x. [DOI] [PubMed] [Google Scholar]
- Kowitz A, Kadmon G, Eckert M, Schirrmacher V, Schachner M, Altevogt P. Expression and function of the neural cell adhesion molecule L1 in mouse leukocytes. Eur J Immunol. 1992;22:1199–1205. doi: 10.1002/eji.1830220514. [DOI] [PubMed] [Google Scholar]
- Kuhn TB, Stoeckli ET, Condrau MA, Rathjen FG, Sonderegger P. Neurite outgrowth on immobilized axonin-1 is mediated by a heterophilic interaction with L1(G4) J Cell Biol. 1991;115:1113–1126. doi: 10.1083/jcb.115.4.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujat R, Miragall F, Krause D, Dermietzel R, Wrobel K-H. Immunolocalization of the neural cell adhesion molecule L1 in non-proliferating epithelial cells of the male urogenital tract. Histochemistry. 1995;103:311–321. doi: 10.1007/BF01457416. [DOI] [PubMed] [Google Scholar]
- Lagenaur C, Lemmon V. An L1-like molecule, the 8D9 antigen, is a potent substrate for neurite extension. Proc Natl Acad Sci USA. 1987;84:7753–7757. doi: 10.1073/pnas.84.21.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leahy DJ, Aukhil I, Erickson HP. 2.0 Å crystal structure of a four-domain segment of human fibronectin encompassing the RGD loop and synergy region. Cell. 1996;84:155–164. doi: 10.1016/s0092-8674(00)81002-8. [DOI] [PubMed] [Google Scholar]
- Lindberg FP, Gresham HD, Schwarz E, Brown EJ. Molecular cloning of integrin-associated protein: an immunoglobulin family member with multiple membrane-spanning domains implicated in αvβ3-dependent ligand binding. J Cell Biol. 1993;123:485–496. doi: 10.1083/jcb.123.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner J, Rathjen FG, Schachner M. L1 mono- and polyclonal antibodies modify cell migration in early postnatal mouse cerebellum. Nature. 1983;305:427–430. doi: 10.1038/305427a0. [DOI] [PubMed] [Google Scholar]
- Linnemann D, Bock E. Cell adhesion molecules in neural development. Dev Neurosci. 1989;11:149–173. doi: 10.1159/000111896. [DOI] [PubMed] [Google Scholar]
- Lüthi A, Laurent J-P, Figurov A, Muller D, Schachner M. Hippocampal long-term potentiation and neural cell adhesion molecules L1 and NCAM. Nature. 1994;372:777–779. doi: 10.1038/372777a0. [DOI] [PubMed] [Google Scholar]
- Martini R, Schachner M. Immunoelectron microscopic localization of neural cell adhesion molecules (L1, N-CAM, and MAG) and their shared carbohydrate epitope and myelin basic protein in developing sciatic nerve. J Cell Biol. 1986;103:2439–2448. doi: 10.1083/jcb.103.6.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawby WJ, Holmes CH, Anstee DJ, Spring FA, Tanner MJ. Isolation and characterization of CD47 glycoprotein: a multispanning membrane protein which is the same as integrin-associated protein (IAP) and the ovarian tumour marker OA3. Biochem J. 1994;304:525–530. doi: 10.1042/bj3040525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milev P, Meyer-Puttlitz B, Margolis RK, Margolis RU. Complex-type asparagine-linked oligosaccharides on phosphacan and protein-tyrosine phosphatase-ζβ mediate their binding to neural cell adhesion molecules and tenascin. J Biol Chem. 1995;270:24650–24653. doi: 10.1074/jbc.270.42.24650. [DOI] [PubMed] [Google Scholar]
- Milner R, Edwards G, Streuli C, ffrench-Constant C. A role in migration for the αvβ1 integrin expressed on oligodendrocyte precursors. J Neurosci. 1996;16:7240–7252. doi: 10.1523/JNEUROSCI.16-22-07240.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura M, Asou H, Kobayashi M, Uyemura K. Functional expression of a full-length cDNA coding for rat neural cell adhesion molecule L1 mediates homophilic intercellular adhesion and migration of cerebellar neurons. J Biol Chem. 1992;267:10752–10758. [PubMed] [Google Scholar]
- Montgomery AMP, Becker JC, Siu C-H, Lemmon VP, Cheresh DA, Pancook JD, Zhao X, Reisfeld RA. Human neural cell adhesion molecule L1 and rat homologue NILE are ligands for integrin αvβ3. J Cell Biol. 1996;132:475–485. doi: 10.1083/jcb.132.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos M, Tacke R, Scherer H, Teplow D, Früh K, Schachner M. Neural adhesion molecule L1 as a member of the immunoglobulin superfamily with binding domains similar to fibronectin. Nature. 1988;334:701–703. doi: 10.1038/334701a0. [DOI] [PubMed] [Google Scholar]
- Moscoso LM, Sanes JR. Expression of four immunoglobulin superfamily adhesion molecules (L1, Nr-CAM/Bravo, neurofascin/ABGP, and NCAM) in the developing mouse spinal cord. J Comp Neurol. 1995;352:321–334. doi: 10.1002/cne.903520302. [DOI] [PubMed] [Google Scholar]
- Mujoo K, Spiro RC, Reisfeld RA. Characterization of a unique glycoprotein antigen expressed on the surface of human neuroblastoma cells. J Biol Chem. 1986;261:10299–10305. [PubMed] [Google Scholar]
- Müller U, Bossy B, Venstrom K, Reichardt LF. Integrin α8β1 promotes attachment, cell spreading, and neurite outgrowth on fibronectin. Mol Biol Cell. 1995;6:433–448. doi: 10.1091/mbc.6.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne HR, Burden SM, Lemmon V. Modulation of growth cone morphology by substrate-bound adhesion molecules. Cell Motil Cytoskeleton. 1992;21:65–73. doi: 10.1002/cm.970210108. [DOI] [PubMed] [Google Scholar]
- Persohn E, Schachner M. Immunoelectron microscopic localization of the neural cell adhesion molecules L1 and N-CAM during postnatal development of the mouse cerebellum. J Cell Biol. 1987;105:569–576. doi: 10.1083/jcb.105.1.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierschbacher MD, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984;309:30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- Poltorak M, Shimoda K, Freed WJ. Cell adhesion molecules (CAMs) in adrenal medulla in situ and in vitro: enhancement of chromaffin cell L1/Ng-CAM expression by NGF. Exp Neurol. 1990;110:52–72. doi: 10.1016/0014-4886(90)90051-s. [DOI] [PubMed] [Google Scholar]
- Poltorak M, Williams JR, Freed WJ. Degradation fragments of L1 antigen enhance tyrosine hydroxylase-immunoreactive neurite outgrowth in mesencephalic cell culture. Brain Res. 1993;619:255–262. doi: 10.1016/0006-8993(93)91619-4. [DOI] [PubMed] [Google Scholar]
- Poole S, Firtel RA, Lamar E, Rowekamp W. Sequence and expression of the discoidin-I gene family in Dictyostelium discoideum. J Mol Biol, 1981;153:273–289. doi: 10.1016/0022-2836(81)90278-3. [DOI] [PubMed] [Google Scholar]
- Prince JT, Alberti L, Healy PA, Nauman SJ, Stallcup WB. Molecular cloning of NILE glycoprotein and evidence for its continued expression in mature rat CNS. J Neurosci Res. 1991;30:567–581. doi: 10.1002/jnr.490300315. [DOI] [PubMed] [Google Scholar]
- Probstmeier R, Martini R, Tacke R, Schachner M. Expression of the adhesion molecule L1, N-CAM, and J1/tenascin during development of the murine small intestine. Differentiation. 1990;44:42–55. doi: 10.1111/j.1432-0436.1990.tb00535.x. [DOI] [PubMed] [Google Scholar]
- Reid RA, Hemperly JJ. Variants of human L1 cell adhesion molecule arise through alternate splicing of RNA. J Mol Neurosci. 1992;3:127–135. doi: 10.1007/BF02919404. [DOI] [PubMed] [Google Scholar]
- Reinhold MI, Lindberg FP, Kersh GJ, Allen PM, Brown EJ. Costimulation of T cell activation by integrin-associated protein (CD47) is an adhesion-dependent, CD28-independent signaling pathway. J Exp Med. 1997;185:1–11. doi: 10.1084/jem.185.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold MI, Lindberg FP, Plas D, Reynolds S, Peters MG, Brown EJ. In vivo expression of alternatively spliced forms of integrin-associated protein (CD47) J Cell Sci. 1995;108:3419–3425. doi: 10.1242/jcs.108.11.3419. [DOI] [PubMed] [Google Scholar]
- Richardson JS. The anatomy and taxonomy of protein structure. Adv Protein Chem. 1981;34:167–339. doi: 10.1016/s0065-3233(08)60520-3. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- Ruppert M, Aigner S, Hubbe M, Yagita H, Altevogt P. The L1 adhesion molecule is a cellular ligand for VLA-5. J Cell Biol. 1995;131:1881–1891. doi: 10.1083/jcb.131.6.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoul K, Sadoul R, Faissner A, Schachner M. Biochemical characterization of different molecular forms of the neural cell adhesion molecule L1. J Neurochem. 1988;50:510–521. doi: 10.1111/j.1471-4159.1988.tb02941.x. [DOI] [PubMed] [Google Scholar]
- Saffell JL, Williams EJ, Mason IJ, Walsh FS, Doherty P. Expression of a domainant negative FGF receptor inhibits axonal growth and FGF receptor phosphorylation stimulated by CAMs. Neuron. 1997;18:231–242. doi: 10.1016/s0896-6273(00)80264-0. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- Sandig M, Rao Y, Siu C-H. The homophilic binding site of the neural cell adhesion molecule NCAM is directly involved in promoting neurite outgrowth from cultured neural retinal cells. J Biol Chem. 1994;269:14841–14848. [PubMed] [Google Scholar]
- Schwartz MA, Brown EJ, Fazeli B. A 50-kDa integrin-associated protein is required for integrin-regulated calcium entry in endothelial cells. J Biol Chem. 1993;268:19931–19934. [PubMed] [Google Scholar]
- Schuch U, Lohse MJ, Schachner M. Neural cell adhesion molecules influence second messenger systems. Neuron. 1989;3:13–20. doi: 10.1016/0896-6273(89)90111-6. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Cadherin cell adhesion receptors as a morphogenic regulator. Science. 1991;251:1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- Takechi T, Tohyama J, Kurashige T, Maruta K, Uyemura K, Ohi T, Matsukura S, Sakuragawa N. A deletion of five nucleotides in the L1CAM gene in a Japanese family with X-linked hydrocephalus. Hum Genet. 1996;97:353–356. doi: 10.1007/BF02185770. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- Thor G, Probstmeier R, Schachner M. Characterization of the cell adhesion molecule L1, N-CAM and J1 in the mouse intestine. EMBO J. 1987;6:2581–2586. doi: 10.1002/j.1460-2075.1987.tb02548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tongiorgi E, Bernhardt RR, Schachner M. Zebrafish neurons express two L1-related molecules during early axonogenesis. J Neurosci Res. 1995;42:547–561. doi: 10.1002/jnr.490420413. [DOI] [PubMed] [Google Scholar]
- Tsao PW, Mousa SA. Thrombospondin mediates calcium mobilization in fibroblasts via its Arg-Gly-Asp and carboxyl-terminal domains. J Biol Chem. 1995;270:23747–23753. doi: 10.1074/jbc.270.40.23747. [DOI] [PubMed] [Google Scholar]
- Venstrom K, Reichardt L. β8 integrins mediate interactions of chick sensory neurons with laminin-1, collagen IV, and fibronectin. Mol Biol Cell. 1995;6:419–431. doi: 10.1091/mbc.6.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vits L, et al. MASA syndrome is due to mutations in the neural cell adhesion gene L1CAM. Nat Genet. 1994;7:408–413. doi: 10.1038/ng0794-408. [DOI] [PubMed] [Google Scholar]
- Volkmer H, Hassel B, Wolff JM, Frank R, Rathjen FG. Structure of the axonal surface recognition molecule neurofascin and its relationship to a neural subgroup of the immunoglobulin superfamily. J Cell Biol. 1992;118:149–161. doi: 10.1083/jcb.118.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EJ, Doherty P, Turner G, Reid RA, Hemperly JJ, Walsh FS. Calcium influx into neurons can solely account for cell contact-dependent neurite outgrowth stimulated by transfected L1. J Cell Biol. 1992;119:883–892. doi: 10.1083/jcb.119.4.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EJ, Furness J, Walsh FS, Doherty P. Activation of the FGF receptor underlies neurite outgrowth stimulated by L1, N-CAM, and N-cadherin. Neuron. 1994a;13:583–594. doi: 10.1016/0896-6273(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Williams EJ, Furness J, Walsh FS, Doherty P. Characterisation of the second messenger pathway underlying neurite outgrowth stimulated by FGF. Development. 1994b;120:1685–1693. doi: 10.1242/dev.120.6.1685. [DOI] [PubMed] [Google Scholar]
- Wong EV, Kenwrick S, Willems P, Lemmon V. Mutations in the cell adhesion molecule L1 cause mental retardation. Trends Neurosci. 1995;18:168–172. doi: 10.1016/0166-2236(95)93896-6. [DOI] [PubMed] [Google Scholar]
- Zhao X, Siu C-H. Colocalization of the homophilic binding site and the neuritogenic acitivity of the cell adhesion molecule L1 to its second Ig-like domain. J Biol Chem. 1995;270:29413–29421. doi: 10.1074/jbc.270.49.29413. [DOI] [PubMed] [Google Scholar]
- Zhao X, Siu C-H. Differential effects of two hydrocephalus/MASA syndrome-related mutations on the homophilic binding and neuritogenic activities of the cell adhesion molecule L1. J Biol Chem. 1996;271:6563–6566. doi: 10.1074/jbc.271.12.6563. [DOI] [PubMed] [Google Scholar]
- Zhou H, Fuks A, Alcaraz A, Bolling TJ, Stanners SP. Homophilic adhesion between Ig superfamily carcinoembryonic antigen molecules involves double reciprocal bonds. J Cell Biol. 1993;122:951–960. doi: 10.1083/jcb.122.4.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Brown EJ. Leukocyte response integrin and integrin-associated protein act as a signal transduction unit in generation of a phagocyte respiratory burst. J Exp Med. 1993;178:1165–1174. doi: 10.1084/jem.178.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuellig RA, et al. The axonally secreted cell adhesion molecule, axonin-1. Primary structure, immunoglobulin-like and fibronectin-type-III-like domains and glycosyl-phosphatidylinositol anchorage. Eur J Biochem. 1992;204:453–463. doi: 10.1111/j.1432-1033.1992.tb16655.x. [DOI] [PubMed] [Google Scholar]