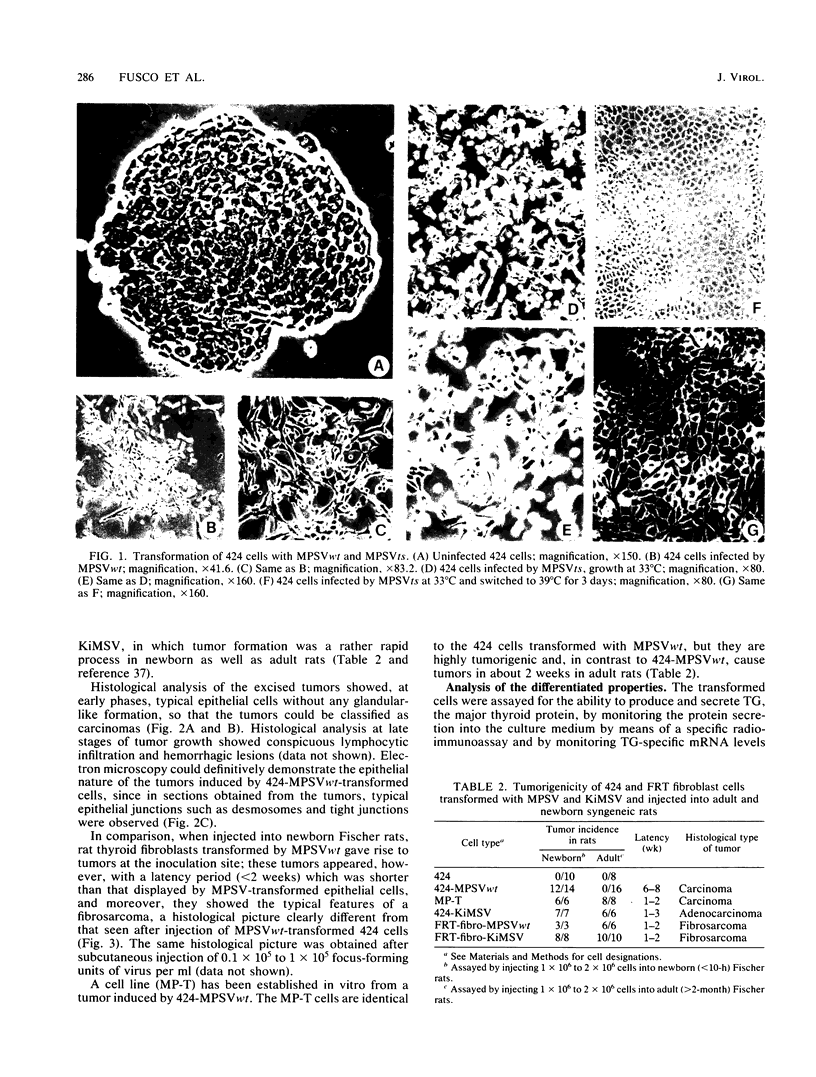
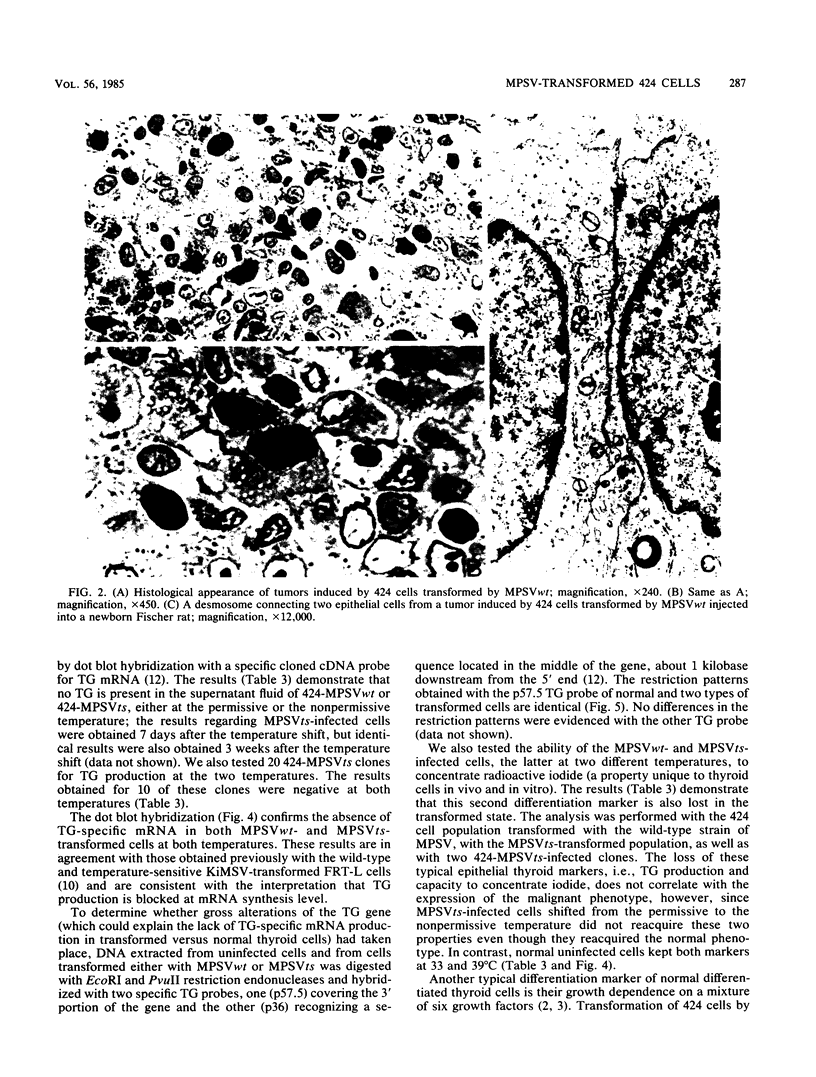
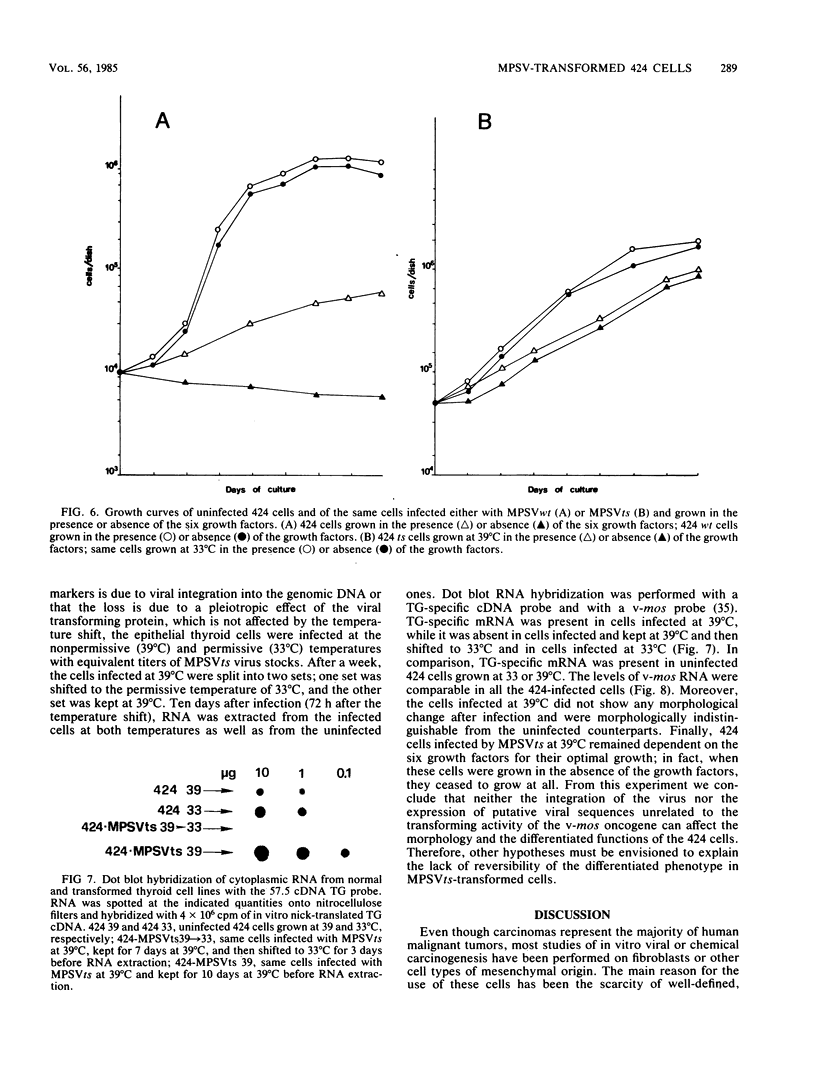
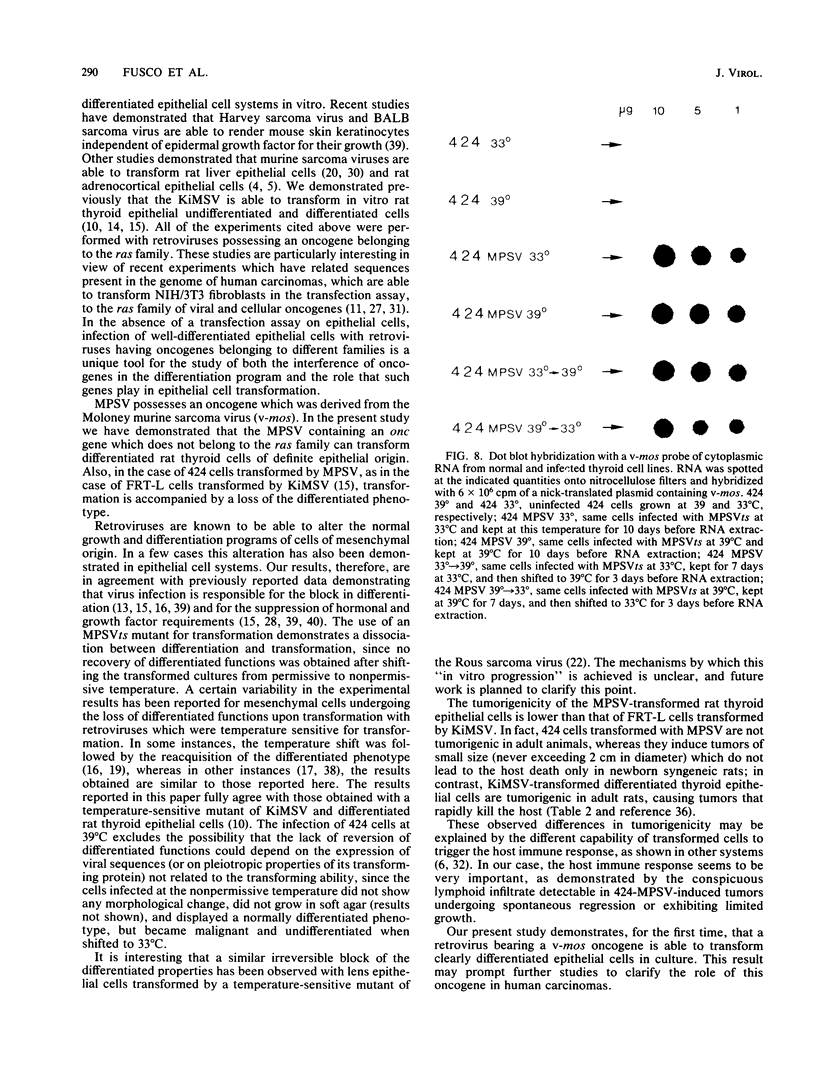
Abstract
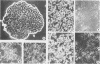
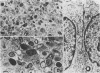
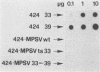
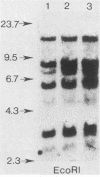
Differentiated, cloned rat thyroid epithelial cells (424 cells) were infected with a wild-type and a temperature-sensitive strain of the myeloproliferative variant of the Moloney murine sarcoma virus. The thyroid cells were productively infected and transformed by both virus strains and displayed some of the typical properties of malignant cells, such as morphological changes, growth in soft agar, and in vivo tumorigenicity. The acquisition of the transformed phenotype by the virus-infected cells was accompanied by a loss of the typical differentiated features of the thyroid epithelium, such as thyroglobulin (TG) secretion, iodide uptake, and dependence for growth on six factors including thyrotropin, the physiological thyroid stimulator. TG mRNA could not be demonstrated in cells transformed by both viral strains, suggesting a block at the level of the TG gene transcription. While the transformed state of the cell clones infected with the temperature-sensitive strain could be reverted by shifting the cultures to the temperature nonpermissive for transformation (39 degrees C), no reversion of the differentiated functions took place after such a shift, showing that the v-mos oncogene irreversibly shuts off the differentiation of thyroid epithelial cells in vitro. These results demonstrate, for the first time, an oncogenic potential of the v-mos oncogene family towards differentiated epithelial cells in vitro.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A. Unique aspects of the interactions of retroviruses with vertebrate cells: C. P. Rhoads memorial lecture. Cancer Res. 1983 Jan;43(1):1–5. [PubMed] [Google Scholar]
- Ambesi-Impiombato F. S., Coon H. G. Thyroid cells in culture. Int Rev Cytol Suppl. 1979;(10):163–172. doi: 10.1016/s0074-7696(08)60619-1. [DOI] [PubMed] [Google Scholar]
- Ambesi-Impiombato F. S., Parks L. A., Coon H. G. Culture of hormone-dependent functional epithelial cells from rat thyroids. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3455–3459. doi: 10.1073/pnas.77.6.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auersperg N. Effects of culture conditions on the growth and differentiation of transformed rat adrenocortical cells. Cancer Res. 1978 Jul;38(7):1872–1884. [PubMed] [Google Scholar]
- Auersperg N., Hudson J. B., Goddard E. G., Klement V. Transformation of cultured rat adrenocortical cells by Kirsten murine sarcoma virus (Ki-MSV). Int J Cancer. 1977 Jan;19(1):81–89. doi: 10.1002/ijc.2910190112. [DOI] [PubMed] [Google Scholar]
- Bernards R., Schrier P. I., Houweling A., Bos J. L., van der Eb A. J., Zijlstra M., Melief C. J. Tumorigenicity of cells transformed by adenovirus type 12 by evasion of T-cell immunity. 1983 Oct 27-Nov 2Nature. 305(5937):776–779. doi: 10.1038/305776a0. [DOI] [PubMed] [Google Scholar]
- Bilello J. A., Colletta G., Warnecke G., Koch G., Frisby D., Pragnell I. B., Ostertag W. Analysis of the expression of spleen focus-forming virus (SFFV)-related RNA and gp55, a Friend and Rauscher virus-specific protein. Virology. 1980 Dec;107(2):331–344. doi: 10.1016/0042-6822(80)90301-3. [DOI] [PubMed] [Google Scholar]
- Chirigos M. A., Scott D., Turner W., Perk K. Biological, pathological and physical characterization of a possible variant of a murine sarcoma virus (Moloney). Int J Cancer. 1968 Mar 15;3(2):223–227. doi: 10.1002/ijc.2910030207. [DOI] [PubMed] [Google Scholar]
- Colletta G., Di Fiore P. P., Ferrentino M., Pietropaolo C., Turco M. C., Vecchio G. Enhancement of viral gene expression in Friend erythroleukemic cells by 12-O tetradecanoylphorbol-13-acetate. Cancer Res. 1980 Sep;40(9):3369–3373. [PubMed] [Google Scholar]
- Colletta G., Pinto A., Di Fiore P. P., Fusco A., Ferrentino M., Avvedimento V. E., Tsuchida N., Vecchio G. Dissociation between transformed and differentiated phenotype in rat thyroid epithelial cells after transformation with a temperature-sensitive mutant of the Kirsten murine sarcoma virus. Mol Cell Biol. 1983 Nov;3(11):2099–2109. doi: 10.1128/mcb.3.11.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der C. J., Krontiris T. G., Cooper G. M. Transforming genes of human bladder and lung carcinoma cell lines are homologous to the ras genes of Harvey and Kirsten sarcoma viruses. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3637–3640. doi: 10.1073/pnas.79.11.3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lauro R., Obici S., Acquaviva A. M., Alvino C. G. Construction of recombinant plasmids containing rat thyroglobulin mRNA sequences. Gene. 1982 Jul-Aug;19(1):117–125. doi: 10.1016/0378-1119(82)90196-2. [DOI] [PubMed] [Google Scholar]
- Durban E. M., Boettiger D. Differential effects of transforming avian RNA tumor viruses on avian macrophages. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3600–3604. doi: 10.1073/pnas.78.6.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusco A., Pinto A., Ambesi-Impiombato F. S., Vecchio G., Tsuchida N. Transformation of rat thyroid epithelial cells by Kirsten murine sarcoma virus. Int J Cancer. 1981 Nov 15;28(5):655–662. doi: 10.1002/ijc.2910280519. [DOI] [PubMed] [Google Scholar]
- Fusco A., Pinto A., Tramontano D., Tajana G., Vecchio G., Tsuchida N. Block in the expression of differentiation markers of rat thyroid epithelial cells by transformation with Kirsten murine sarcoma virus. Cancer Res. 1982 Feb;42(2):618–626. [PubMed] [Google Scholar]
- Graf T., Ade N., Beug H. Temperature-sensitive mutant of avian erythroblastosis virus suggests a block of differentiation as mechanism of leukaemogenesis. Nature. 1978 Oct 12;275(5680):496–501. doi: 10.1038/275496a0. [DOI] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Gross J. L., Rifkin D. B. The effect of avian retroviruses on limb bud chondrogenesis in vitro. Cell. 1979 Nov;18(3):707–718. doi: 10.1016/0092-8674(79)90125-9. [DOI] [PubMed] [Google Scholar]
- Holtzer H., Biehl J., Yeoh G., Meganathan R., Kaji A. Effect of oncogenic virus on muscle differentiation. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4051–4055. doi: 10.1073/pnas.72.10.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikawa Y. Transformation of cultured rat liver cells by murine sarcoma viruses. Bibl Haematol. 1975;(40):165–177. doi: 10.1159/000397530. [DOI] [PubMed] [Google Scholar]
- Ikekubo K., Pervos R., Schneider A. B. Clearance of normal and tumor-related thyroglobulin from the circulation of rats: role of the terminal sialic acid residues. Metabolism. 1980 Jul;29(7):673–681. doi: 10.1016/0026-0495(80)90113-4. [DOI] [PubMed] [Google Scholar]
- Jones R. E., DeFeo D., Piatigorsky J. Initial studies on cultured embryonic chick lens epithelial cells infected with a temperature-sensitive Rous sarcoma virus. Vision Res. 1981;21(1):5–9. doi: 10.1016/0042-6989(81)90130-9. [DOI] [PubMed] [Google Scholar]
- Le Bousse-Kerdiles M. C., Smadja-Joffe F., Klein B., Caillou B., Jasmin C. Study of a virus-induced myeloproliferative syndrome associated with tumor formation in mice. Eur J Cancer. 1980 Jan;16(1):43–51. doi: 10.1016/0014-2964(80)90106-1. [DOI] [PubMed] [Google Scholar]
- MACPHERSON I., MONTAGNIER L. AGAR SUSPENSION CULTURE FOR THE SELECTIVE ASSAY OF CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Jun;23:291–294. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- Ostertag W., Freshney M., Vehmeyer K., Jasmin C., Rutter G. Action of temperature-sensitive mutants of myeloproliferative sarcoma virus suggests that fibroblast-transforming and hematopoietic transforming viral properties are related. J Virol. 1984 Jan;49(1):253–261. doi: 10.1128/jvi.49.1.253-261.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostertag W., Vehmeyer K., Fagg B., Pragnell I. B., Paetz W., Le Bousse M. C., Smadja-Joffe F., Klein B., Jasmin C., Eisen H. Myeloproliferative virus, a cloned murine sarcoma virus with spleen focus-forming properties in adult mice. J Virol. 1980 Feb;33(2):573–582. doi: 10.1128/jvi.33.2.573-582.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada L. F., Tabin C. J., Shih C., Weinberg R. A. Human EJ bladder carcinoma oncogene is homologue of Harvey sarcoma virus ras gene. Nature. 1982 Jun 10;297(5866):474–478. doi: 10.1038/297474a0. [DOI] [PubMed] [Google Scholar]
- Pierce J. H., Aaronson S. A. BALB- and Harvey-murine sarcoma virus transformation of a novel lymphoid progenitor cell. J Exp Med. 1982 Sep 1;156(3):873–887. doi: 10.1084/jem.156.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pragnell I. B., Fusco A., Arbuthnott C., Smadja-Joffe F., Klein B., Jasmin C., Ostertag W. Analysis of the myeloproliferative sarcoma virus genome: limited changes in the prototype lead to altered target cell specificity. J Virol. 1981 Jun;38(3):952–957. doi: 10.1128/jvi.38.3.952-957.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhim J. S., Kim C. M., Okigaki T., Huebner R. J. Transformation of rat liver epithelial cells by Kirsten murine sarcoma virus. J Natl Cancer Inst. 1977 Nov;59(5):1509–1518. doi: 10.1093/jnci/59.5.1509. [DOI] [PubMed] [Google Scholar]
- Santos E., Tronick S. R., Aaronson S. A., Pulciani S., Barbacid M. T24 human bladder carcinoma oncogene is an activated form of the normal human homologue of BALB- and Harvey-MSV transforming genes. Nature. 1982 Jul 22;298(5872):343–347. doi: 10.1038/298343a0. [DOI] [PubMed] [Google Scholar]
- Schrier P. I., Bernards R., Vaessen R. T., Houweling A., van der Eb A. J. Expression of class I major histocompatibility antigens switched off by highly oncogenic adenovirus 12 in transformed rat cells. 1983 Oct 27-Nov 2Nature. 305(5937):771–775. doi: 10.1038/305771a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Topp W. C. Normal rat cell lines deficient in nuclear thymidine kinase. Virology. 1981 Aug;113(1):408–411. doi: 10.1016/0042-6822(81)90168-9. [DOI] [PubMed] [Google Scholar]
- Tronick S. R., Robbins K. C., Canaani E., Devare S. G., Andersen P. R., Aaronson S. A. Molecular cloning of Moloney murine sarcoma virus: arrangement of virus-related sequences within the normal mouse genome. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6314–6318. doi: 10.1073/pnas.76.12.6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M. J., Friis R. R. Dissociation of transformation parameters using temperature-conditional mutants of Rous sarcoma virus. Cell. 1979 Jan;16(1):25–32. doi: 10.1016/0092-8674(79)90184-3. [DOI] [PubMed] [Google Scholar]
- Weissman B. E., Aaronson S. A. BALB and Kirsten murine sarcoma viruses alter growth and differentiation of EGF-dependent balb/c mouse epidermal keratinocyte lines. Cell. 1983 Feb;32(2):599–606. doi: 10.1016/0092-8674(83)90479-8. [DOI] [PubMed] [Google Scholar]
- Yuspa S. H., Vass W., Scolnick E. Altered growth and differentiation of cultured mouse epidermal cells infected with oncogenic retrovirus: contrasting effects of viruses and chemicals. Cancer Res. 1983 Dec;43(12 Pt 1):6021–6030. [PubMed] [Google Scholar]